Abstract
In Alzheimer’s disease (AD), fibrillar β-amyloid protein (fAβ) accumulates in the walls of cerebral vessels associated with vascular smooth muscle cells (SMCs), endothelium, and pericytes, and with microglia and astrocytes in plaques in the brain parenchyma. Scavenger receptor class A (SR-A) and class B, type I (SR-BI) mediate binding and ingestion of fAβ by cultured human fetal microglia, microglia from newborn mice, and by cultured SMCs. Our findings that SR-BI participates in the adhesion of cultured microglia from newborn SR-A knock-out mice to fAβ-coated surfaces, and that microglia secrete reactive oxygen species when they adhere to these surfaces prompted us to explore expression of SR-BI in vivo. We report here that astrocytes and SMCs in normal adult mouse and human brains and in AD brains express SR-BI. In contrast, microglia in normal adult mouse and human brains and in AD brains do not express SR-BI. These findings indicate that SR-BI may mediate interactions between astrocytes or SMCs and fAβ, but not of microglia and fAβ, in AD, and that expression of SR-BI by rodent microglia is developmentally regulated. They suggest that SR-BI expression also is developmentally regulated in human microglia.
Scavenger receptor class A (SR-A) is expressed by mononuclear phagocytes (monocytes, macrophages, microglia and Mato cells, follicular dendritic cells in germinal centers, high-endothelial venular cells in lymphoid organs, and in the endoplasmic reticulum and Golgi membranes of fibroblasts and smooth muscle cells (SMCs). 1-5 SR-A mediates adhesion of macrophages and microglia to fibrillar β-amyloid protein (fAβ)-containing matrices and ingestion of fAβ by these cells.
Scavenger receptor class B, type I (SR-BI) was first identified as a receptor for high-density lipoproteins on hepatocytes, adipocytes, and nonplacental steroidogenic cells. 6 Subsequently, it has been identified on the surfaces of monocytes, macrophages, 7-9 and in endosomes of cultured SMCs from brain. 5 Paresce and colleagues’ report 10 that Chinese hamster ovary (CHO) cells transfected with SR-BI bind and endocytose fAβ showed that SR-BI, like SR-A, has the capacity to promote cellular interactions with fAβ.
In studying interactions between microglia from mice whose class A scavenger receptors had been genetically disrupted (SR-A−/− mice) and fAβ, we discovered that cultured microglia from newborn SR-A−/− mice and from wild-type (SR-A+/+) mice express SR-BI. 36 This led us to investigate the expression of this receptor in brain cells of normal adult mice and humans and of patients with Alzheimer’s disease (AD). Our findings that SR-BI is expressed by astrocytes and vascular SMCs, but not by microglia, in the brains of normal adult mice and humans and of patients with AD indicate that SR-BI is developmentally regulated in mice. They suggest that expression of SR-BI by microglia is down-regulated during postnatal development in mice and probably in humans as well, and that SR-BI mediates interactions between astrocytes and fAβ in senile plaques and between SMCs and fAβ in amyloid angiopathy.
Materials and Methods
Blocks of frozen human brain and 5-μm-thick formalin-fixed paraffin-embedded human brain sections from control (n = 4) and AD patients (n = 4) were provided by the Columbia University Brain Bank (Columbia University, New York, NY). Brains from adult mice (BALB/c, 6 to 8 weeks of age; Jackson Laboratory, Bar Harbor, ME) were fixed in 10% formalin in phosphate-buffered saline (PBS) for 24 hours, embedded in paraffin, and 5-μm sections were prepared. Cryosections (8 μm) were fixed in ice-cold acetone (Sigma Chemical Co., St. Louis, MO) for 10 minutes, and stored at −80°C until used. Formalin-fixed paraffin-embedded samples were treated with DeWax (InnoGenex, San Ramon, CA) according to the manufacturer’s instructions, washed in PBS, incubated in 1 mmol/L Na-Citrate (pH 6.0) in double-distilled water for 30 minutes at 93 to 98°C to facilitate antigen renaturation, and washed in PBS. For immunocytochemistry, antibodies were diluted in PBS supplemented with 3% goat serum (Vector Laboratories, Burlingame, CA). Sections were incubated in PBS supplemented with 20% goat serum for 20 minutes, incubated with primary antibody as indicated in Table 1 ▶ and in the figure legends, washed three times in PBS, incubated with secondary antibody as indicated in Table 1 ▶ and in the figure legends, and washed three times in PBS, all at room temperature. Peroxidase-coupled secondary antibodies were visualized with diaminobenzidine (Sigma Chemical Co.) as chromogen according to the manufacturer’s instructions. Some sections were doubly stained to visualize peroxidase- and alkaline phosphatase-labeled antibodies. In these instances, Tris-buffered saline (Sigma Chemical Co.) supplemented with 3% goat serum was used for incubations and washes, and alkaline phosphatase was visualized with BCIP/NBT (DAKO, Carpinteria, CA) as chromogen according to the manufacturer’s instructions. After incubation with primary antibody, frozen sections were incubated in 1% aqueous thioflavin S solution (Sigma Chemical Co.) for 10 seconds, rinsed in 80% alcohol, and washed in PBS.
Table 1.
