Abstract
Pulmonary delivery of peptidomimetic antibiotics is frequently used for local drug therapy in pulmonary infections. Identification of transport pathways into airway epithelia can lead to new strategies of therapy. Here we describe the distribution of the β-lactam-transporting high-affinity proton-coupled peptide transporter PEPT2 in mammalian lungs. Using reverse transcriptase-polymerase chain reaction and Northern blot analysis, PEPT2-mRNA was detected in lung extracts. The expression of PEPT2-mRNA and protein was localized to alveolar type II pneumocytes, bronchial epithelium, and endothelium of small arteries of rat lung by nonisotopic in situ hybridization and immunohistochemistry. In addition, transport studies using murine whole-organ preparations revealed transporter-mediated uptake of a fluorophore-conjugated dipeptide derivative into bronchial epithelial cells and type II pneumocytes. This transport was competitively inhibited by cephalosporins and dipeptides that are reported as PEPT2-carried substrates. Cell specificity of the PEPT2-mediated uptake pattern was confirmed by double labeling with Lycopersicon esculentum lectin. Together these data suggest that PEPT2 is the molecular basis for the transport of peptides and peptidomimetics in pulmonary epithelial cells. In conclusion PEPT2 may be an interesting target for pulmonary delivery of peptides and peptidomimetics.
In addition to its role in regulating airway tone and production of alveolar lining fluid, the airway epithelium is an important barrier between higher organisms and their environment. The large surface area of ∼140 m 2 in adult human lungs can be efficiently used for the administration of different drugs. Indeed, a multitude of transport systems that mediate active uptake for water, 1 glucose, 2 antioxidants, 3 and amino acids 4 has been characterized in respiratory epithelia in the past years. Various functional studies have demonstrated a di- and tripeptide transport suggesting the presence of an oligopeptide transport system. 5-8
Recently, the cDNAs encoding two families of proton-coupled oligopeptide transporters have been cloned 9-15 from epithelial cells of intestine (PEPT1) and kidney cortex (PEPT2). Whereas PEPT1 is expressed in the intestine 16-18 and to a smaller extent in kidney 19,20 but not lung, 11,14 PEPT2 is expressed in the kidney, 10,15 central nervous system, 21-23 and in a variety of peripheral tissues including lung. 10,24 Both isoforms possess 12 membrane-spanning domains and share an identity of ∼47% at the protein level. The carrier proteins mediate electrogenic uphill peptide transport by coupling substrate translocation to the movement of H+/H3O+ with the transmembrane electrochemical proton gradient as the driving force. In addition to di- and tripeptides, both isoforms transport several peptidomimetics such as aminocephalosporins, aminopenicllins, bestatin, delta aminolevulinic acid (δ-ALA), and selected angiotensin-converting enzyme inhibitors as substrate. 25
Because some of these agents may be applied as aerosolic drugs, information about the localization and function of the oligopeptide transporter PEPT2 in the respiratory tract may be useful for the development of new therapeutic strategies.
Materials and Methods
In total, 18 adult Sprague-Dawley rats and 12 BALB/c mice housed under standard laboratory conditions and fed ad libitum were used. For each of the following techniques tissue samples of eight animals were used.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
RT-PCR was performed as described previously. 24 In brief, total RNA was isolated from rat lung, rat kidney, and rat intestine, and digested with DNase, followed by cDNA synthesis by reverse transcription. PCR amplification was performed for 35 cycles with 94°C denaturation for 1 minute, annealing for 1 minute (55°C for PEPT2, 55°C for PEPT1, and 61°C for GAPDH), 72°C extension for 1 minute and 72°C end-synthesis for 10 minutes. PEPT2-specific primers representing the nucleotides 111 to 134 (5′-GCTGCCTACTGAAGCCAAATGCTTG-3′) and 437 to 417 (5′-AGAGGCTGCTGAAGGCATGGT-3′) of the protein-coding region of PEPT2. PEPT1-specific primers representing the nucleotides 1105 to 1125 (5′-GGTTTCAACTTCACCTCCCTG-3′) and 1859 to 1839 (5′-CACTGTCTCTTCTGGTGGAGC-3′) of the open reading frame of PEPT1. GAPDH-specific primers representing the nucleotides 558 to 579 (5′-GACCACAGTCCATGACATCACT-3′) and 1010 to 990 (5′-TCCACCACCCTG-TTGCTGTAG-3′) of the open reading frame. A 1/10 volume of each sample was separated on a 1% agarose gel and stained by ethidium bromide. PCR controls were performed by using H2O instead of cDNA. The PCR products were sequenced and compared with the published sequences of PEPT2 and PEPT1 from rat kidney 15 and intestine. 14
PEPT2-cRNAs Probes
Digoxigenin-labeled PEPT2-specific cRNA probes were produced as following: a rat-specific PEPT2 PCR fragment (nucleotides 51 to 290 of the open reading frame of PEPT2) was ligated into the PCRII expression vector (Invitrogen, Leck, The Netherlands). The plasmid was linearized with EcoRI (for sense probe) or NotI (for antisense probe) and used as template to synthesize digoxigenin-labeled sense and antisense RNA according to the manufacturer’s manual (Boehringer Mannheim, Mannheim, Germany).
