Abstract
Provision of adequate T cell costimulation is critical for the development of acute and chronic allograft rejection. We have previously reported that early blockade of CD28-B7 T cell costimulation prevents the development of graft arteriosclerosis, in the LEW into F344 rat cardiac transplant model. In this study, we used the same model to examine the requirement for CD28-B7-mediated T cell costimulation in the progression of established chronic rejection and examined the individual roles of B7–1 (CD80) and B7–2 (CD86) costimulatory molecules. Late blockade of CD28-B7 T cell costimulation by the fusion protein CTLA4Ig, which binds both CD80 and CD86, attenuated the development of transplant arteriosclerosis, mononuclear cell infiltration, and parenchymal fibrosis in this model. Selective blockade of CD80 using the mutant fusion protein Y100F was as effective as CTLA4Ig in this regard. In contrast to CTLA4Ig, blockade of CD80 alone by Y100F was ineffective at preventing early graft loss and prolonging graft survival when given early after transplantation. This study is the first to demonstrate that late blockade of CD28-B7 T cell costimulation interrupts chronic cardiac allograft rejection, and it indicates the importance of continued T cell activation in this process. This study further defines functional differences between CD80 and CD86 costimulatory molecules in vivo.
Chronic rejection is the major cause of late graft loss in solid organ transplantation. 1 It is characterized histologically by the development of transplant arteriosclerosis, a vasculopathy consisting of concentric fibrointimal thickening in large and medium-sized arteries and arterioles. 2 Other cardinal histological features of chronic rejection include perivascular and interstitial mononuclear cell infiltrates and parenchymal fibrosis. The etiology of chronic rejection remains undefined, but increasing evidence suggests that it may represent a common immune-mediated injury response to multiple etiological factors. This is exemplified by the many disparate risk factors that predispose to its development, including alloantigen-dependent factors such as major histocompatibility complex (MHC) incompatibility and alloantigen-independent factors such as perioperative ischemic graft damage, viral infections, and hyperlipidemia. 3
We are currently investigating the role of the CD4+ T cell in the initiation and coordination of the immune response underlying chronic rejection. T lymphocytes require two signals to become fully activated: an antigen-dependent signal induced by ligation of the T cell receptor and a costimulatory signal induced predominantly by ligation of T cell CD28 with a member of the B7 family of molecules (CD80 or CD86) on antigen-presenting cells. 4 Ligation of CD28 is necessary for maximal CD4+ T cell cytokine production, proliferation and prevention of activation-induced apoptosis. CD80 and CD86 are the only ligands for T cell CD28 and interactions between both ligands and CD28 are interrupted by CTLA4Ig, 5 a recombinant fusion protein that contains the extracellular domain of human CTLA4, a homologue of CD28, fused to a human IgG1 heavy chain. Blockade of CD28-B7 interactions using CTLA4Ig prevents CD4+ T cell activation in response to alloantigen and leads to antigen-specific T cell anergy. 6 CTLA4Ig is effective in preventing allograft rejection and inducing transplantation tolerance in several experimental transplant models. 7 Thus, costimulatory blockade using CTLA4Ig provides an experimental tool to examine the role of T cell activation in the initiation and progression of chronic allograft rejection.
We have previously shown that inhibition of T cell activation early after transplantation by blocking T cell costimulation with CTLA4Ig results in long term survival in the majority of grafts and prevents the development of graft arteriosclerosis in the LEW to F344 rat heterotopic cardiac transplant model. 2,8 In these studies, we wished to determine the role of continued T cell activation on the progression of chronic rejection in this model by examining the effects of late T cell costimulation blockade. Furthermore, we used the mutant form of CTLA4Ig, Y100F, to determine the effects of selective CD80 blockade at early and late time points post-transplant in the development of chronic rejection.
Materials and Methods
Animals, Reagents, and Experimental Protocols
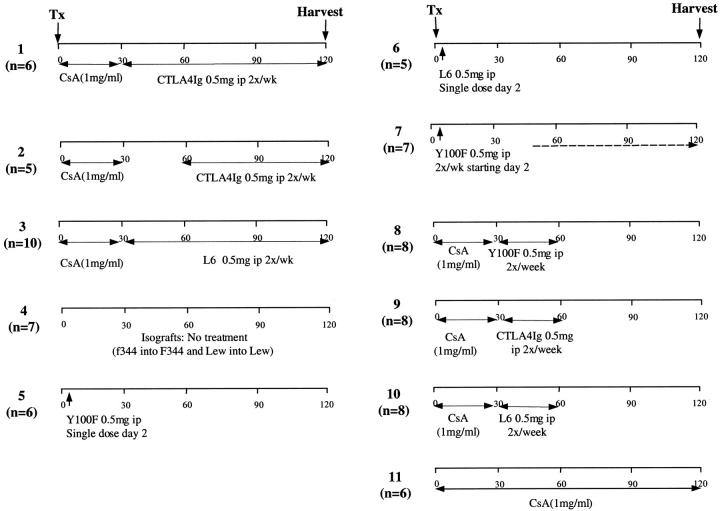
Inbred adult male LEW and F344 rats were purchased from Harlan Sprague-Dawley (Indianapolis, IN). F344 rats served as recipients of LEW cardiac allografts. Hearts were transplanted to the infrarenal great vessels by standard microvascular techniques as described previously 2,9 under ether anesthesia. F344-to-F344 and LEW-to-LEW isografts served as controls. Graft function was monitored by daily palpation of the transplanted heart; time of rejection was defined as the day of complete cessation of myocardial contraction. Allografts were harvested routinely at day 120 or after rejection. Survival estimates were calculated using the Kaplan Meier test and the log rank test was used to compare differences in survival between groups. CTLA4Ig, mutant CTLA4Ig Y100F and control fusion protein L6 (Bristol-Myers Squibb Pharmaceutical Research Institute, Princeton, NJ) 10 were used to study the role of CD28-B7 T cell costimulatory molecule blockade on the course of chronic rejection. Y100F is a mutant form of CTLA4Ig that selectively binds CD80. Figure 1 ▶ shows the dose and time course of treatment in each group.