List of Primary and Secondary Antibodies and Control Antibodies Used in First (I) and Second (II) Staining Cycle for Double Labeling
| Figure ▶ | |
|---|---|
| I. Primary antibody | |
| Polyclonal rabbit anti-SR-BI (1:100), (Novus Biologicals, Littleton, CO) | 1a, 1g, 1j, 2a–h |
| Polyclonal goat anti-GFAP (1:100) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) | 1e |
| Polyclonal rabbit anti-SR-BI/BII (RED-I; 1:100) (Novus Biologicals) | 2k |
| I. Secondary antibody | |
| Alexa 488-labeled goat anti-rabbit IgG (1:1000) (Molecular Probes, Eugene, OR) | 1a, 1g, 1j |
| Alexa 568-labeled donkey anti-goat IgG (1:1000) (Molecular Probes) | 1e |
| HRP-conjugated goat anti-rabbit IgG (1:20) (Sigma) | 2a–g |
| Alexa 594-labeled goat anti-rabbit IgG (1:1000) (Molecular Probes) | 2h, 2k |
| II. Primary antibody | |
| Monoclonal mouse anti-GFAP (IgG1, 1:400) (Chemicon, Temecula, CA) | 1b |
| Monoclonal mouse anti-CD68 (IgG3,κ, undiluted) (DAKO) | 1h, 2a, 2c–g |
| Polyclonal rabbit anti-SR-BI (1:100) (Novus Biologicals) | 1d |
| Monoclonal rat anti-mouse CD11b (IgG2b, 1:50) (Chemicon) | 1k |
| Monoclonal mouse anti-NFT (IgG2b, 1:50) (BioMarkers, Fremont, CA) | 2i |
| II. Secondary antibody | |
| Alexa 594-labeled goat anti-mouse IgG (1:1000) (Molecular Probes) | 1b, 1h |
| AP-conjugated goat anti-mouse IgG (1:20) (Sigma) | 2a, 2c–g |
| Alexa 594-labeled goat anti-rat IgG (1:1000) (Molecular Probes) | 1k |
| Alexa 488-labeled goat anti-rabbit IgG (1:1000) (Molecular Probes) | 1d |
| Alexa 488-labeled goat anti-mouse IgG (1:1000) (Molecular Probes) | 2i |
| Control antibodies | |
| Rabbit IgG (Protein A-purified rabbit serum), (Serotec); rat IgG2b, (Pharmingen); mouse IgG3,κ, (DAKO); | |
| Mouse IgG1, (Pharmingen); mouse IgG2b, (Pharmingen) |
To confirm the specificity for SR-BI of the commercial polyclonal rabbit anti-SR-BI antisera used in these experiments we performed two types of control experiments. 1) We absorbed diluted rabbit anti-SR-BI serum (1:100) and rabbit anti-SR-BI/II serum (RED-I) (1:100) with 106/ml mock-transfected, or SR-BI-transfected CHO cells (a generous gift from Dr. M. Krieger, Massachusetts Institute of Technology) for 20 minutes at room temperature, pelleted the cells, collected the supernatant and repeated the absorption step with fresh cells four more times. 2) We stained unfixed mock-transfected, or SR-BI-transfected CHO cells with these antisera as follows. SR-BI-transfected or mock-transfected CHO cells (10 4 cells/50 μl) were suspended in Krebs-Ringer buffer supplemented with 1 mmol/L glucose (Sigma Chemical Co.) and 0.1% bovine serum albumin (Sigma Chemical Co.) (KRBG-A), and plated on multispot glass slides (Shandon, Pittsburgh, PA) for 2 hours at 37°C in a 5%CO2/95% air atmosphere. The cells were blocked by incubation with KRBG-A containing 20% goat serum (Vector Laboratories) for 30 minutes at 4°C, and incubated with absorbed (as described above) or nonabsorbed rabbit anti-SR-BI serum (1:100) or with rabbit anti-SR-BI/II serum (RED-I) (1:100), washed, incubated with 1 μg/ml of biotinylated goat anti-rabbit IgG, fixed in 10% formalin in PBS for 20 minutes at room temperature, washed in PBS, and incubated with 2 μg/ml avidin-conjugated Alexa 568 in PBS at room temperature. Antisera were diluted in KRBG-A containing 3% goat serum.
For bright-field microscopy, sections were dehydrated in graded alcohol and xylenes (Sigma Chemical Co.), mounted with Permount (Fisher Scientific, Fair Lawn, NJ), and viewed with a Nikon E800 model microscope (Nikon, Garden City, NY). For fluorescence microscopy, sections were mounted with GelMount (Biomeda, Foster City, CA) and illuminated with a mercury lamp on the same microscope. Pictures were taken with a digital camera (Spot Diagnostic, Inc., Sterling Heights, MI). Overlay pictures were created using Adobe Photoshop 4.0 for Macintosh computers. All immunocytochemical findings reported are representative of data obtained in multiple sections from each of the four normal and four AD brains and from at least four adult mouse brains.
Results
Specificity of Rabbit Anti-SR-B Sera
Unfixed, nonpermeabilized CHO cells transfected with SR-BI stained intensely with rabbit anti-SR-BI serum and rabbit anti-SR-BI/II serum (RED-I), whereas virtually no staining was seen in mock-transfected CHO cells, or in SR-BI-transfected or mock-transfected CHO cells incubated with absorbed rabbit anti-SR-BI serum or rabbit anti-SR-BI/II serum (RED-I) (not shown). These findings indicate that the antibodies in these rabbit sera react with SR-BI, and that both antisera recognize epitopes on SR-BI that are on the extracellular face of the plasma membranes of viable cells.