Northern Blot
Ten μg of total RNA prepared from rat lung was separated by agarose gel electrophoresis and transferred onto nylon membranes (Boehringer Mannheim). Lanes were hybridized with the digoxigenin-labeled PEPT2-specific cRNA probe. Hybridization was performed overnight at 65°C in the presence of 50% deionized formamide, 5× standard saline citrate (SSC), 0.1% (w/v) N-lauroylsarcosine, 0.02% (w/v) sodium dodecyl sulfate, and 2% blocking reagent (Boehringer Mannheim). After hybridization, membranes were washed twice for 15 minutes at 65°C in 2× SSC containing 0.1% sodium dodecyl sulfate and twice for 15 minutes at 65°C in 0.5× SSC containing 0.1% sodium dodecyl sulfate. For detection of the digoxigenin-labeled hybrids, membranes were washed briefly in phosphate-buffered saline (PBS) (1 time), blocked with blocking reagent (1 hour at room temperature; Boehringer Mannheim) and incubated with alkaline phosphatase-conjugated anti-digoxigenin antibody (diluted 1:400; 2 hours at room temperature; Boehringer Mannheim). Unbound antibody was removed by two washing steps in 100 mmol/L maleic acid, 150 mmol/L NaCl, and 0.3% Tween. Subsequent development was performed according to the manufacturer’s digoxigenin detection kit for glycoconjugate and protein analysis protocol (developing times, 2 to 4 hours; Boehringer Mannheim).
In Situ Hybridization
Detection of PEPT2-mRNA was performed by using nonradioactive in situ hybridization. Cryostat sections (8 μm) of rat lung were mounted on silane-precoated glass slides and fixed by immersion for 10 minutes in 4% paraformaldehyde. Tissue sections were treated with 0.1 N HCl (10 minutes), washed in 1× PBS and air-dried for 20 minutes. Each section was covered with 100 μl hybridization buffer (50% formamide, 1× Denhardt’s, 10 mmol/L triethanolamine, 5 mmol/L ethylenediaminetetraacetic acid, 6.25% dextransulfate, 0.3 mol/L NaCl, 1 mg/ml tRNA) containing 100 ng/100 μl PEPT2-specific digoxigenin-labeled sense or antisense cRNA probe. After hybridization, sections were washed twice for 15 minutes at 60°C in 5× SSC and twice for 15 minutes at 65°C in 1× SSC, followed by two 15-minute washes at 60°C in 0.1× SSC. Subsequently, the sections were treated with 20 μg/ml of RNase A to remove unhybridized single-stranded RNA. The detection and development of hybridization signals were performed as described above and in the manufacturer’s commercial digoxigenin-detection kit protocol (Boehringer Mannheim). Slides were mounted in 50% glycerol in 1× PBS (pH 7.4).
Immunohistochemistry
Immunohistochemistry was performed on 4% paraformaldehyde-fixed rat and murine lung specimens. Cryostat sections (8 μm) were washed several times in 1× PBS and preincubated for 1 hour at room temperature with 2% low-fat milk powder in Tris-buffered saline containing 1% Tween 20, pH 7.4. Sections were incubated with polyclonal anti-rabbit PEPT2 serum, 26 diluted 1:1,000 in the preincubation solution overnight. As secondary antibody an anti-rabbit indocarbocyanin (Cy3)-antibody (1:1,000; Dianova, Hamburg, Germany) was used. Specificity of the antibody reaction was verified in parallel sections that were incubated either with the primary antiserum that had been preabsorbed with the corresponding antigenic peptide (concentration 20 μg protein/ml diluted antiserum) or only the secondary antibodies. Slides were coverslipped in carbonate-buffered glycerol (pH 8.6) and viewed using epifluorescence microscopy.
Ex Vivo Uptake Studies
Mice of both sexes were killed by chloroform inhalation. The lung was rapidly removed and stored in Eagle’s minimum essential medium (MEM-21011; GIBCO, Karlsruhe, Germany) (37°C, gassed with 95% O2/5% CO2). Uptake experiments were performed by instillation of 1.0 ml MEM containing 25 μmol/L (d)-Ala-(l)-Lys-N-ε-7-amino-4-methylcoumarin-3-acetic acid (d-Ala-Lys-AMCA) 27 into the trachea. For inhibition studies 1.0 ml MEM containing 25 μmol/L d-Ala-Lys-AMCA and 1 mmol/L unlabeled glycyl-(l)-glutamine or 1 mmol/L unlabeled cefadroxil was used (Figure 1) ▶ . Controls were performed by incubation at 4°C or by omitting the labeled dipeptide conjugate. Incubation was stopped after 20 minutes by perfusion of the trachea with ice-cold MEM for 2 ×10 minutes. Lungs were fixed in 4% paraformaldehyde (in 0.1 mol/L phosphate buffer, pH 7.4) for 4 hours. Fixed tissues were rinsed several times in 1× PBS, pH 7.4, and incubated in 1× PBS containing 18% sucrose overnight. After freezing in liquid nitrogen-cooled isopentane the sections were cut to 8-μm cryostat sections and examined.
Figure 1.