Figure 1.
Treatment and control groups.
Morphometric and Histological Analyses
Coronal sections of graft tissue were fixed in 10% formaldehyde, embedded in paraffin, sectioned, and stained with Verhoeff’s elastin (arteriosclerosis scoring) or Masson’s trichrome (fibrosis and infiltrate scoring). Arteriosclerosis was assessed by light microscopy and percentage luminal occlusion by intimal thickening determined using the scoring system (scale, 0–5) described by Adams et al. 11 All arteries seen were examined and scored by two blinded examiners. A total of 325 vessels were analyzed with an average of 14 scored per graft. Only vessels that were cut orthogonally and displayed a clear internal elastic lamina were scored.
Matched trichrome-stained sections were also scored by a pathologist (RM) blinded for the treatment groups to look for fibrosis, interstitial cellular infiltration, and vasculitis. Each of these categories was graded, and the average grade was calculated for each group. Fibrosis was quantified by collagen deposition highlighted by trichrome stain and scored on a 0–3 scale where 0 indicated no fibrosis, and 1, 2, and 3 indicated mild, moderate, and severe fibrosis, respectively. A scale of 0–4 was used for scoring the degree of interstitial mononuclear cell infiltration, where 0 indicated no cellular infiltration and 1, 2, 3, and 4 indicated minimal, mild, moderate, and severe cellular infiltration, respectively. Vasculitis was assessed by the degree of inflammatory cell adhesion to the endothelial surface and infiltration of the muscle layer, which was scored 0 (up to 10% of all vessels were involved), 1 (10–50%), or 2 (>50%).
Nonparametric statistical tests were used to compare arteriosclerosis scores and fibrosis scores. The Kruskal-Wallis analysis of variance test was used to assess variance between groups and Dunn’s Post test was then used to determine differences between individual groups.
Isolation of RNA and Reverse Transcription Polymerase Chain Reaction (PCR) Analysis
Total RNA was extracted from frozen tissue specimens using the Ultraspec RNA isolation system (Biotecx, Houston, TX) and was quantified by spectrophotometry. cDNA was prepared by reverse transcription of 5 μg of RNA using random hexamer primers (100 ng/μl) and Moloney murine leukemia virus reverse transcriptase (50 u/μl) (Stratagene, La Jolla, CA) in a 50-μl reaction. Ten microliters of cDNA were used for each PCR amplification reaction. PCR was performed with Taq DNA polymerase using the buffer supplied by the manufacturer (Boehringer Mannheim, Indianapolis, IN). Primers and PCR conditions used were, for monocyte chemotactic peptide-1(MCP-1): 5′-ATGCAGGTCTCTGTCACG-3′ and 3′-CTAGTTCTCTGTCATACT-5′ (50°C, 28 cycles); for inducible nitric oxide synthase: 5′-TGCCAGGGTCACAACTTTACAGG-3′ and 3′-GGTCGATGTCACATGCAGCTTGTC-5′ (60°C, 35 cycles); and for transforming growth factor-β (TGF-β) 5′-TGAACCAAGGAGACGGAATACAGG-3′ and 3′-TACTGTGTGTCCAGGCTCCAAATG-5′ (57°C, 26 cycles; Integrated DNA Technologies, Coralville, IA). The PCR conditions were 94°C for 30 seconds, 50–60°C for 30 seconds, and 72°C for 2 minutes. PCR products were analyzed by ethidium bromide staining in 1.5% agarose gels using standard techniques. The densitometric band densities were measured using computerized imaging software (ALpha-Imager, version 0.1.12, Alpha Innotech Corp, San Leandro, CA). Glyceraldehyde-3-phosphate dehydrogenase gene products were simultaneously measured and used to correct for variations in cDNA amounts between samples. 2,12
Rat Carotid Balloon Injury Model
Twenty male Sprague-Dawley rats (275–300 g) underwent endothelial denudation of the left carotid artery (Zivic Miller, Zelienople, PA). 13 Rats were randomized to daily i.p. injections of 1 mg CTLA4Ig (Bristol-Myers Squibb, Seattle, WA) diluted in phosphate-buffered saline (1 mg/ml) or vehicle control for 14 days, beginning on the day of injury. On day 14, the left (injured) and right (uninjured) carotids were dissected and removed. The center piece of the carotid artery was fixed in methyl carnoys and embedded in paraffin. Four-micron sections were treated with Verhoeff’s stain to identify the elastic lamina. 14 Using Scion Image 1.55, morphometric analysis was completed on elastin-stained sections by measuring the area of the lumen (L), internal elastic lamina (IEL), and external elastic lamina (EEL). These measurements were then used to determine the percentage of luminal occlusion, (IEL-L/IEL) × 100, and intima-to-media ratio, IEL/(EEL-IEL). The measurements were tabulated for three separate sections per carotid. Mean scores were pooled for the two subgroups and are reported as the mean levels ± SD. Results were subjected to analysis of variance to evaluate significant differences between CTLA4Ig and control groups.