The finding that the rabbit anti-SR-BI antiserum from Novus stains viable SR-BI-transfected CHO cells is surprising because this antiserum was reported by the manufacturer to have been raised by immunization with a peptide encompassing residues 496 to 509 of SR-BI. According to Babitt and colleagues, 11 these residues are contained in the portion of SR-BI’s carboxy terminus that lies in the cytoplasm and therefore should be inaccessible to antibodies. Although our studies provide no insight into the reasons a rabbit anti-SR-BI serum raised by immunization with this 496 to 509 peptide reacts with SR-BI on viable cells, they show that both the anti-SR-BI and the anti-SR-BI/II (RED-I) antisera used in these experiments react with SR-BI, and that in the case of RED-I, all reactivity against mouse and human brain can be removed by absorption with SR-BI-transfected CHO cells. For these reasons we conclude that these antisera provide an accurate assessment of sites of SR-BI expression in tissues.
Astrocytes and Vascular SMCs, but Not Microglia, Express SR-BI in Situ in Normal Adult Human and Mouse Brain and in Brain of AD Patients
Astrocytes, identified by staining with mouse anti-human glial fibrillary acidic protein (GFAP) IgG (Figure 1b) ▶ , in brains of patients with AD (Figure 1a ▶ , open arrows, and yellow-colored cells in the overlay in Figure 1c ▶ ), and in normal human brain (not shown) stained specifically for SR-BI (Figure 1a ▶ , open arrows, and yellow-colored cells in the overlay in Figure 1c ▶ ). GFAP-positive cells in mouse brain (Figure 1e) ▶ also stained strongly for SR-BI (Figures 1d and 2b ▶ ▶ , open arrows), identifying these cells as astrocytes (Figure 1f ▶ , overlay). In contrast, microglia identified in human brain by staining with mouse anti-human CD68 (Figure 1h ▶ , filled arrowheads), and in adult mouse brain by staining with rat anti-mouse CD11b (Figure 1k ▶ , filled arrowheads), did not stain for SR-BI.
Figure 1.
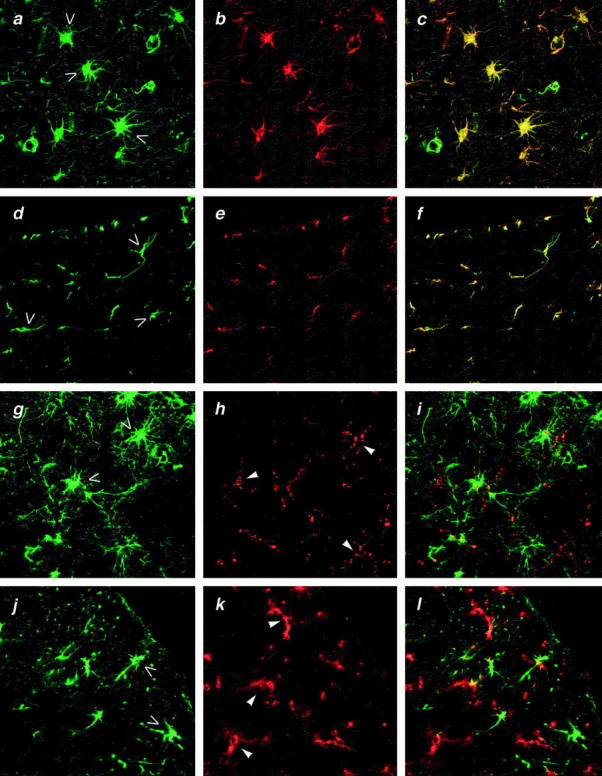
Immunostaining of brain from patients with AD and from normal adult mice for SR-BI. a, b, and c: Human AD brain (cortex) doubly stained with rabbit anti-SR-BI and Alexa 488-conjugated goat F(ab)2 anti-rabbit IgG (green) (a) and mouse anti-GFAP and Alexa 594-conjugated goat F(ab)2 anti-mouse IgG (red) (b). Yellow color in the overlay picture (c) indicates co-localization of SR-BI and GFAP and identifies these doubly stained cells as astrocytes. d, e, and f: Adult mouse brain (cortex) doubly stained with rabbit anti-SR-BI and Alexa 488-conjugated goat F(ab)2 anti-rabbit IgG (green) (d) and goat anti-GFAP and Alexa 568-conjugated donkey anti-goat IgG (red) (e). Yellow color in the overlay picture (f) indicates co-localization of SR-BI and GFAP and identifies these doubly stained cells as astrocytes. g, h, and i: Human AD brain (cortex) doubly stained as in a, b, and c with anti-SR-BI for astrocytes (green) (g) and with mouse anti-CD68 and Alexa 594-conjugated goat F(ab)2 anti-mouse IgG (red) (h) for microglia. Absence of yellow color in the overlay (i) indicates that anti-SR-BI and anti-CD68 stain different cells. j, k, and l: Adult mouse brain (cortex) doubly stained as in d, e, and f with anti-SR-BI (green) (j) for astrocytes and with rat anti-CD11b and Alexa 594-conjugated goat anti-rat IgG (red) (k) for microglia. Absence of yellow color indicates that anti-SR-BI and anti-CD11b stain different cells. Original magnifications, ×60. Results shown are representative of all four normal human or mouse brains and four AD brains examined; all samples were formalin-fixed paraffin-embedded tissues.