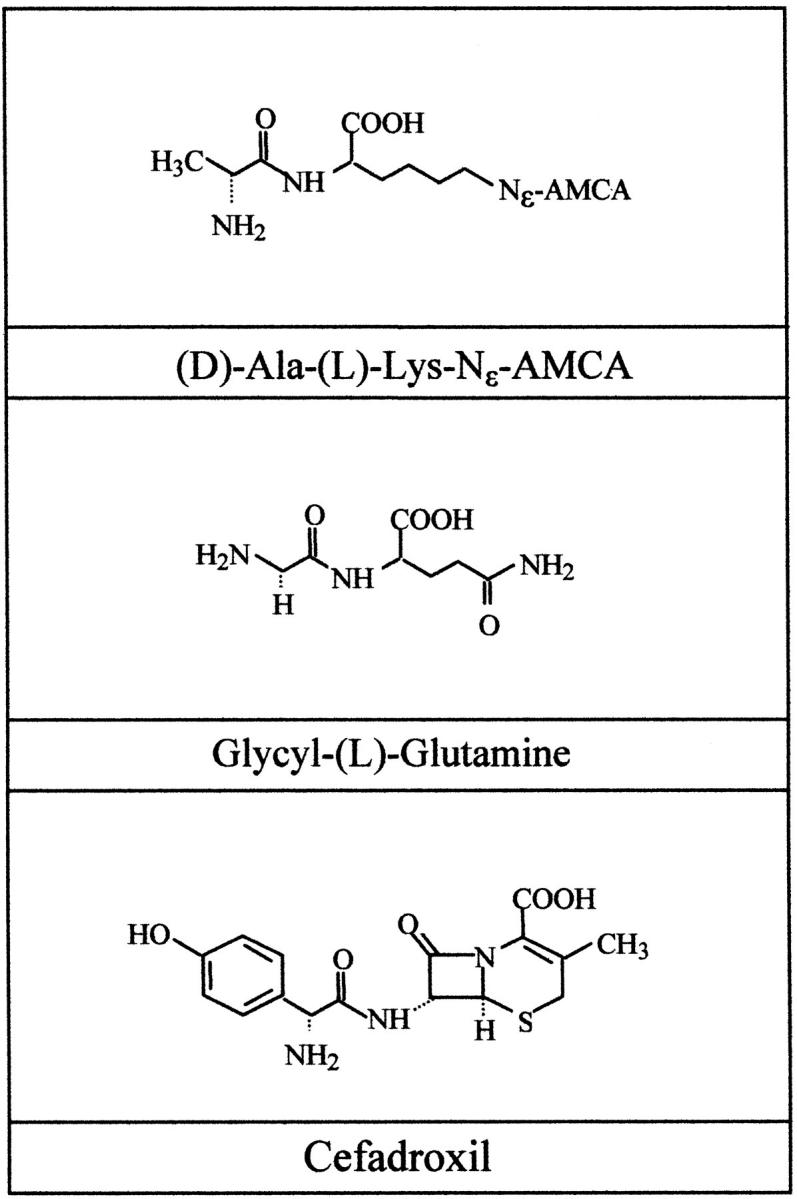
Substrates of ex vivo uptake studies. The fluorophore-conjugated dipeptide d-Ala-Lys-AMCA, unlabeled glycyl-(l)-glutamine, and unlabeled cefadroxil served as substrates for the in situ uptake studies.
Combined Ex Vivo Uptake and Histochemistry Studies
To demonstrate specifically the cellular identity of uptake displaying cells, a combined application of ex vivo uptake studies and histochemistry was established. Murine lung preparations were first used for uptake studies as described in the regular ex vivo uptake protocol above and then subjected to lectin histochemistry. Biotinylated Lycopersicon esculentum lectin (LEA) (Vector Laboratories, Burlingame, CA) was used as a marker for type I pneumocytes. 28,29 In brief, after the uptake protocol was completed, the sections were washed in PBS and preincubated with 2% low-fat milk powder in Tris-buffered saline containing 1% Tween 20, pH 7.4, for 30 minutes and methyl-α-d-mannopyranoside. Overnight incubation with LEA (1:160 diluted in the preincubation solution) was followed by the removal of the lectin by rinsing twice in PBS for 10 minutes each. Detection was performed with Texas Red (Dianova, Hamburg, Germany) and slides were coverslipped in carbonate-buffered glycerol (pH 8.6) and examined with fluorescence microscopy.
Results
Detection of PEPT2-mRNA in Lungs
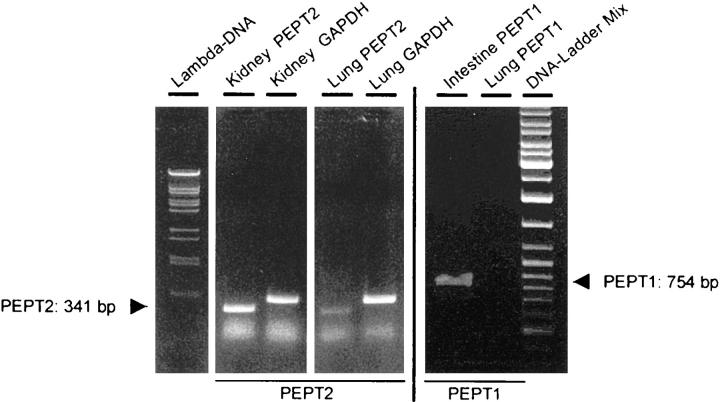
To demonstrate the expression of the PEPT2-mRNA in mammalian lungs, RT-PCR and Northern blot experiments were performed. Using mRNA from rat lung and kidney PEPT2-specific amplification products with a length of 341 bp were detected in both kidney and lung. PEPT1-specific products (754 bp) were found in the small intestine but not in lung extracts. Expression of the housekeeping gene GAPDH was positive in all probes (Figure 2) ▶ . The PCR product identities were confirmed by direct sequencing that revealed identity with the published sequences from rat kidney 14 and intestine. 15
Figure 2.
Detection of PEPT2-mRNA in rat lung by RT-PCR. Five μg of total RNA from rat lung and kidney were subjected to RT-PCR using primer pairs specific for PEPT2, PEPT1, or GAPDH. The RT-PCR products were separated by agarose gel electrophoresis and visualized with ethidium bromide.