Results
Late Blockade of CD4+ T Cell Costimulation with CTLA4Ig Interrupts the Development of Chronic Rejection
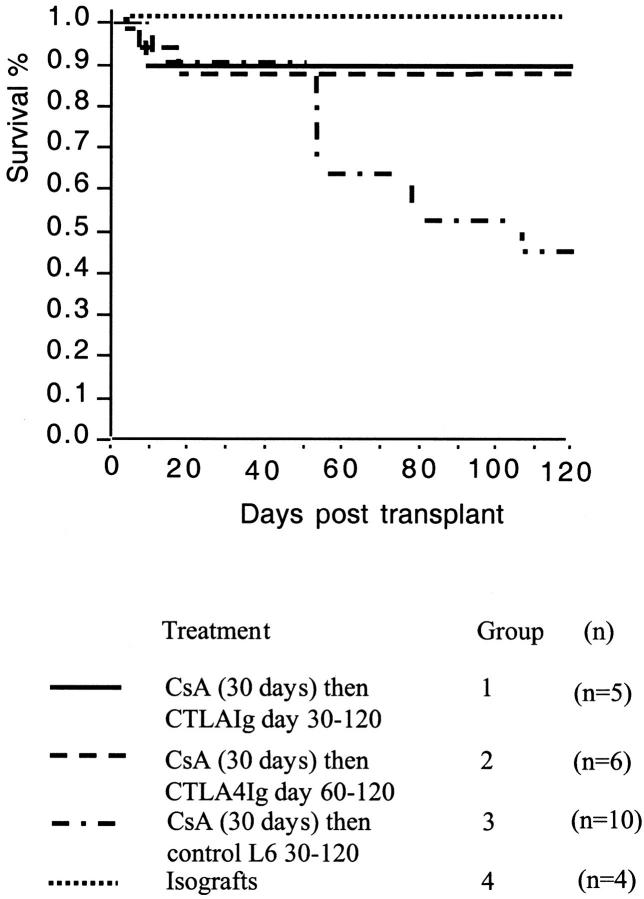
We have previously shown that a single injection of CTLA4Ig on day 2 post-transplantation prevented development of graft arteriosclerosis in the LEW into F344 model of chronic cardiac allograft rejection. 2 We now report the effects of late CD28-B7 blockade by administration of CTLA4Ig starting on day 30 or 60 post-transplantation. This is a clinically relevant strategy, because it addresses the issue of interruption of progression of chronic rejection and the therapy of established disease. Illustrated in Figure 2 ▶ is the percentage of survival of allografts within each experimental group plotted against time. Whereas approximately 50% of grafts from the control group 3 rejected before 120 days, all isografts and the majority of grafts from CTLA4Ig-treated animals (groups 1 and 2) survived to >120 days (time of harvest; P < 0.05 by Kaplan Meier). Thus, blocking T cell costimulation by CTLA4Ig late after acute graft injury was effective in preventing chronic graft loss.
Figure 2.
Kaplan-Meier survival plot for cardiac allografts from groups 1 to 4. Allografts surviving to 120 days were routinely harvested. Survival for each group was graphed together for the first 30 days post-transplant, after which time treatment regimes diverged. There was no loss of any of the transplants post-day 30 in the isograft (group 4) or CTLA4 Ig-treated groups (groups 1 and 2). This was significantly different from the control L6-treated (group 3) in which 50% of the allografts surviving to day 30 had stopped by day 120 (P < 0.05 by Kaplan-Meier), giving an overall survival of 45%.
Grafts surviving >100 days were examined histologically for graft arteriosclerosis. Shown in Table 1 ▶ are the mean arteriosclerosis scores for each group. Control animals (group 3) develop significant arteriosclerosis (mean intimal thickening score was 1.68 ± 0.81 with 55.93 ± 26.06% of the total number of vessels found to be diseased). In comparison, isografts do not develop arteriosclerosis (mean intimal thickening score for isografts was 0.14 ± 0.14 with 2.78 ± 4.81% of vessels diseased; P < 0.001). Late blockade of T cell costimulation with CTLA4Ig starting on day 30 or day 60 is associated with a significant attenuation of graft arteriosclerosis as compared to controls (P < 0.001). Interestingly, more prolonged costimulatory blockade (90 days in group 1 vs. 60 days in group 2) was associated with greater attenuation of graft arteriosclerosis. Representative vessels from isografts and allografts from control and CTLA4Ig-treated groups are shown in Figure 3 ▶ .
Table 1.
CTLA4Ig Interrupts Progression of Transplant Arteriosclerosis
| Group | n | Vessel score | Diseased vessel (%) |
|---|---|---|---|
| CTLA4Ig 30–120 | 6 | 0.39 ± 0.30* | 16.32 ± 13.21* |
| CTLA4Ig 60–120 | 5 | 0.57 ± 0.35* | 25.35 ± 12.76* |
| Control Ig | 6 | 1.68 ± 0.81 | 55.93 ± 26.06 |
| Isograft | 7 | 0.14 ± 0.14* | 2.78 ± 4.81* |
Comparison of group versus control Ig-treated group. Values expressed as means ± SD.
*P < 0.001 using an analysis of variance with Bonferroni correction.
Figure 3.
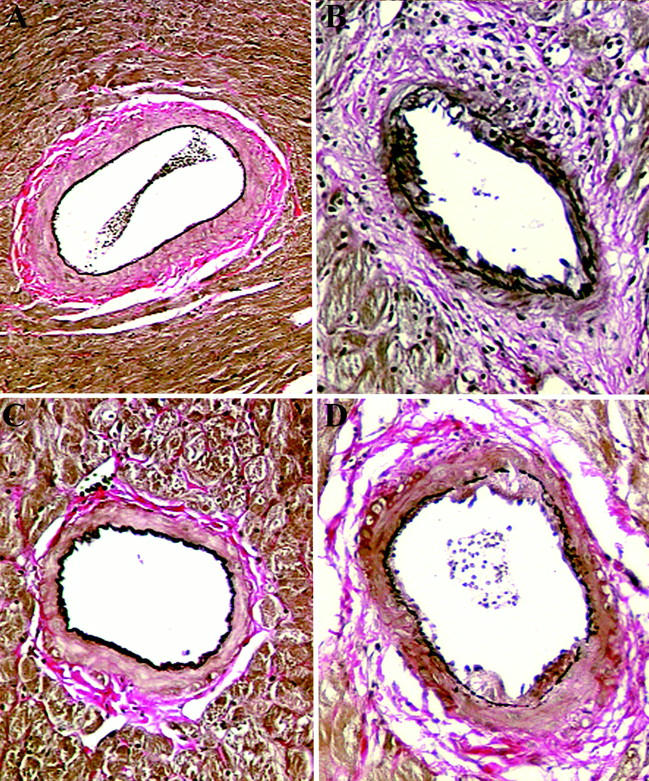
Representative paraffin sections of rat cardiac transplants at 120 days post-transplant demonstrating transplant arteriosclerosis (Van Gieson stain) from (A) isografts (group 4), (B) control L6-treated (group 3), (C) 90 day CTLA4Ig-treated animals (group 1), and (D) 60 day CTLA4Ig-treated animals (group 2).