Figure 2.
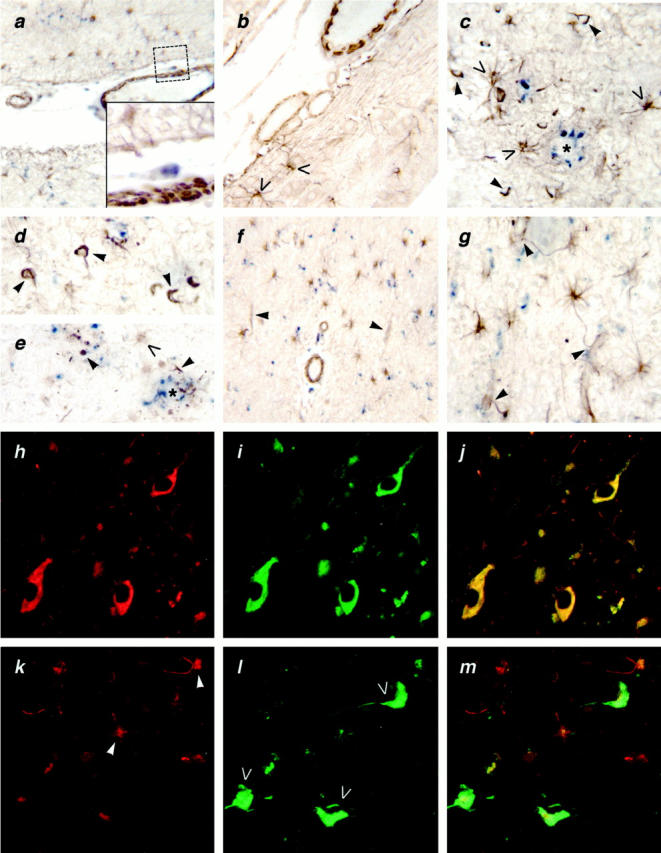
a: Human AD brain doubly stained with rabbit anti-SR-BI (brown) and mouse anti-CD68 (blue) for microglia (original magnification, ×25). Inset: ×60 original magnification of boxed area in upper right corner. Note anti-SR-BI (brown) staining of SMCs in the wall of this pial vessel. Blue color identifies anti-CD68-stained microglia and perivascular macrophages. b: Adult mouse brain stained with anti-SR-BI (brown). Note strongly stained vascular SMCs and astrocytes (open arrows) (original magnification, ×40). c, d, and e: Human AD brain incubated with rabbit anti-SR-BI serum and HRP-conjugated goat-anti-rabbit IgG, and with mouse anti-human CD68 and alkaline phosphatase-conjugated goat anti-mouse IgG. Note Aβ-containing plaque (asterisks) surrounded by anti-SR-BI (brown) stained astrocytes (open arrows), some of whose processes penetrate the plaque. Anti-SR-BI IgG (brown) also stains NFT-like structures (arrowheads) associated with plaques as well as similar structures in the parenchyma. Anti-CD68 (blue) stains plaque-associated microglial cells (original magnification, ×60). f: Human AD brain (white matter) stained as in a with anti-CD68 (blue) for microglial cells and anti-SR-BI (brown). Note strong anti-SR-BI staining of large vessels and astrocytes and weak anti-SR-BI staining on capillaries (arrowheads) (original magnification, ×25). g: Human AD brain (white matter)stained as in g with anti-CD68 (blue) for microglia and anti-SR-BI (brown). Note strong anti-SR-BI staining of astrocytes and of astrocyte processes sheathing the capillaries (arrowheads) (original magnification, ×60). h, i, and j: Human AD brain incubated with rabbit anti-SR-BI serum (h) and with mouse anti-tau (i) for NFTs. Yellow color in the overlay picture (j) indicates co-localization of SR-BI and tau and identifies these doubly stained structures as NFTs (original magnification, ×100). Results shown are representative of four normal human or mouse brains and four AD brains examined; all samples were formalin-fixed paraffin-embedded tissues. k, l, and m: Immunostaining of acetone-fixed cryosections of AD brain for SR-BI/II. Human AD brain (cortex) doubly stained with rabbit anti-SR-BI/II and Alexa 594-conjugated goat F(ab)2 anti-rabbit IgG (red) (k) and thioflavin S for NFTs (green; open arrows) (l). Cells with astrocyte morphology stain with antibody RED-I for SR-BI/II (k; arrowheads). Absence of yellow color in the overlay (m) indicates lack of NFT staining with RED-I (original magnification, ×100). Results shown are representative of four normal human brains and four AD brains examined.
SR-BI-positive astrocytes (Figure 2, c and e ▶ , open arrows) were detected around β-amyloid plaques in AD brains (Figure 2, c and e ▶ , asterisk), and some of the processes of these cells extended into the plaques. Plaques also contained CD68-positive microglial cells (Figure 2 ▶ ; c, d, and e, blue). Like the microglia found outside of plaques, these plaque-associated microglia also lacked SR-BI.