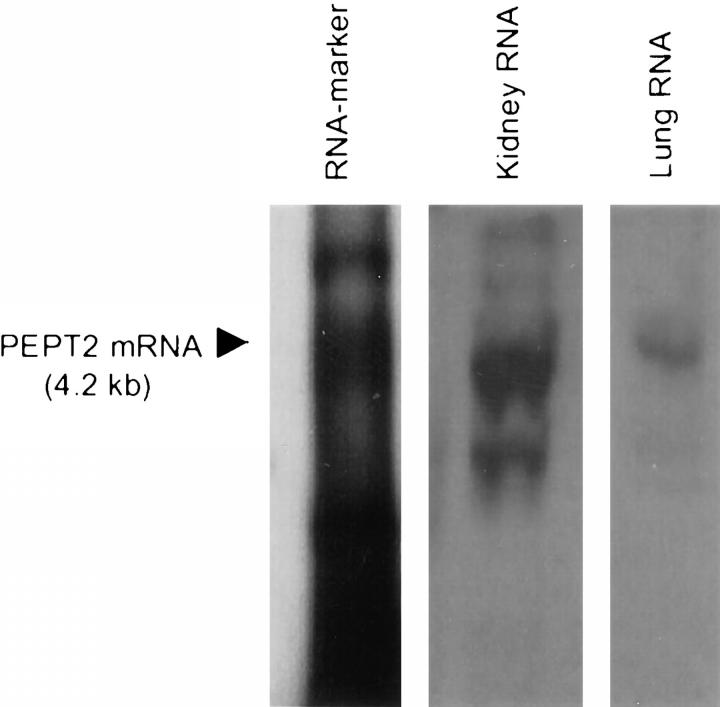
The PEPT2-mRNA was also identified by Northern blot analysis using a PEPT2-specific digoxigenin-labeled mRNA probe. The size (4.2 kb) of the hybridization signal obtained from lung RNA was identical to those of the kidney (Figure 3) ▶ .
Figure 3.
Detection of PEPT2-mRNA in rat lung by Northern blot analysis. Samples of total RNA (10 μg) from rat lung and kidney were separated by agarose gel electrophoresis, blotted, and hybridized with a specific PEPT2 antisense probe.
Distribution of PEPT2-mRNA in the Rat Lung
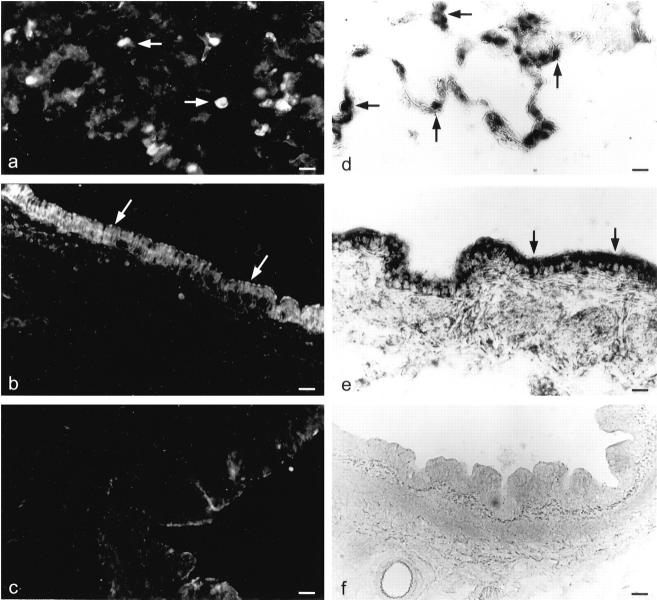
In the lower respiratory tract of rat, PEPT2-mRNA was localized to cells of the respiratory epithelium of large bronchi (Figure 4e) ▶ . No signals were present in connective tissue or smooth muscle bundles. In peripheral lung transcription signal was found in type II pneumocytes that are characterized by their prominent shape in the alveolar lumen (Figure 4d) ▶ . Also, endothelial cells of some smaller vessels revealed positive staining. Positive staining was reproducibly detected after hybridization with antisense probe. Controls with equivalent amounts of sense probe using the same hybridization and washing stringency were unstained on alternate sections and demonstrated the specificity of antisense signals (Figure 4f) ▶ . Omission of labeled cRNA probes from the hybridization mixture also resulted in unstained sections, identical to results obtained when RNA was digested before hybridization with RNase incubation.
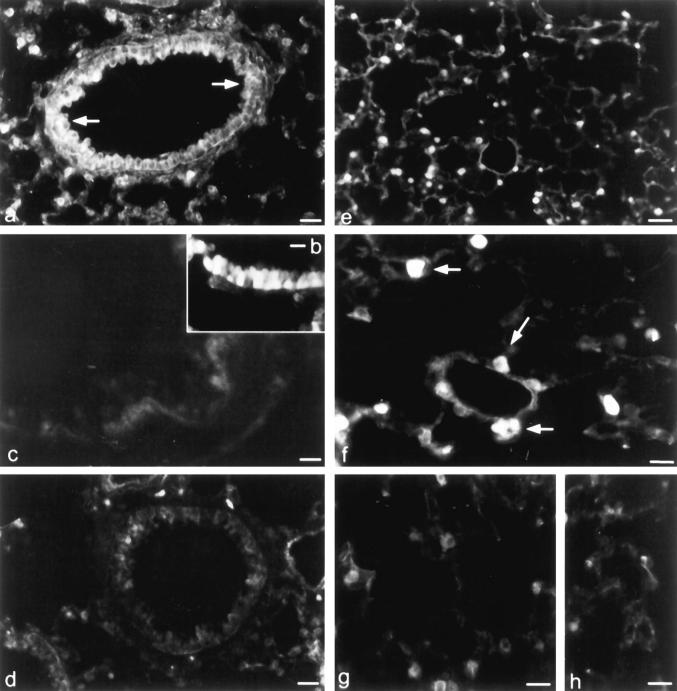
Figure 4.
Localization of PEPT2-mRNA and PEPT2-like immunoreactivity in the rat lung. Localization of PEPT2-like immunoreactivity in type II pneumocytes (a) and bronchial epithelium (b) of rat lung. Sections incubated with the PEPT2-antibody in the presence of the antigen peptide do not exhibit specific immunoreactivity (c). Distribution of PEPT2-mRNA in rat lung was detected by nonradioactive in situ hybridization. Cryostat sections (8 μm) of rat lung were hybridized with digoxigenin-labeled antisense (d and e) or sense (f) PEPT2-cRNA probe. Hybridization signals were obtained in bronchial epithelium (e) and type II pneumocytes (d). Scale bars: 22.5 μm (a), 45 μm (b), 34 μm (c), 16 μm (d), and 30 μm (e and f).