Grafts surviving >100 days were examined histologically for parenchymal fibrosis, leukocyte infiltration, and vasculitis, and severity scores were determined blindly by an experienced pathologist. Scores for each treatment group are shown in Table 2 ▶ . The amount of fibrosis was significantly greater in allografts from control group 3 as compared to isografts (group 4) and allografts from animals treated with CTLA4Ig from day 30 (Group 1; P < 0.05). Although allografts from animals treated with CTLA4Ig from day 60 (group 2) had an intermediate degree of fibrosis; this did not reach statistical difference when compared with control L6-treated animals (group 3). Similar results were also obtained when the amount of cellular infiltration within allografts was compared. Grafts from control L6-treated animals (group 3) demonstrated marked cellular infiltration, which was significantly less in grafts from animals treated with CTLA4Ig from day 30 (group 1; P < 0.05). Consistent with previous findings using this model, acute vasculitic changes were minimal in all groups. Representative histology showing fibrosis and inflammatory infiltration are shown in Figure 4 ▶ .
Table 2.
Effect of T Cell Costimulatory Blockade on Fibrosis, Interstitial Infiltration, and Vasculitis
| Group | n | Fibrosis | Inflammation | Vasculitis |
|---|---|---|---|---|
| CTLA4Ig 30–120 | 6 | 0.33 ± 0.21* | 0* | 0.33 ± 0.33 |
| CTLA4Ig 60–120 | 5 | 1.6 ± 0.51 | 1.6 ± 0.68 | 0.6 ± 0.25 |
| Control Ig | 6 | 2.17 ± 0.17 | 3.0 ± 0.37 | 0.83 ± 0.4 |
| Isograft | 7 | 0.29 ± 0.18* | 0.14 ± 0.14* | 0.29 ± 0.29 |
Comparison of group versus control Ig-treated group. Values expressed as means ± SD.
*P < 0.001 using an analysis of variance with Bonferroni correction.
Figure 4.
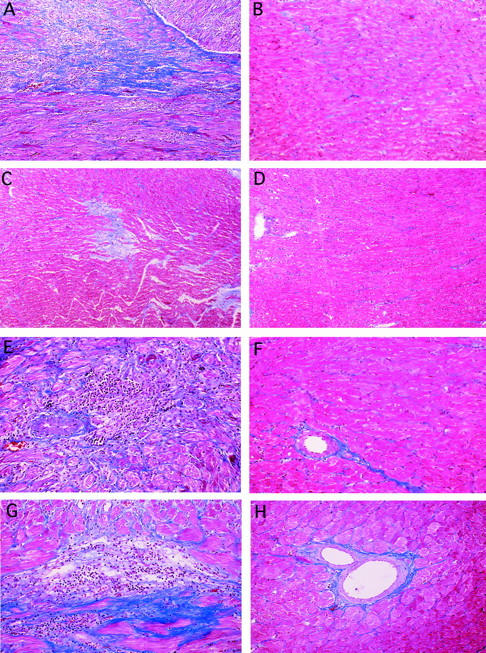
Representative paraffin sections of rat cardiac transplants at 120 days post-transplant demonstrating graft fibrosis (Trichrome stain) from (A) control L6 treated (group 3), (B) isografts (group 4), (C) 60 day CTLA4Ig animals (group 2), and (D) 90 day CTLA4Ig treated animals (group 1) and interstitial inflammatory infiltrate (E) control L6-treated (group 3), (F) isografts (group 4), (G) 60 day CTLA4Ig-treated animals (group 2), and (H) 90 day CTLA4Ig-treated animals (group 1).
Effects of Early and Late Selective Blockade of CD80 on the Development of Chronic Rejection
Evidence suggests that CD80 and CD86 may have functionally distinct roles in T cell activation. In general, CD86 is constitutively expressed and rapidly up-regulated on antigen-presenting cells, whereas CD80 is not usually expressed until after prolonged (72 hours) stimulation. These differences may suggest that CD86 is important in mediating initial T cell alloactivation and CD80 is important in perpetuation of the immune response, although this has not been established in an experimental transplant model.
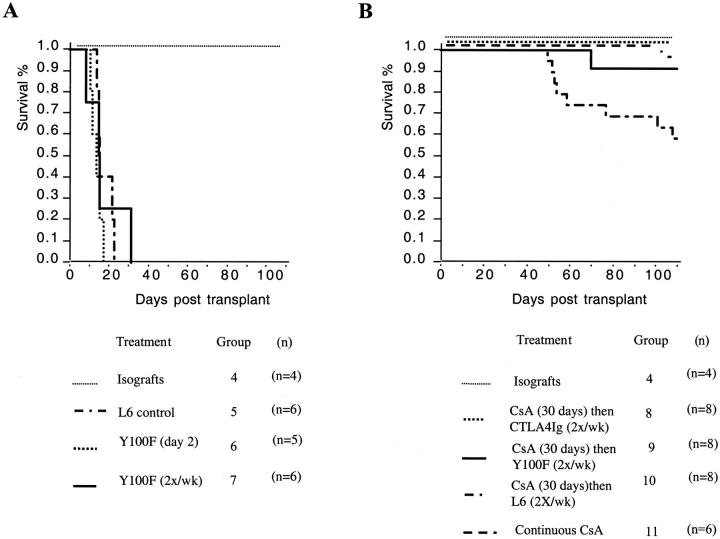
To examine the effects of interrupting CD80-mediated T cell costimulation at early and late time points post-transplantation in this model, we used the mutant CTLA4Ig Y100F, an agent that selectively binds CD80. Administration of Y100F (0.5 mg i.p.) as a single dose on day 2 or as repeated twice-weekly doses post-transplantation did not prolong graft survival (Figure 5A) ▶ . This is in striking contrast to the effects of CTLA4Ig (0.5 mg i.p.) given on day 2, which resulted in prolonged graft survival (>100 days) in over 60% of recipients. 14 We next examined whether late administration of Y100F was effective in attenuating chronic rejection in this model. In these experiments we compared Y100F to CTLA4Ig in a modified “interruption” treatment protocol consisting of twice-weekly injections of Y100F from day 30 to day 60. In contrast to the lack of efficacy of Y100F early post-transplantation, we find that Y100F is as effective as CTLA4Ig when given late post-transplantation in interrupting chronic rejection. Illustrated in Figure 5B ▶ is the percentage survival of allografts in Y100F-treated (group 8), CTLA4Ig-treated (group 9), and control L6-treated groups (group 10). Both Y100F and CTLA4Ig result in a significant prolongation of allograft survival (P < 0.05).