Large blood vessels, such as the pial vessels, in human AD brain stained for SR-BI (Figure 2a) ▶ . At higher magnification it was evident that the anti-SR-BI IgG reacted with vascular SMCs surrounding these vessels (Figure 2a ▶ , insert). Vascular SMCs were also strongly reactive for SR-BI in normal adult human (not shown) and mouse brain (Figure 2b) ▶ . Large blood vessels and astrocytes in white matter in normal human brain (not shown) and in AD brain also stained for SR-BI (Figure 2f) ▶ .
Capillaries in cortical and subcortical areas in normal adult human and mouse brain (not shown), and in AD brain (Figure 2f) ▶ , stained with anti-SR-BI IgG. On close examination it was evident that the anti-SR-BI IgG reacted with astrocyte processes associated with the capillary sheaths rather than with the capillary endothelial cells (Figure 2g) ▶ . Consistent with this interpretation, we observed no SR-BI staining of endothelial cells in human or mouse brain.
To confirm expression of SR-BI on SMCs (Figure 2a) ▶ , and astrocytes (Figure 1a) ▶ we stained acetone-fixed cryosections of normal adult mouse and human brain and AD brain with RED-I, another antibody against SR-BI/BII. RED-I stained cells with astrocytic morphology (Figure 2k) ▶ and vascular SMCs (not shown) in normal adult mouse and human brain and AD brain. These findings confirm that astrocytes and vascular SMCs in normal adult mouse and human brain and AD brain express SR-BI.
Neurofibrillary Tangles (NFTs) Do Not Contain SR-BI
Structures resembling NFTs reacted strongly with rabbit polyclonal anti-SR-BI serum in human AD brain, both in association with plaques and in plaque-free areas in the brain parenchyma (Figure 2; c, d, and e ▶ , arrowheads). To confirm that the structures stained with rabbit anti-SR-BI serum were indeed NFTs, we incubated AD brain sections with both rabbit anti-SR-BI serum (Figure 2h ▶ , open arrows), and mouse anti-human tau IgG (Figure 2i) ▶ , and visualized these primary antibodies with Alexa 594-conjugated goat anti-rabbit IgG and with Alexa 488-conjugated goat anti-mouse IgG (see Table 1 ▶ ). As shown in Figure 2j ▶ (overlay), rabbit anti-SR-BI serum and mouse anti-tau antibody reacted with the same structures.
Because neurons do not express SR-BI, we were suspicious that the rabbit anti-SR-BI serum contained IgG that cross-reacted with NFTs. Indeed, NFTs did not stain with RED-I (Figure 2k ▶ and overlay in Figure 2m ▶ ). They did, however, stain with thioflavin S (Figure 2i) ▶ , confirming their identity as NFTs. To further confirm that NFTs do not contain SR-BI, we absorbed rabbit anti-SR-BI serum with either mock-transfected, or SR-BI-transfected CHO cells, and then incubated formalin-fixed, paraffin-embedded normal mouse and human brain and AD brain with these absorbed sera. Rabbit anti-SR-BI serum, and rabbit anti-SR-BI/II serum (RED-I) absorbed with SR-BI-transfected CHO cells showed markedly reduced staining of astrocytes and SMCs in normal mouse and human brain and in AD brain. However, even after absorption with SR-BI-expressing CHO cells, there was no reduction in NFT staining in AD brain by rabbit anti-SR-BI serum. As expected, absorption of rabbit anti-SR-BI serum, and rabbit anti-SR-BI/II serum (RED-I) with mock-transfected CHO cells had no effect on the ability of these anti-sera to stain astrocytes, SMCs, and NFTs in AD brain. Therefore, staining of NFTs by rabbit anti-SR-BI serum reflects nonspecific cross-reactivity of this rabbit anti-serum with NFT proteins. It does not indicate the presence of SR-BI in NFTs.
Discussion
This is the first report of SR-BI expression by different cell-types in the central nervous systems of adult mice and humans and of humans with AD. SR-BI is expressed by astrocytes and vascular SMCs, and absent on microglia, in normal adult mouse and human brain, and in AD brain. The presence of SR-BI in the brain raises many questions relevant to brain physiology in health and disease, four of which we think are of special relevance to the findings reported here. They are:
Is SR-BI Expression by Mouse and Human Microglia Developmentally Regulated?
SR-BI is expressed by microglia in the brains of newborn mice, 36 consistent with the origin of these cells from blood monocytes. 12 Similarly, the absence of SR-BI on microglia in the brains of adult mice is consistent with reduced entry of blood monocytes into the brain after the maturation of the blood-brain barrier. Although further work is required to document the precise age at which SR-BI expression by mouse and human microglia is extinguished, and the mechanism(s) responsible for its extinction, our findings with respect to SR-BI expression suggest that this receptor’s expression on microglia is developmentally regulated. A similar situation prevails for SR-A expression by microglia in normal brain. That is, SR-A is expressed by microglia in the brains of newborn mice, 13 but is not expressed by microglia in adult mouse brain or by microglia in the brains of normal human adults.
Are SR-A and SR-BI Expressed Independently of One Another by Human Microglia?