Distribution of PEPT2-Like Immunoreactivity in Rat and Murine Lungs
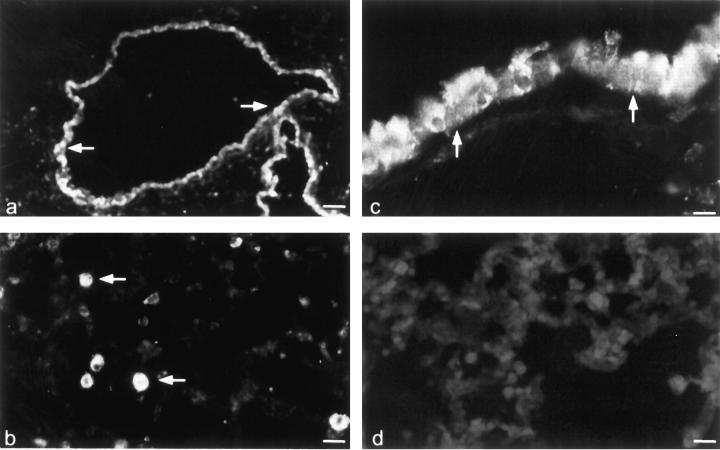
Immunohistochemistry with anti-PEPT2 serum was performed on sections from rat (Figure 4) ▶ and murine (Figure 5) ▶ lungs. In sections of rat lung, positive staining for PEPT2-like immunoreactivity was seen in tracheal, bronchial, and smaller airway epithelia, and it was especially strong in the apical border (Figure 4b) ▶ . In alveolar space, type II pneumocytes were stained cytoplasmatically (Figure 4a) ▶ . The endothelium of small vessels was also found to express PEPT2 immunoreactivity whereas there was no staining of bronchial glands. A similar distribution was obtained in murine lung with PEPT2-like immunoreactivity localized to bronchial epithelium (Figure 5, a and c) ▶ , type II pneumocytes (Figure 5b) ▶ , and endothelium of small vessels. Positive staining was not observed in sections of rat and murine lung when anti-PEPT2 serum was preabsorbed with the corresponding antigenic peptide sequence, showing the specificity of the immunostaining (Figures 4c and 5d) ▶ ▶ .
Figure 5.
Localization of PEPT2-like immunoreactivity in the murine lung. Immunofluorescence localization of PEPT2-like immunoreactivity in the bronchial epithelium (a and c) and type II pneumocytes (b) of murine lung. d: A control section stained for PEPT2-like immunoreactivity in the presence of the antigen peptide. Scale bars: 80 μm (a), 16 μm (b), 10 μm (c), and 14 μm (d).
Fluorophore-Conjugated Dipeptide Uptake in the Murine Lung
To assess whether the lung tissues also exhibit physiological peptide transport activity, the fluorophore-conjugated dipeptide d-Ala-Lys-AMCA was used as a reporter substrate. Incubation of murine lungs with 25 μmol/L d-Ala-Lys-AMCA in Eagle’s MEM solution containing different single amino acids revealed uptake and intracellular accumulation of AMCA fluorescence in type II pneumocytes (Figure 6, e and f) ▶ and epithelial cells of trachea and bronchi (Figure 6, a and b) ▶ . Incubation of lungs with 25 μmol/L d-Ala-Lys-AMCA in the presence of either 1 mmol/L unlabeled cefadroxil (Figure 6, d and g) ▶ or glycyl-(l)-glutamine (Figure 6, c and h) ▶ reduced fluorescence signals to a minimal extend. Control incubations at 4°C or without the labeled dipeptide revealed completely unstained samples.
Figure 6.
Uptake studies in murine whole-organ preparations. d-Ala-Lys-AMCA uptake was restricted to bronchial epithelial cells (arrows in a). There was no visible uptake as a result of adding 1 mmol/L of either cefadroxil (d) or Gly-(l)-Gln (c) to the d-Ala-Lys-AMCA solution. In the alveolar space, fluorescence accumulation was detected in type II pneumocytes (e, arrows in f) but signal was absent in type I cells (f). Inhibition studies with cefadroxil (g) or Gly-(l)-Gln (h) lead to a reduction of the signal in the type II pneumocytes. Scale bars: 60 μm (a, d, and e), 12 μm (f), 22 μm (b and c), 18 μm (g and h).