Figure 5.
Kaplan Meier survival plots showing effects of administration of Y100F early (A) and late (B) post-transplantation. Number of animals in each group is shown in parentheses.
When grafts surviving >100 days were examined for arteriosclerosis, grafts from Y100F-treated animals had significantly less arteriosclerosis than controls (P < 0.001) (Figure 6A) ▶ . There was a trend toward less arteriosclerosis in grafts from Y100F- compared to CTLA4Ig-treated groups. Representative vessels from allografts from control and Y100F- and CTLA4Ig-treated groups are shown in Figure 7 ▶ . Furthermore, late blockade of CD80/CD28 interactions with Y100F is associated with reduced parenchymal fibrosis (Figure 6B) ▶ and inflammatory cell infiltration (data not shown) compared to controls. In keeping with these observations we find lower levels of mRNA expression of the chemotactic agent MCP-1 and the fibrogenic cytokine TGF-β in grafts from Y100F treated recipients compared to controls (Figure 6C) ▶ . We also find lower levels of expression of the Th-1 cytokine interferon-γ in grafts from Y100F animals. This may reflect either a deviation from Th1 differentiation with CD80 blockade or simply a reduction in immune cell infiltration.
Figure 6.
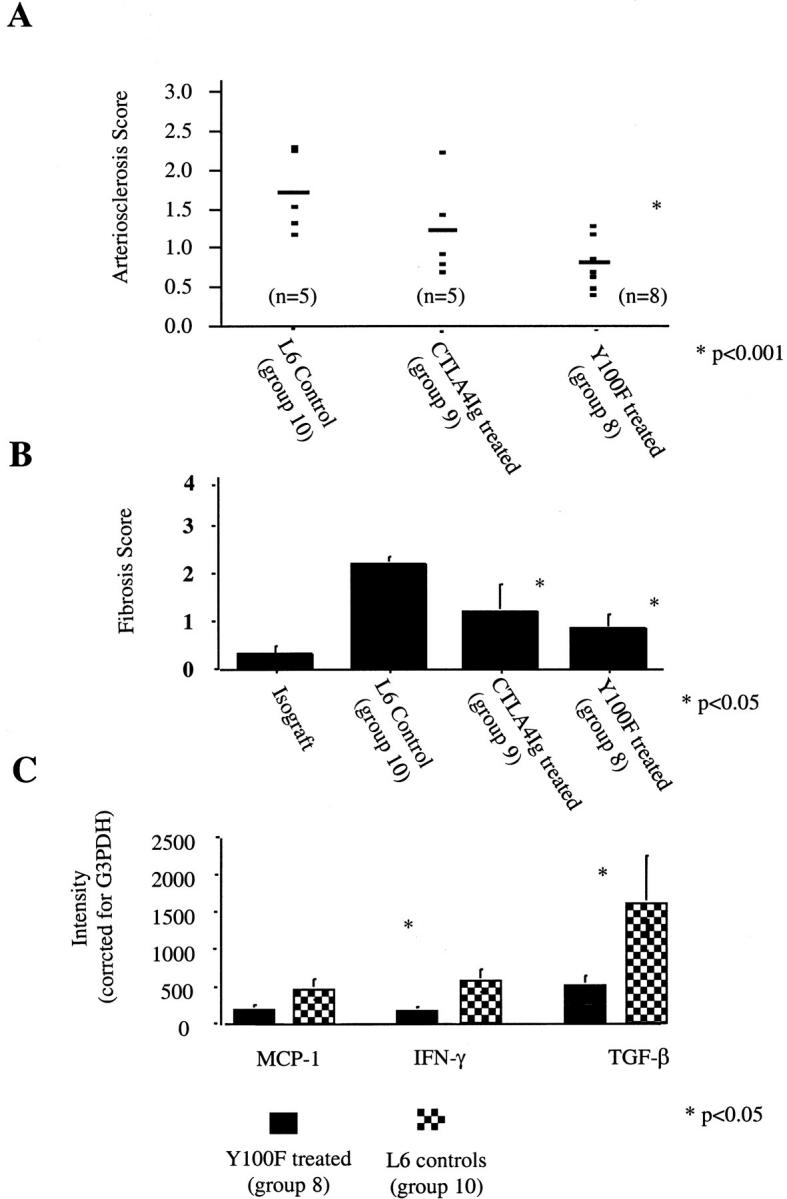
A: Arteriosclerosis scores in treatment groups. Mean number of vessels counted per graft was 9.1. Shown are mean arteriosclerosis scores for each animal and mean for each group. Percentage of diseased vessels for each group were (i) Y100F-treated (group 8): 57 ± 19%; (ii) CTLA4Ig-treated (group 9): 72 ± 22%; and (iii) L6-treated (group 10) 87 ± 16%. B: Mean graft fibrosis score for each group as calculated by experienced pathologist. C: Levels of expression of graft mRNA for MCP-1, interferon-γ, and TGF-β in Y100F- and control L6-treated groups. Levels are expressed in arbitrary units of fluorescence intensity obtained by optical imaging.
Figure 7.