The findings that both SR-A and SR-BI are expressed by monocytes and macrophages from adult mice and humans, 1,7-9 by microglia from newborn mice, 36 and Bell et al 13 , but not by adult mouse or human microglia, and that SR-A, but not SR-BI, is expressed on microglia associated with senile plaques in AD brains, 2,3 indicate that microglia can regulate SR-A and SR-BI expression by independent mechanisms. They suggest that reactive oxygen species, 14,15 growth factors, 16 and/or pro-inflammatory cytokines, 15,16 produced as a result of interactions of microglia, neurons, and/or astrocytes with Aβ, and presumed to be responsible for expression of SR-A by plaque-associated microglia in AD, are either incapable of inducing SR-BI expression, or suppress it.
Does SR-BI Play a Role in Lipid Trafficking within the Normal and/or Diseased Central Nervous System?
SR-BI is a lipid transfer protein that is most highly expressed in tissues that synthesize large amounts of cholesterol (ie, liver), or use large amounts of cholesterol for steroid hormone synthesis (ie, adrenal cortex and ovary). 6 SR-BI is also expressed on monocytes and macrophages 7-9 where it may participate in scavenger functions via binding of oxidatively modified low-density lipoproteins, senescent or apoptotic cells, 9 and anionic phospholipids. 6 On all of these cells, SR-BI binds apolipoprotein E (apoE) and other apoproteins on high-density lipoprotein (HDL), 17 and promotes cholesterol and phospholipid exchange between HDL and the plasma membranes of SR-BI-expressing cells. 6
ApoE-containing HDL-like lipoprotein particles are thought to facilitate lipid transport within the central nervous system. 18 Under normal conditions, astrocytes are the primary central nervous system cells that synthesize and secrete apoE-containing lipoproteins. 19 ApoE mRNA is expressed in cultured rat microglia, 20 and apoE- and apoJ-containing lipoproteins are secreted by cells of the BV2 mouse microglial line. 21 Cole and colleagues 22 reported that microglia from newborn rats secrete as much apoE in vitro as astrocytes. Immunocytochemistry of AD brain reveals intense apoE staining in senile plaques and in microglial cells associated with them. 23 Whether the material in plaque-associated microglia that reacts with anti-apoE IgG is apoE synthesized by microglia, or reflects endocytosis of apoE by microglia, is not known and cannot be determined from immunocytochemical studies. Whether microglia synthesize apoE in normal adult human brain or in AD brain also is unknown.
Neurons and glia express several receptors for lipoproteins, many of which bind apoE. 24-26 Mice genetically deficient in SR-BI appear normal (ie, weight, general appearance, and behavior) with no reported signs of central nervous system pathology, 27 indicating either that astrocytes and SMCs do not need to bind lipoproteins to maintain lipid homeostasis, or, more likely, that the absence of SR-BI is compensated by other lipoprotein receptors.
Is SR-BI Involved in Binding, Uptake, and Metabolism of Aβ by Astrocytes and Vascular SMCs and Does It Play a Role in Aβ-Mediated Signaling of Astrocytes to Secrete Pro-Inflammatory Cytokines?
Wisniewski and Wegiel 28 reported that astrocyte processes interact with both diffuse and fibrillar Aβ in AD brains. Kurt and colleagues, 29 and Yamaguchi and colleagues 30 described Aβ-immunoreactive material within astrocytes in AD brain, and proposed that its presence within these cells indicates Aβ engulfment by them. Our own studies, 36 and those of Paresce and colleagues 10 show that SR-BI binds and mediates the ingestion of Aβ peptides by wild-type and SR-A−/− microglia from newborn mice, and by SR-BI-transfected CHO cells, respectively. Thus, it seems likely that SR-BI mediates Aβ uptake by astrocytes.
Hu and van Eldik 31 and Johnstone and colleagues 32 reported that fibrillar Aβ activates cultured astrocytes to synthesize and secrete pro-inflammatory substances (eg, interleukin 1b, MCP-1, RANTES). We 36 have shown that SR-BI mediates adhesion of microglia from newborn SR-A knockout mice to matrices containing fibrillar Aβ, that these cells produce reactive oxygen species when they adhere to these matrices, and that antibodies that block SR-BI inhibit both adhesion and production of reactive oxygen species by these cells. Although these findings confirm a role for SR-BI in adhesion of microglia to Aβ-containing matrices, and suggest that SR-BI plays a similar role in mediating astrocyte adhesion to Aβ-containing matrices, they do not tell us whether interaction of SR-BI with Aβ signals formation and secretion of pro-inflammatory substances. Indeed, we do not yet know whether binding of HDL to SR-BI activates cellular-signaling pathways. Similarly, cerebrovascular SMCs express SR-BI, and Prior and colleagues 5 have described fAβ uptake by SR-BI-expressing cerebrovascular SMCs. Whether SR-BI-mediated binding and/or uptake of fAβ by these cells contributes to the clearance and digestion of fAβ and/or to the angiopathy of AD also is unknown.
Note Added in Proof:
We report here that SR-BI is espressed on cerebral arterial smooth muscle cells of mouse and human cerebral arteries, including leptomeningeal arteries. However, SR-BI is not expressed by mouse or human smooth muscle cells (SMC) of extracerebral arteries (ie aorta, heart, liver, skeletal muscle), or of intra- or extracerebral veins (not shown). SR-BI expression has been noted previously in cultured canine leptomeningeal SMC 5 and in macrophages in atherosclerotic lesions but not in SMC in the adjacent tunica media 7,33,34 or in cultured rat aortic SMC. 35 Expression of SR-BI by cerebral arterial smooth muscle cells suggests participation in cerebral amyloid angioipathy in AD.
