Numerous adult age differences have been reported in intentional learning and working memory (WM) processes, but relatively few age effects have been found in relation to implicit memory (for reviews see Fabiani & Gratton, 2005; Fleischman & Gabrieli, 1998; Reuter-Lorenz, 2000). More recently, an increasing number of studies have found age-related differences in perceptual priming tasks such as repetition priming (Fleischman & Gabrieli, 1998; La Voie & Light, 1994).
Repetition priming represents a common form of memory and learning where repeated encounters with an item result in faster and more efficient processing of the item. The effect is also known as adaptation, and is preserved even among amnesia patients (for a review see Gabrieli, 1998). Thus, repetition priming seems to be an automatic process in the absence of awareness, which is in stark contrast to conscious or explicit memory.
Behavioral studies typically demonstrate repetition priming by means of faster response times, decreased bias, and gains in accuracy. Single-cell and fMRI studies have shown that reductions in neural activation to repeated stimuli, known as repetition suppression, can at least partially account for repetition priming effects (e.g., Bucker et al., 1998; Grill-Spector & Malach, 2001; Jiang, Haxby, Martin, Ungerleider, & Parasuraman, 2000, van Turennout, Ellmore, & Martin, 2000). The underlying brain mechanisms of repetition suppression, however, are still under debate and can not be clearly explained by a single neural model (for reviews see Grill-Spector, Henson, & Martin, 2006; Schacter & Buckner, 1998). Furthermore, recent evidence suggests that repetition suppression can be moderated by both perceptual and response-related processes including exposure duration (Zago, Fenske, Aminoff, & Bar, 2005), attention (Vuilleumier, Henson, Driver, & Dolan, 2002; Vuilleumier, Schwartz, Duhoux, Dolan, & Driver, 2005; Yi & Chun, 2005), lag time (e.g., Henson, Rylands, Ross, Vuilleumeir, & Rugg, 2004; Wagner, Maril, & Schacter, 2000), emotion (Bentley, Vuilleumier, Thiel, Driver, & Dolan, 2003; Ishai, Pessoa, Bikle, & Ungerleider, 2004), and task performance (Jiang et al., 2000; Sawamura, Orban & Vohels, 2006; Wig, Grafton, Demos, & Kelley, 2005).
Changes in brain activation reflecting repetition effects have also been indexed with event-related potentials (ERPs), which are distinguished by their high temporal resolution. From this body of research, at least two ERP repetition effects have emerged (e.g. Rugg & Doyle, 1994; Grill-Spector et al., 2006). Some researchers have reported a repetition effect beginning as early as 160 msec when no intervening stimuli are present (Henson et al., 2004). This early effect has been shown to occur with the repetition of both words and non-words (e.g., Rugg, 1987; Rugg, Soardi, & Doyle, 1995), and may reflect more efficient perceptual processing from the immediate repetition of stimuli (Nagy & Rugg, 1989).
A second ERP repetition effect beginning around 300 msec has been more readily observed. This component is related to lexical memory (Rugg & Nagy, 1987) and is modulated by several factors including semantic (e.g., Pickering & Schweinberger, 2003) and decision processes (e.g., Bentin & McCarty, 1994). Several researchers have suggested that this positive-going repetition effect is actually composed of two ERP components: a decrease in N400 activation and increased activation of the Late Positive Component (LPC), also known as the P300 (Friedman, 2000; Rugg, 1990; Swick & Knight, 1999). The N400 is elicited by stimuli that are incongruent with the current semantic context. The LPC is associated with several distinct neural processes, but most often interpreted in relation to brain mechanisms involved in processing capacity and WM (for review see Kok, 2001).
ERP studies examining adult aging have reported conflicting results regarding age differences in repetition priming. Some studies have found clear patterns of age-related differences (Friedman, Hamberger, & Ritter, 1993; Friedman, Hamberger, Stern, & Marder, 1992; Joyce, Paller, McIsaac, & Kutas, 1998: Rugg, Ruth, Gilchrist, & Roberts, 1997) while others have not (Friedman, Ritter, & Snodgrass, 1996; Karayanidis, Andrews, Ward, & McConaghy, 1993; Pfutze, Sommer, & Schweinberger, 2002; Rugg, Pearl, Walker, Roberts, & Holdstock, 1994). Such inconsistencies in findings may be due to influences from explicit memory.