Combined Ex Vivo Uptake and Immunohistochemistry Studies
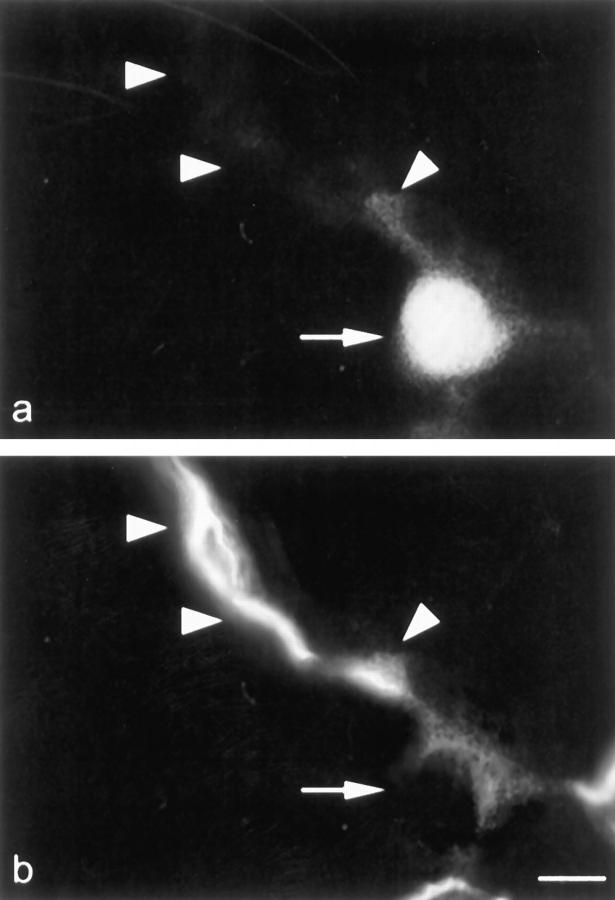
To validate the observation of substrate uptake and PEPT2 expression in type II but not type I pneumocytes, a combined uptake and immunohistochemical protocol was established. Incubation of lung preparations led to intracellular accumulation of d-Ala-Lys-AMCA fluorescence in bronchial epithelium and type II cells (Figure 7a) ▶ as reported for the regular uptake protocol. Consecutive immunohistological with LEA lectin stained type I pneumocytes specifically (Figure 7b) ▶ and revealed a pattern of immunofluorescence that was different to the PEPT2-mediated d-Ala-Lys-AMCA uptake and PEPT2-mRNA and protein expression.
Figure 7.
Combined ex vivo uptake and immunohistochemistry studies. d-Ala-Lys-AMCA fluorescence was not present in type I pneumocytes of the peripheral lung. Double labeling of the same section with LEA (b), a specific marker for type I cells, revealed the mutually exclusive presence. The uptake of d-Ala-Lys-AMCA (a) was restricted to type II pneumocytes (arrow) that are flanked by type I cell membranes (arrowheads) that displayed LEA-activity (b). Scale bar: 15 μm.
Discussion
This study demonstrates the presence of the high-affinity peptide transporter PEPT2 in alveolar type II pneumocytes, bronchial epithelium, and endothelium of small vessels in mammalian lungs. The existence of a specific transport mechanism for oligopeptides in the lung has been suggested by studies demonstrating an uptake for di- and tripeptides. 5-8 RT-PCR revealed expression of PEPT2 but not PEPT1 in lung tissues as reported earlier for rat tissues in the expression cloning studies. 14,15 The localization of both PEPT2-mRNA by nonradioactive in situ hybridization and PEPT2 protein by immunohistochemistry allowed us to identify the transporter and to determine the exact cellular location of the transporter. Extending these observations, we developed experiments that allowed the visualization of cell types that possess a functional peptide transporter. The used reporter molecule d-Ala-Lys-AMCA has previously been shown to serve as a substrate for PEPT2 in renal LL-CPK1 cells 27 and in yeast cells expressing heterologously PEPT2. 26 Specific uptake of the reporter substrate into type II cells as well as into bronchial and tracheal epithelium confirmed the morphological data of immunohistochemistry and in situ hybridization. The simultaneous demonstration of the presence of PEPT2-mRNA and protein together with the functional characteristics of a PEPT2-mediated uptake provides circumstantial evidence to identify the transport route as a PEPT2-mediated process.
Because type I cells are susceptible to distortion of their shape by tissue processing, combined uptake and histochemical studies were established. The type I cell-specific LEA was used as marker. 28,29 Double labeling of the same area with LEA revealed a mutually exclusive presence and therefore validated the morphological findings of the different protocols for in situ hybridization, immunohistochemistry, and uptake studies. The absence of PEPT2-mRNA and protein- and transporter-mediated uptake in type I cells gives evidence for suggestions by earlier functional studies. 5-7
The lack of fluorescence in endothelial cells that possess the PEPT2 message may be explained by the route of administration of the substrate through the airways that may not allow the substrate to reach the endothelium in sufficient quantities, or by a different subcellular expression of the transporter. In this respect, the high capacity transporter has been localized to nuclei and lysosomes of pancreatic exocrine cells earlier. 30
Recent studies revealed a variety of PEPT2 substrates with therapeutic interests. In this respect the transporter was characterized to mediate uptake of a variety of β-lactam antibiotics. 31,32 The pulmonary route is an attractive alternative to oral application of peptides and peptidomimetics because of low proteolytic activity and bypassed hepatic metabolism. 33-35 Clinical trials demonstrated benefits when antibiotics were administered by inhalation. 36 The demonstration of competitive inhibition of d-Ala-l-Lys-AMCA uptake in murine lung by cefadroxil is strong evidence for its transport by PEPT2. Cefadroxil, a semisynthetic cephalosporine that has proved effective against gram-negative and gram-positive pulmonary infections 37 has previously been reported to act as a substrate for PEPT2. 10 Therefore, PEPT2 expression in mammalian lungs may present a novel target for delivery of antibiotic therapeutics via the airways.
The previous demonstration of δ-ALA as a substrate for PEPT2 24 is a new finding with a number of physiological and pharmacological implications in airway tissue. On the basis of being a substrate for heme synthesis, δ-ALA may play a role in the pulmonary production of carbon monoxide, a possible signaling molecule produced by the stress-inducible heat-shock protein heme oxygenase I and its constitutional isoform heme oxygenase II. 38 Carbon monoxide is discussed as a marker for chronic airway inflammations such as asthma. 39 δ-ALA’s therapeutical relevance is based on photodynamic therapy 40,41 that uses accumulation of porphyrins after administration of δ-ALA to induce tissue necrosis and apoptosis. As δ-ALA is discussed for aerosol administration in lung tumors we provide data for the possible uptake mechanisms and determine the cellular site of uptake in airway tissues. Further investigation will be needed to determine the expression of PEPT2 in lung tumor cells to reveal the therapeutic value of the transporter in neoplasms.