Acknowledgments
We thank Dr. Steven Chin (Director, Brain Bank of the Department of Pathology, Columbia University) for the human brain tissue used in this study.
Footnotes
Address reprint requests to Jens Husemann, M.D., Columbia University, College of Physicians and Surgeons, Department of Physiology and Cellular Biophysics, 630 West 168th St., New York, NY 10032. E-mail: jh577@columbia.edu.
Supported in part by grants AI20516 from the NIAID, HL52145 from the NHLBI, and RG1-96-067 from the Alzheimer’s Disease Foundation.
References
- 1.Krieger M, Herz J: Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP). Annu Rev Biochem 1994, 63:601-637 [DOI] [PubMed] [Google Scholar]
- 2.Honda M, Akiyama H, Yamada Y, Kondo H, Kawabe Y, Takeya M, Takahashi K, Suzuki H, Doi T, Sakamoto A, Ookawara S, Mato M, Gough PJ, Greaves DR, Gordon S, Kodama T, Matsushita M: Immunohistochemical evidence for a macrophage scavenger receptor in Mato cells and reactive microglia of ischemia and Alzheimer’s disease. Biochem Biophys Res Commun 1998, 245:734-740 [DOI] [PubMed] [Google Scholar]
- 3.Christie RH, Freeman M, Hyman BT: Expression of the macrophage scavenger receptor, a multifunctional lipoprotein receptor, in microglia associated with senile plaques in Alzheimer’s disease. Am J Pathol 1996, 148:399-403 [PMC free article] [PubMed] [Google Scholar]
- 4.Naito M, Kodama T, Matsumoto A, Doi T, Takahashi K: Tissue distribution, intracellular localization, and in vitro expression of bovine macrophage scavenger receptors. Am J Pathol 1991, 139:1411-1423 [PMC free article] [PubMed] [Google Scholar]
- 5.Prior R, Wihl G, Urmoneit B: Apolipoprotein E, smooth muscle cells and the pathogenesis of cerebral amyloid angiopathy: the potential role of impaired cerebrovascular A beta clearance. Ann NY Acad Sci 2000, 903:180-186 [DOI] [PubMed] [Google Scholar]
- 6.Krieger M: Charting the fate of the “good cholesterol”: identification and characterization of the high-density lipoprotein receptor SR-BI. Annu Rev Biochem 1999, 68:523-558 [DOI] [PubMed] [Google Scholar]
- 7.Hirano K, Yamashita S, Nakagawa Y, Ohya T, Matsuura F, Tsukamoto K, Okamoto Y, Matsuyama A, Matsumoto K, Miyagawa J, Matsuzawa Y: Expression of human scavenger receptor class B type I in cultured human monocyte-derived macrophages and atherosclerotic lesions. Circ Res 1999, 85:108-116 [DOI] [PubMed] [Google Scholar]
- 8.Buechler C, Ritter M, Quoc CD, Agildere A, Schmitz G: Lipopolysaccharide inhibits the expression of the scavenger receptor Cla-1 in human monocytes and macrophages. Biochem Biophys Res Commun 1999, 262:251-254 [DOI] [PubMed] [Google Scholar]
- 9.Murao K, Terpstra V, Green SR, Kondratenko N, Steinberg D, Quehenberger O: Characterization of CLA-1, a human homologue of rodent scavenger receptor BI, as a receptor for high density lipoprotein and apoptotic thymocytes. J Biol Chem 1997, 272:17551-17557 [DOI] [PubMed] [Google Scholar]
- 10.Paresce DM, Ghosh RN, Maxfield FR: Microglial cells internalize aggregates of the Alzheimer’s disease amyloid beta-protein via a scavenger receptor. Neuron 1996, 17:553-565 [DOI] [PubMed] [Google Scholar]
- 11.Babitt J, Trigatti B, Rigotti A, Smart EJ, Anderson RG, Xu S, Krieger M: Murine SR-BI, a high density lipoprotein receptor that mediates selective lipid uptake, is N-glycosylated and fatty acylated and colocalizes with plasma membrane caveolae. J Biol Chem 1997, 272:13242-13249 [DOI] [PubMed] [Google Scholar]
- 12.Perry VH, Hume DA, Gordon S: Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience 1985, 15:313-326 [DOI] [PubMed] [Google Scholar]
- 13.Bell MD, Lopez-Gonzalez R, Lawson L, Hughes D, Fraser I, Gordon S, Perry VH: Upregulation of the macrophage scavenger receptor in response to different forms of injury in the CNS. J Neurocytol 1994, 23:605-613 [DOI] [PubMed] [Google Scholar]
- 14.El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD: Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature 1996, 382:716-719 [DOI] [PubMed] [Google Scholar]
- 15.Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr, Baron P, Villalba M, Ferrari D, Rossi F: Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature 1995, 374:647-650 [DOI] [PubMed] [Google Scholar]