Several researchers have recently proposed that explicit contamination, or explicit modulation, of priming effects may account for the inconsistent age effects found with repetition priming. Mitchell and Bruss (2003) found behavioral age differences in explicit memory but not in implicit memory when novel geometric figures were used. Such findings led the authors to conclude that age differences in implicit memory were likely due to explicit memory contamination, since minimizing the likelihood of explicit contamination also eliminated any age effects in repetition priming. Using evoked potentials, Joyce et al. (1998) also attributed their age modulated repetition effect to explicit contamination since this ERP component was also affected by different levels of processing (i.e., deep versus shallow encoding).
The purpose of the present study was to examine the brain and psychological mechanisms that constitute age-related differences in repetition priming during a working memory task that focused on the physical identification of common objects. Participants performed a matching task involving common objects. Some of the objects were intentionally studied prior to the matching task and all objects were repeated within the task. We were specifically interested in determining the potential cortical interplay, if any, between: a) adult aging and repetition, and b) whether any age differences in repetition were modulated by prior intentional learning. Based upon previous research, we hypothesized that explicit mechanisms would at least partially account for age differences in repetition.
Method
Participants
Fourteen younger (ages 19 – 28, M = 22.3, SD = 3.1) and 14 older (ages 66 – 74, M = 69.7, SD = 3.0) informed and consenting healthy adults were recruited for this study. Younger participants included five males and nine females while elderly subjects included six males and eight females. The data for one additional younger participant could not be used because of excessive EEG artifact. Younger subjects were recruited by means of flyers posted on the University of Kentucky campus and older subjects were recruited via a community research pool. All subjects were right handed, native English speakers, with no history of neurological disease or head injury, and had normal or corrected to normal vision. All older subjects met or surpassed the normal range on screening measures of cognitive functioning as assessed by the Folestein Mini-Mental State Exam (M = 26.6, SD = 1.4, range = 24 – 29), WAIS-II: Vocabulary score (M = 49.1, SD = 6.8), and WMS: Logical Memory I (M = 7.42, SD = 2.9) and II (M = 4.75, SD = 2.9) subtests. Younger and older participants were similar with respect to years of education (M younger = 14.1, SD = 1.8; M old = 15.0, SD = 2.2). All subjects received monetary compensation for their participation.
Visual Stimuli
Stimuli consisted of 240 two dimensional pictures of common objects taken from Snodgrass and Vanderwart (1980). Each object was presented in white-black within a rectangular area of approximately, 8.3 by 5.8 cm, with a 65 cm viewing distance, and at a visual angle of approximately 7 degrees. Also, target objects were presented with a 6 mm green border at the beginning of a trial. The 240 object stimuli were divided into 2 groups with 60 “studied” objects being intentionally studied by participants and 180 “new” objects not previously studied. The 60 studied objects were used as both studied targets and studied distractors. The 180 new objects were subdivided into 60 objects that served as new targets and 120 objects that served as new distractors. Each object group was normed for familiarity and complexity.
Working Memory (WM) Task
The short-term memory task consisted of 120 trials separated into 12 blocks of 10 trials each. Each trial began with the presentation of the sample target object (for 3000 msec) distinguished by having a green border (see Figure 1). A single tone presented at the onset of the sample target further distinguished it from subsequent test objects. The sample target was followed by 9 successive test objects with an ISI of 700 msec per object. All objects were divided by a fixation cross with an ISI of 1100± 100 msec. Each trial lasted approximately 21 seconds.
Figure 1.