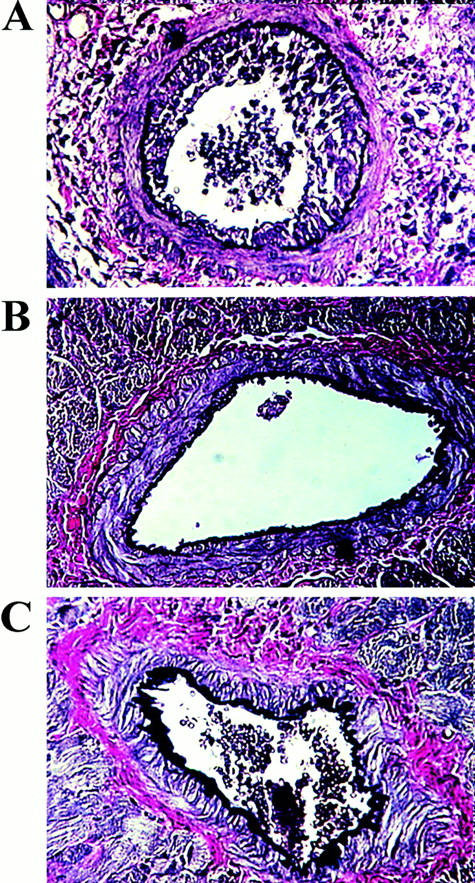
Representative paraffin sections demonstrating graft arteriosclerosis (Van Gieson stain) in rat cardiac transplants surviving >100 days post-transplant from (A) L6-treated controls (group 10), (B) CTLA4Ig-treated animals (group 9), and (C) Y100F-treated animals (group 8).
To investigate whether the observed effect of CTLA4Ig or Y100F is due merely to increased immunosuppression versus a specific effect of T cell costimulatory activation blockade, we examined the effects of continuous immunosuppression with low-dose CsA in this model. Whereas continuous CsA treatment prolonged graft survival, we found that mean transplant arteriosclerosis scores were no different from controls (1.73 ± 1.53; P = n.s.). Furthermore, grafts exhibited marked parenchymal fibrosis, consistent with the ability of CsA to induce expression of the potent fibrogenic growth factor TGF-β 15 (data not shown).
Effect of Blocking T Cell Costimulation on the Development of Intimal Proliferation in a Rat Carotid Balloon Injury Model
We next wished to confirm that the effects of CTLA4Ig were a direct consequence of prevention of T cell costimulation/activation rather than a nonspecific anti-inflammatory effect or an effect on monocytes/macrophages or endothelial cell activation that is independent of T cell activation. To address this issue, we studied the effect of CTLA4Ig on the development of arteriosclerosis in a vascular endothelial injury model where activated T lymphocytes do not play a role in the pathogenesis of lesion formation. In this model, rat carotid arteries undergo endothelial injury by balloon angioplasty. The animals develop arteriosclerosis secondary to endothelial and monocyte/macrophage activation. Carotid arteries from CTLA4Ig and control saline-treated animals were analyzed histologically and percentage of luminal occlusion determined as described above in Methods. 13 Mean percent luminal occlusion was 56 ± 12% in CTLA4Ig-treated rats compared to 49 ± 25% in saline-treated rats (P = 0.44). Intima-to-media ratios were also similar: 1.2 ± 0.3 in CTLA4Ig-treated rats compared with 0.9 ± 0.4 in saline-treated group (P = n.s.). Thus, blockade of T cell costimulation did not inhibit the development of intimal thickening in this model.
Discussion
The pathophysiology of chronic rejection remains poorly understood. Early graft injury, secondary to either acute rejection or graft ischemia, is an important risk factor for later development of chronic rejection. It is suggested that injury augments the immunogenicity of the graft via up-regulation of class II MHC and costimulatory molecule expression. 16 Subsequent CD4+ T cell activation initiates a complex immunological reaction involving alloantibody production and macrophage activation that is responsible for insidious graft injury, and ultimately the development of graft arteriosclerosis, interstitial fibrosis, and loss of graft function. 17 The importance of CD4+ T cell activation in chronic rejection is demonstrated by the prevention of graft arteriosclerosis and prolongation of allograft survival in the LEW-to-F344 rat cardiac transplant model by blockade of T cell costimulation with a single injection of CTLA4Ig on day 2 post-transplantation. 14
In this report we have shown that blocking T cell costimulation late post-transplantation, after initial graft injury, interrupts the development of chronic cardiac allograft rejection, evidenced by reduced graft arteriosclerosis, interstitial infiltration and fibrosis, and improved graft survival. These data suggest that ongoing T cell recognition of alloantigen and activation is an important component of the chronic rejection process. 18 Although there is no question that persistent macrophage activity is crucial in the pathogenesis of these lesions, the continuing role of T cells has been less certain. 19-21 In support of a role for continued T cell activation, Chandraker et al interrupted the development of chronic graft dysfunction and glomerulosclerosis with T cell costimulatory blockade in a rat model of chronic renal allograft rejection. 18 Taken together, these findings suggest that chronic rejection proceeds as a result of continuous immune stimulation rather than an irreversible cascade of events arising from initial injury. 22 More importantly, these data show that that chronic allograft rejection may be amenable to modification by T cell costimulatory blockade even after the process has been initiated.