- 16.Du Yan S, Zhu H, Fu J, Yan SF, Roher A, Tourtellotte WW, Rajavashisth T, Chen X, Godman GC, Stern D, Schmidt AM: Amyloid-beta peptide-receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage-colony stimulating factor: a proinflammatory pathway in Alzheimer disease. Proc Natl Acad Sci USA 1997, 94:5296-5301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arai T, Rinninger F, Varban L, Fairchild-Huntress V, Liang CP, Chen W, Seo T, Deckelbaum R, Huszar D, Tall AR: Decreased selective uptake of high density lipoprotein cholesteryl esters in apolipoprotein E knock-out mice. Proc Natl Acad Sci USA 1999, 96:12050-12055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaDu MJ, Gilligan SM, Lukens JR, Cabana VG, Reardon CA, Van Eldik LJ, Holtzman DM: Nascent astrocyte particles differ from lipoproteins in CSF. J Neurochem 1998, 70:2070-2081 [DOI] [PubMed] [Google Scholar]
- 19.Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW: Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta 1987, 917:148-161 [DOI] [PubMed] [Google Scholar]
- 20.Nakai M, Kawamata T, Taniguchi T, Maeda K, Tanaka C: Expression of apolipoprotein E mRNA in rat microglia. Neurosci Lett 1996, 211:41-44 [DOI] [PubMed] [Google Scholar]
- 21.Xu Q, Li Y, Cyras C, Sanan DA, Cordell B: Isolation and characterization of apolipoproteins from murine microglia: identification of a low density lipoprotein-like apolipoprotein J-rich but E-poor spherical particle. J Biol Chem 2000, 275:31770-31777 [DOI] [PubMed] [Google Scholar]
- 22.Cole GM, Beech W, Frautschy SA, Sigel J, Glasgow C, Ard MD: Lipoprotein effects on Abeta accumulation and degradation by microglia in vitro. J Neurosci Res 1999, 57:504-520 [PubMed] [Google Scholar]
- 23.Uchihara T, Duyckaerts C, He Y, Kobayashi K, Seilhean D, Amouyel P, Hauw JJ: ApoE immunoreactivity and microglial cells in Alzheimer’s disease brain. Neurosci Lett 1995, 195:5-8 [DOI] [PubMed] [Google Scholar]
- 24.Pitas RE, Boyles JK, Lee SH, Hui D, Weisgraber KH: Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J Biol Chem 1987, 262:14352-14360 [PubMed] [Google Scholar]
- 25.Christie RH, Chung H, Rebeck GW, Strickland D, Hyman BT: Expression of the very low-density lipoprotein receptor (VLDL-r), an apolipoprotein-E receptor, in the central nervous system and in Alzheimer’s disease. J Neuropathol Exp Neurol 1996, 55:491-498 [DOI] [PubMed] [Google Scholar]
- 26.Rebeck GW, Reiter JS, Strickland DK, Hyman BT: Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron 1993, 11:575-580 [DOI] [PubMed] [Google Scholar]
- 27.Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M: A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci USA 1997, 94:12610-12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wisniewski HM, Wegiel J: Spatial relationships between astrocytes and classical plaque components. Neurobiol Aging 1991, 12:593-600 [DOI] [PubMed] [Google Scholar]
- 29.Kurt MA, Davies DC, Kidd M: beta-Amyloid immunoreactivity in astrocytes in Alzheimer’s disease brain biopsies: an electron microscope study. Exp Neurol 1999, 158:221-228 [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi H, Sugihara S, Ogawa A, Saido TC, Ihara Y: Diffuse plaques associated with astroglial amyloid beta protein, possibly showing a disappearing stage of senile plaques. Acta Neuropathol (Berl) 1998, 95:217-222 [DOI] [PubMed] [Google Scholar]
- 31.Hu J, Van Eldik LJ: Glial-derived proteins activate cultured astrocytes and enhance beta amyloid-induced glial activation. Brain Res 1999, 842:46-54 [DOI] [PubMed] [Google Scholar]
- 32.Johnstone M, Gearing AJ, Miller KM: A central role for astrocytes in the inflammatory response to beta-amyloid; chemokines, cytokines and reactive oxygen species are produced. J Neuroimmunol 1999, 93:182-193 [DOI] [PubMed] [Google Scholar]
- 33.Ji Y, Jian B, Wang N, Sun Y, Moya ML, Phillips MC, Rothblat GH, Swaney JB, Tall AR: Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J Biol Chem 1997, 272:20982-20985 [DOI] [PubMed] [Google Scholar]
- 34.Chinetti G, Gbaguidi FG, Griglio S, Mallat Z, Antonucci M, Poulain P, Chapman J, Fruchart JC, Tedgui A, Najib-Fruchart J, Staels B: CLA-1/SR-BI is expressed in atherosclerotic lesion macrophages and regulated by activators of peroxisome proliferator-activated receptors. Circulation 2000, 101:2411-2417 [DOI] [PubMed] [Google Scholar]
- 35.Francis GA, Tsujita M, Terry TL: Apolipoprotein AI efficiently binds to and mediates cholesterol and phospholipid efflux from human but not rat aortic smooth muscle cells. Biochemistry 1999, 38:16315-16322 [DOI] [PubMed] [Google Scholar]
- 36.Husemann J, Loike JD, Kodama T, Silverstein SD: J Neuroimmunol (in press) [DOI] [PubMed]