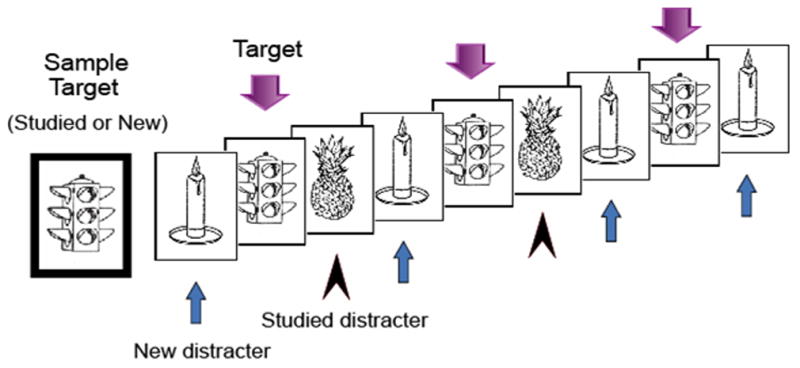
Each trial of the modified delayed match-to-sample task included a sample target object followed by 9 successive test pictures that matched (targets) or did not match (distractors) the sample target. Both targets and distractors included prior studied (old) and new objects, and each object type was presented between 2 and 4 times in a trial.
The 120 trials included 60 trials having a studied target and 60 trials having a new target. The order of studied and new target trials was balanced in a pseudo-random sequence that was consistent across subjects. Within trials, test objects were classified into one of three groups: (a) targets, (b) studied distractors, and (c) new distractors. Each of the studied objects served as a studied target in one trial and as a studied distractor in a later trial. New objects, whether serving as a target or distractor, were not used in any subsequent trials. The test portion of each trial contained a pseudo-random presentation of targets, studied distractors, and new distractors, with each being repeated one to three times, making up a total of nine test objects per trial.
Procedure
Participants were instructed to study and memorize 60 objects that were individually displayed on a computer screen for approximately 10 seconds each (10 minutes total). Subjects also continued to study these objects in paper form during the placement of an EEG cap which lasted about 20 minutes. Subjects were told to relate the objects to personal experiences and that they would be tested after placement of the EEG cap. Participants subsequently performed a recognition test that included the 60 studied objects along with 60 new objects that were not used further in the study. All participants performed well on the recognition test with accuracy of no less than 96% (mean accuracy = 98.2%).
For the WM task, participants were shown a sample target object to hold in mind and were directed to indicate whether the following 9 test objects were the same or different from the sample target by pressing one of two buttons with their right or left hand. Assignment of hands to indicate a target versus distractor was counterbalanced across subjects. Participants were instructed to forget the previous sample target object when a new sample target object appeared, indicating the beginning of a new trial. All participants performed at least 10 practice trials prior to data collection and the WM task lasted approximately 60 minutes overall.
ERP Recordings
Electroencephalographic recordings were made from 62 scalp sites using Ag/AgCI electrodes embedded in an elastic cap at locations designed to provide even coverage across the scalp. Two additional channels were used for monitoring horizontal and vertical eye movements. Trials with incorrect responses and trials contaminated by electro-ocular artifacts were excluded from ERP analyses. A left mastoid reference electrode was used online and the reference was changed offline to the average of left and right mastoid recordings. Impedance was less than 9KΩ. EEG signals were filtered with a band-pass of 0.05–40 Hz and sampled at a rate of 500 Hz. Each epoch lasted 1000 msec with an additional 100 msec recorded prior to stimulus onset to allow for baseline correction.
Statistical Analyses
Behavioral effects were indexed using mean response times (RT) of correct responses and response accuracy data for each condition. ERPs were averaged correct responses elicited by each target or distractor condition recorded during the WM task. Also, preliminary topographic analyses indicated that the midline sites Fpz, Fz, FCz, Cz, CPz, Pz, POz, and Oz provided a good index of neural activation from all 64 scalp locations, and good comparison with previous studies.
Based on the visual inspection of repetition effects for each condition, ERP mean amplitude data were gathered at time segments 100 – 200, 200–300, 300–400, 400–550, 550–700, and 700–850 msec for distractor and target objects relative to the mean amplitude of the pre-stimulus baseline (−100—0 msec set to 0 μV). The initial time interval indexed N2 activation and the latter four intervals indexed Late Positive Component (LPC) activation.