This study further defines the individual functions of CD80 and CD86 in alloimmune responses. Both CD80 and CD86 deliver potent signals through the T cell molecule CD28. CD28 ligation optimizes T cell interleukin-2 production in the presence of antigen stimulation and prevents T cell apoptosis. 23 CD80 and CD86 also bind CTLA4, an Ig superfamily member with homology to CD28, which delivers a negative signal to activated T cells. 24 However, CD80 and CD86 share only 25% amino acid homology, have distinct binding kinetics to CD28 and CTLA4, 25 and differ in their spatial and temporal expression. These differences have led to the suggestion that CD80 and CD86 are functionally distinct; this appears to be corroborated by differential effects of blocking CD80 and CD86 in experimental models of autoimmunity and transplantation. 26
We find that blocking CD80 costimulation at early time points post-transplantation does not effect graft survival, whereas combined blockade leads to long term graft survival and inhibition of chronic rejection in the majority of recipients. This suggests either that CD86 is the dominant costimulatory molecule responsible for inducing initial alloactivation of T cells in this model or that both costimulatory molecules must be blocked to inhibit T cell activation. It is likely that CD86 is dominant early post-transplantation because it is expressed at earlier time points than CD80 in alloimmune responses. In a mouse model of cardiac transplantation, strong expression of CD86 was seen at 24 hours post-transplantation, whereas CD80 was not detected until day 3. 27 Pearson et al found that both anti-CD80 mAbs and anti-CD86 mAbs modestly prolonged allograft survival, whereas combined blockade was most effective. 28 Studies using CD80 knockout mice have supported these findings. 29
In contrast, late blockade of CD80 resulted in prolonged graft survival and a striking reduction in the histological features of chronic rejection (mononuclear cell infiltration, parenchymal fibrosis, and graft arteriosclerosis). Indeed, Y100F was as good, if not more effective, than CTLA4Ig in this regard. This suggests that CD80 is the dominant costimulatory molecule mediating T cell activation in chronic rejection in this model. This may be due to either preferential expression or unique functional characteristics of this molecule in chronic rejection. A dominant cell type in chronically rejecting grafts is the monocyte/macrophage. In addition to causing tissue destruction, monocyte/macrophages express class II MHC and costimulatory molecules and may be responsible for mediating persistent CD4+ T cell activation required for maintenance of the rejection response. A recent report shows that although activated human monocytes express both CD80 and CD86, only CD80 is functional in inducing CD4+ T cell proliferation and interferon-γ production. 30 Furthermore, some evidence suggests that CD80 predominantly costimulates Th1 differentiation whereas CD86 costimulates Th2 differentiation. 31 Long term graft survival and tolerance induction in the LEW-to-F344 cardiac transplant model is associated with deviation of the cytokine profile within the graft from a Th-1 to Th-2 pattern. 32 Thus, we speculate that advantage of isolated CD80 blockade compared to combined CD80 and CD86 blockade may be to allow for CD4+ T cell differentiation into the Th2 pathway. Consistent with these observations, we find reduced levels of interferon-γ in grafts from Y100F-treated recipients (Figure 6c) ▶ . Interleukin-4 transcript levels were low and similar in control and Y100F-treated groups (data not shown). In this study we have not assessed the role of CD154-CD40 interactions in chronic rejection, although this is an area of current investigation in our laboratory. Ligation of CD40 on antigen-presenting cells induces expression of CD80 and CD86, and CD154-CD40 interactions are important in facilitating B cell antibody production, an important effector mechanism in chronic rejection. 18
Finally, we show that blockade of T cell costimulation has no effect on the development of intimal thickening in a model of mechanical injury-induced arteriosclerosis. Arterial intimal thickening after balloon catheter injury is a rapid process involving migration of smooth muscle cells into the intima, smooth muscle cell proliferation, and matrix production. These events are initiated by growth factors released by damaged endothelium and by activated monocytes/macrophages and platelets, but not by activated T cells, thus accounting for the lack of efficacy of CTLA4Ig in this model. These data support the proposition that the beneficial effect of CTLA4Ig on transplant arteriosclerosis arises via a direct inhibitory effect on T cell activation rather than through inhibition of other cellular processes underlying lesion formation.
In summary, this is the first demonstration that late blockade of T cell costimulation can interrupt the development of arteriosclerosis and interstitial fibrosis in a model of chronic cardiac allograft rejection. Furthermore, these data support a crucial role for continued T cell activation in chronic rejection and provides additional evidence for a differential function of CD80 and CD86 in induction of alloimmune responses.
Footnotes
Address reprint requests to Mohamed H. Sayegh, M.D., Laboratory of Immunogenetics and Transplantation, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis St., Boston, MA 02115. E-mail: msayegh@rics.bwh.harvard.edu.
Supported by research grants R01 A134963, A140629, and A140152 from the National Institutes of Health.
K. S. K. and M. D. D. contributed equally to this work.
References
- 1.Weis M, von-Scheidt W: Cardiac allograft vasculopathy: a review. Circulation 1997, 96:2069-2077 [DOI] [PubMed] [Google Scholar]
- 2.Russell ME, Hancock WW, Akalin E, Wallace AF, Glysing-Jensen T, Willett TA, Sayegh MH: Chronic cardiac rejection in the LEW to F344 rat model. J Clin Invest 1995, 97:833-838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tullius S, Tilney N: Both alloantigen-dependent and -independent factors influence chronic allograft rejection. Transplantation 1995, 59:313-318 [PubMed] [Google Scholar]
- 4.Janeway C, Bottomly K: Signals and signs for lymphocyte responses. Cell. 1994, 76:275-285 [DOI] [PubMed] [Google Scholar]