In summary, we have identified the cellular sites of the PEPT2 expression and provided functional data about this transport system in the lung. Together with recent findings on the molecular requirements of peptide transporter substrates, 42 our findings may provide a basis for the development of novel therapeutic strategies using PEPT2-specific drugs delivered via aerosolic administration for the treatment of infectious and neoplastic respiratory diseases.
Footnotes
Address reprint requests to Axel Fischer, M.D., Dept. of Pediatric Pneumology and Immunology, Charite-Virchow Klinikum, Humboldt-University, Augustenburger Platz 1, D-13353 Berlin, Germany. E-mail: axel.fischer@charite.de.
Supported by the DFG (SFB547).
References
- 1.Folkesson HG, Matthay MA, Hasegawa H, Kheradmand F, Verkman AS: Transcellular water transport in lung alveolar epithelium through mercury-sensitive water channels. Proc Natl Acad Sci USA 1994, 91:4970-4974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kemp PJ, Boyd CA: Pathways for glucose transport in type II pneumocytes freshly isolated from adult guinea pig lung. Am J Physiol 1992, 263:L612-L616 [DOI] [PubMed] [Google Scholar]
- 3.Bai C, Brown LA, Jones DP: Glutathione transport by type II cells in perfused rat lung. Am J Physiol 1994, 267:L447-L455 [DOI] [PubMed] [Google Scholar]
- 4.Brown SE, Kim KJ, Goodman BE, Wells JR, Crandall ED: Sodium-amino acid cotransport by type II alveolar epithelial cells. J Appl Physiol 1985, 59:1616-1622 [DOI] [PubMed] [Google Scholar]
- 5.Helliwell PA, Meredith D, Boyd CA, Bronk JR, Lister N, Bailey PD: Tripeptide transport in rat lung. Biochim Biophys Acta 1994, 1190:430-434 [DOI] [PubMed] [Google Scholar]
- 6.Meredith D, Boyd CA: Dipeptide transport characteristics of the apical membrane of rat lung type II pneumocytes. Am J Physiol 1995, 269:L137-L143 [DOI] [PubMed] [Google Scholar]
- 7.Morimoto K, Yamahara H, Lee VH, Kim KJ: Dipeptide transport across rat alveolar epithelial cell monolayers. Pharm Res 1993, 10:1668-1674 [DOI] [PubMed] [Google Scholar]
- 8.Yamashita F, Kim KJ, Lee VH: Dipeptide uptake and transport characteristics in rabbit tracheal epithelial cell layers cultured at an air interface. Pharm Res 1998, 15:979-983 [DOI] [PubMed] [Google Scholar]
- 9.Boll M, Markovich D, Weber WM, Korte H, Daniel H, Murer H: Expression cloning of a cDNA from rabbit small intestine related to proton-coupled transport of peptides, beta-lactam antibiotics and ACE-inhibitors. Pflugers Arch 1994, 429:146-149 [DOI] [PubMed] [Google Scholar]
- 10.Boll M, Herget M, Wagener M, Weber WM, Markovich D, Biber J, Clauss W, Murer H, Daniel H: Expression cloning and functional characterization of the kidney cortex high-affinity proton-coupled peptide transporter. Proc Natl Acad Sci USA 1996, 93:284-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fei YJ, Kanai Y, Nussberger S, Ganapathy V, Leibach FH, Romero MF, Singh SK, Boron WF, Hediger MA: Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature 1994, 368:563-566 [DOI] [PubMed] [Google Scholar]
- 12.Liang R, Fei YJ, Prasad PD, Ramamoorthy S, Han H, Yang-Feng TL, Hediger MA, Ganapathy V, Leibach FH: Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J Biol Chem 1995, 270:6456-6463 [DOI] [PubMed] [Google Scholar]
- 13.Liu W, Liang R, Ramamoorthy S, Fei YJ, Ganapathy ME, Hediger MA, Ganapathy V, Leibach FH: Molecular cloning of PEPT 2, a new member of the H+/peptide cotransporter family, from human kidney. Biochim Biophys Acta 1995, 1235:461-466 [DOI] [PubMed] [Google Scholar]
- 14.Saito H, Okuda M, Terada T, Sasaki S, Inui K: Cloning and characterization of a rat H+/peptide cotransporter mediating absorption of beta-lactam antibiotics in the intestine and kidney. J Pharmacol Exp Ther 1995, 275:1631-1637 [PubMed] [Google Scholar]
- 15.Saito H, Terada T, Okuda M, Sasaki S, Inui K: Molecular cloning and tissue distribution of rat peptide transporter PEPT2. Biochim Biophys Acta 1996, 1280:173-177 [DOI] [PubMed] [Google Scholar]
- 16.Tanaka H, Miyamoto KI, Morita K, Haga H, Segawa H, Shiraga T, Fujioka A, Kouda T, Taketani Y, Hisano S, Fukui Y, Kitagawa K, Takeda E: Regulation of the PepT1 peptide transporter in the rat small intestine in response to 5-fluorouracil-induced injury. Gastroenterology 1998, 114:714-723 [DOI] [PubMed] [Google Scholar]
- 17.Freeman TC, Bentsen BS, Thwaites DT, Simmons NL: H+/di-tripeptide transporter (PepT1) expression in the rabbit intestine. Pflugers Arch 1995, 430:394-400 [DOI] [PubMed] [Google Scholar]
- 18.Ogihara H, Suzuki T, Nagamachi Y, Inui K, Takata K: Peptide transporter in the rat small intestine: ultrastructural localization and the effect of starvation and administration of amino acids. Histochem J 1999, 31:169-174 [DOI] [PubMed] [Google Scholar]
- 19.Smith DE, Pavlova A, Berger UV, Hediger MA, Yang T, Huang YG, Schnermann JB: Tubular localization and tissue distribution of peptide transporters in rat kidney. Pharm Res 1998, 15:1244-1249 [DOI] [PubMed] [Google Scholar]
- 20.Shen H, Smith DE, Yang T, Huang YG, Schnermann JB, Brosius FC, III: Localization of PEPT1 and PEPT2 proton-coupled oligopeptide transporter mRNA and protein in rat kidney. Am J Physiol 1999, 276:F658-F665 [DOI] [PubMed] [Google Scholar]
- 21.Dieck ST, Heuer H, Ehrchen J, Otto C, Bauer K: The peptide transporter PepT2 is expressed in rat brain and mediates the accumulation of the fluorescent dipeptide derivative beta-Ala-Lys-Nepsilon-AMCA in astrocytes. Glia 1999, 25:10-20 [DOI] [PubMed] [Google Scholar]
- 22.Dringen R, Hamprecht B, Broer S: The peptide transporter PepT2 mediates the uptake of the glutathione precursor CysGly in astroglia-rich primary cultures. J Neurochem 1998, 71:388-393 [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Fei YJ, Ganapathy V, Leibach FH: Electrophysiological characteristics of the proton-coupled peptide transporter PEPT2 cloned from rat brain. Am J Physiol 1998, 275:C967-C975 [DOI] [PubMed] [Google Scholar]
- 24.Doring F, Walter J, Will J, Focking M, Boll M, Amasheh S, Clauss W, Daniel H: Delta-aminolevulinic acid transport by intestinal and renal peptide transporters and its physiological and clinical implications. J Clin Invest 1998, 101:2761-2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniel H, Herget M: Cellular and molecular mechanisms of renal peptide transport. Am J Physiol 1997, 273:F1-F8 [DOI] [PubMed] [Google Scholar]
- 26.Doring F, Michel T, Rosel A, Nickolaus M, Daniel H: Expression of the mammalian renal peptide transporter PEPT2 in the yeast Pichia pastoris and applications of the yeast system for functional analysis. Mol Membr Biol 1998, 15:79-88 [DOI] [PubMed] [Google Scholar]
- 27.Wenzel U, Diehl D, Herget M, Kuntz S, Daniel H: Regulation of the high-affinity H+/peptide cotransporter in renal LLC-PK1 cells. J Cell Physiol 1999, 178:341-348 [DOI] [PubMed] [Google Scholar]
- 28.Kasper M, Rudolf T, Hahn R, Peterson I, Muller M: Immuno- and lectin histochemistry of epithelial subtypes and their changes in a radiation-induced lung fibrosis model of the mini pig. Histochemistry 1993, 100:367-377 [DOI] [PubMed] [Google Scholar]
- 29.Fehrenbach H, Kasper M, Tschernig T, Pan T, Schuh D, Shannon JM, Muller M, Mason RJ: Keratinocyte growth factor-induced hyperplasia of rat alveolar type II cells in vivo is resolved by differentiation into type I cells and by apoptosis. Eur Respir J 1999, 14:534-544 [DOI] [PubMed] [Google Scholar]
- 30.Bockman DE, Ganapathy V, Oblak TG, Leibach FH: Localization of peptide transporter in nuclei and lysosomes of the pancreas. Int J Pancreatol 1997, 22:221-225 [DOI] [PubMed] [Google Scholar]
- 31.Daniel H: Function and molecular structure of brush border membrane peptide/H+ symporters. J Membr Biol 1996, 154:197-203 [DOI] [PubMed] [Google Scholar]
- 32.Inui K, Terada T: Dipeptide transporters. Pharm Biotechnol 1999, 12:269-288 [DOI] [PubMed] [Google Scholar]
- 33.Niven RW, Rypacek F, Byron PR: Solute absorption from the airways of the isolated rat lung. III. Absorption of several peptidase-resistant, synthetic polypeptides: poly-(2-hydroxyethyl)-aspartamides. Pharm Res 1990, 7:990-994 [DOI] [PubMed] [Google Scholar]
- 34.Byron PR, Patton JS: Drug delivery via the respiratory tract. J Aerosol Med 1994, 7:49-75 [DOI] [PubMed] [Google Scholar]
- 35.Hoover JL, Rush BD, Wilkinson KF, Day JS, Burton PS, Vidmar TJ, Ruwart MJ: Peptides are better absorbed from the lung than the gut in the rat. Pharm Res 1992, 9:1103-1106 [DOI] [PubMed] [Google Scholar]
- 36.Nolan G, Moivor P, Levison H, Fleming PC, Corey M, Gold R: Antibiotic prophylaxis in cystic fibrosis: inhaled cephaloridine as an adjunct to oral cloxacillin. J Pediatr 1982, 101:626-630 [DOI] [PubMed] [Google Scholar]
- 37.Chisholm DR, DeRegis RG, Behr DA: Therapeutic efficacy of cefadroxil and cephalexin for pneumonia in a rat test model. Antimicrob Agents Chemother 1986, 30:105-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maines MD: Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J 1988, 2:2557-2568 [PubMed] [Google Scholar]
- 39.Horvath I, Donnelly LE, Kiss A, Paredi P, Kharitonov SA, Barnes PJ: Raised levels of exhaled carbon monoxide are associated with an increased expression of heme oxygenase-1 in airway macrophages in asthma: a new marker of oxidative stress. Thorax 1998, 53:668-672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q: Photodynamic therapy. J Natl Cancer Inst 1998, 90:889-905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rowe PM: Photodynamic therapy begins to shine. Lancet 1998, 351:1496. [DOI] [PubMed] [Google Scholar]
- 42.Doring F, Will J, Amasheh S, Clauss W, Ahlbrecht H, Daniel H: Minimal molecular determinants of substrates for recognition by the intestinal peptide transporter. J Biol Chem 1998, 273:23211-23218 [DOI] [PubMed] [Google Scholar]