- 5.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA: CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med 1991, 174:561-569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gimmi C, Freeman G, Gribben J, Gray G, Nadler L: Human T cell anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci USA 1993, 90:6586-6590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA: Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science 1992, 257:789-792 [DOI] [PubMed] [Google Scholar]
- 8.Adams D, Russell M, Hancock W, Sayegh M, Wyner L, Karnovsky M: Chronic rejection in experimental cardiac transplantation: studies in the Lewis-F344 model. Immunol Rev 1993, 134:5-19 [DOI] [PubMed] [Google Scholar]
- 9.Sayegh M, Sablinski T, Tanaka K, Kut J, Kwok C, Tilney N, Kupiec-Weglinski J, Milford E: Effects of BWH-4 anti-CD4 monoclonal antibody on rat vascularized cardiac allografts before and after engraftment. Transplantation 1991, 51:296-299 [DOI] [PubMed] [Google Scholar]
- 10.Chandraker A, Takada M, Nadeau K, Peach R, Tilney N, Sayegh M: CD28–B7 blockade in organ dysfunction secondary to cold ischemia-reperfusion injury. Kidney Int 1997, 52:1678-1684 [DOI] [PubMed] [Google Scholar]
- 11.Adams D, Tilney N, Collins J, Karnovsky M: Experimental graft arteriosclerosis I. The Lewis to F-344 allograft model. Transplantation 1992, 53:1115-1119 [PubMed] [Google Scholar]
- 12.Knoflach A, Azuma H, Magee C, Denton M, Murphy B, Iyenger A, Buelow R, Sayegh M: Immunomodulatory functions of low-molecular weight hyaluronate in an acute rat renal allograft rejection model. J Am Soc Nephrol 1999, 10:1059-1066 [DOI] [PubMed] [Google Scholar]
- 13.Hancock W, Adams D, Wyner L, Sayegh M, Karnovsky M: CD4+ mononuclear cells induce cytokine expression, vascular smooth muscle cell proliferation and arterial occlussion after endothelial injury. Am J Pathol 1994, 145:1008-1014 [PMC free article] [PubMed] [Google Scholar]
- 14.Russel M, Hancock W, Akalin E, Wallace A, Glysing-Jensoen T, Willet T, Sayegh M: Chronic cardiac rejection in the Lewis to F344 rat model: blockade of CD28–B7 costimulation by CTLA4Ig modulates T cell and macrophage activation and attenuates arteriosclerosis. J Clin Invest 1995, 97:833-838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prashar Y, Khanna A, Sehajpal P, Sharma V, Suthanthiran M: Stimulation of transforming growth factor-beta 1 transcription by cyclosporine. FEBS Lett 1995, 358:109-112 [DOI] [PubMed] [Google Scholar]
- 16.Goes N, Urmson J, Ramassar V, Halloran P: Ischemic acute tubular necrosis induces an extensive local cytokine response: evidence for induction of interferon-g, transforming growth factor-b1, granulocyte-macrophage colony stimulating factor, interleukin-2 and interleukin-10. Transplantation 1995, 59:565-572 [PubMed] [Google Scholar]
- 17.Sayegh M, Turka L: The role of T cell costimulatory activation pathways in transplant rejection. N Engl J Med 1998, :1813-1821 [DOI] [PubMed] [Google Scholar]
- 18.Chandraker A, Azuma H, Nadeau K, Carpenter C, Tilney N, Hancock W, Sayegh M: Late Blockade of T cell costimulation interrupts progression of experimental chronic allograft rejection. J Clin Invest 1998, 101:2307-2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong C, Redenbach D, Wood S, Battistini B, Wilson JE, McManus BM: The pathogenesis of cardiac allograft vasculopathy. Curr Opin Cardiol 1996, 11:183-190 [DOI] [PubMed] [Google Scholar]
- 20.Shi C, Lee WS, He Q, Zhang D, Fletcher DJ, Newell JB, Haber E: Immunologic basis of transplant-associated arteriosclerosis. Proc Natl Acad Sci USA 1996, 93:4051-4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Croker BP, Clapp WL, Abu SA, Kone BC, Peterson JC: Macrophages and chronic renal allograft nephropathy. Kidney Int Suppl 1996, 57:S42-S49 [PubMed] [Google Scholar]
- 22.Halloran PF: Impact of the new immunossuppressives on chronic rejection: potential for reduction of graft injury. Transplant Vascular Sclerosis. Edited by Orosz CG, Sedmak DD, Ferguson RM. Austin, TX, R.G. Landes Company, 1995, pp 202–217
- 23.Linsley P, Ledbetter J: The role of the CD28 receptor during T cell responses to antigen. Ann Rev Immunol 1993, 11:191-212 [DOI] [PubMed] [Google Scholar]
- 24.Walunas T, Bakker C, Bluestone J: CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med 1996, 183:2541-2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linsley P, Greene J, Brady W, Bayorath J, Ledbetter J, Peach R: Human B7–1(CD80) and B7–2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA4 receptors. Immunity 1994, 1:793-801 [DOI] [PubMed] [Google Scholar]
- 26.Kuchroo V, Prabhu Das M, Brown J, Ranger A, Zamvil S, Sobel R, Weiner H, Nabavi N, Glimcher L: B7–1 and B7–2 costimulatory molecules differentially activate the Th1/Th2 developmental pathways: application to autoimmune therapy. Cell 1995, 80:707-718 [DOI] [PubMed] [Google Scholar]
- 27.Hancock WW, Sayegh MH, Zheng XG, Peach R, Linsley PS, Turka LA: Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci USA 1996, 93:13967-13972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearson T, Alexander D, Corbascio M, Hendrix R, Ritchie S, Linsley P, Faherty D, Larsen C: Analysis of the B7 costimulatory pathway in allograft rejection. Transplantation 1997, 63:1463-1469 [DOI] [PubMed] [Google Scholar]
- 29.Zheng X, Sayegh M, Zheng X, Li Y, Linsley P, Peach R, Borriello F, Strom T, Sharpe A, Turka L: The role of donor and recipient B7–1 (CD80) in allograft rejection. J Immunol 1997, :1169-1173 [PubMed] [Google Scholar]
- 30.Fleisher J, Soeth E, Reiling N, Grage-Griebenow E, Flad H, Ernst M: Differential expression and function of CD80 (B7–1) and CD86 (B7–2) on human peripheral blood monocytes. Immunology. 199, 89:592–598 [DOI] [PMC free article] [PubMed]
- 31.Freeman G, Boussiotis V, Anumanthan A, Bernstein G, Ke X, Rennert P, Gray G, Gribben J, Nadler L: B7–1 and B7–2 do not deliver identical costimulatory signals, since B7–2 but not B7–1 preferentially costimulates the initial production of IL-4. Immunity 1995, 2:523-532 [DOI] [PubMed] [Google Scholar]
- 32.Sayegh M, Akalin E, Hancock W, Russel M, Carpenter C, Turka L: CD28–B7 blockade after alloantigenic challange in vivo inhibits Th1 cytokines but spares Th2. J Exp Med 1995, 181:1869-1874 [DOI] [PMC free article] [PubMed] [Google Scholar]