To examine age related effects in repetition, omnibus four-way analysis of variance (ANOVA) tests for two age groups (younger, older), two study types (new, studied), three repetition types (none, once, two or three times), and eight electrode sites (Fz, FCz, Cz, CPz, Pz, Oz; for ERP analyses) were conducted separately for responses to targets and distractors and for each dependant condition (i.e., RA, RT, each ERP mean amplitude segment).
Since target objects were held in mind at the beginning of each trial, the first presentation of a target during the test phase of each trial was actually the second time that the target was perceived. This initial processing of targets was expected to differentially affect repetition processes in comparison to the repetition of distractors. Significance tests, therefore, did not include the direct comparison of targets and distractors. Also, preliminary examination repeated items for each condition revealed that 3rd and 4th presentations did not noticeably differ, and thus, were grouped together for significance testing.
All ANOVAs had a level of significance set at 0.05 and were supplemented with Bonferroni pairwise comparisons or simple main effects comparisons when appropriate. Greenhouse-Geisser corrections were reported with all effects having two or more degrees of freedom in the numerator. In instances where significant interactions occur, main effects may not be reported.
Results
Preliminary analyses of data from older adults grouped by MMSE performance determined that neither behavioral nor ERP scores were differentially affected (p > .10) by individual differences in this cognitive measure. Also, d′ values for younger (d′ = 3.50) and older (d′ = 3.51) adults did not significantly differ (p = .953) with respect to response bias as determined by an independent-samples t-test.
Behavioral Results
The percentage of correct responses and mean response time (RT) for both targets and distractors and each condition type are reported in Table 1. Analysis of response accuracy for targets revealed a main effect of repetition [F(2, 52) = 8.83, p = .001] such that accuracy was higher for repeated targets than when they were initial presented. An opposite trend in accuracy was found for distractors, with a main effect of repetition [F(2, 52) = 5.19, p = .011] indicating more accurate responses to the initial presentation than subsequent repetitions. Also, an age by repetition effect for distractors [F(2, 52) = 3.60, p = .039] showed that younger adults responded more accurately during the 3rd/4th presentations than older adults.
Table 1.
Mean response time (msec) and percentage of correct responses for each condition.
| Younger Adults | Older Adults | |||
|---|---|---|---|---|
| Targets | ||||
| Presentation | Studied | New | Studied | New |
| initial | 509(89.7%) | 504(90.2%) | 584(91.0%) | 572(92.0%) |
| 2nd | 471(91.5%) | 471(92.3%) | 515(93.5%) | 515(95.5%) |
| 3rd/4th | 478(91.2%) | 478(91.2%) | 530(93.7%) | 524(95.2%) |
| Distractors | ||||
| initial | 481(98.2%) | 489(98.5%) | 582(98.0%) | 588(97.3%) |
| 2nd | 454(96.8%) | 451(97.5%) | 533(96.5%) | 526(98.3%) |
| 3rd/4th | 451(97.2%) | 453(98.5%) | 529(96.2%) | 531(97.2%) |
For all subjects, RT data revealed main effects of repetition for targets [F(2, 52) = 77.70, p < .0005] and distractors [F(2, 52) = 109.76, p < .0005]. For both stimulus types, responses were faster for repeated objects than their initial presentation. For distractors only, a repetition by study type interaction [F(2, 52) = 5.29, p = .011] also indicated faster responses to studied than new distractors during the initial presentation only. Main RT effects of age group were found for target [F(1, 26) = 7.40, p = .011] and distractor [F(1, 26) = 15.55, p = .001] objects. For both object types, older adults responded slower than younger adults. Age by repetition interactions were also found for targets [F(2, 52) = 5.90, p = .012] and distractors [F(2, 52) = 7.30, p = .006]. As Figure 2 illustrates, reductions in RT due to repetition were greater for older than younger adults with overall reductions of 9.7 % and 6.6 %, respectively.
Figure 2.
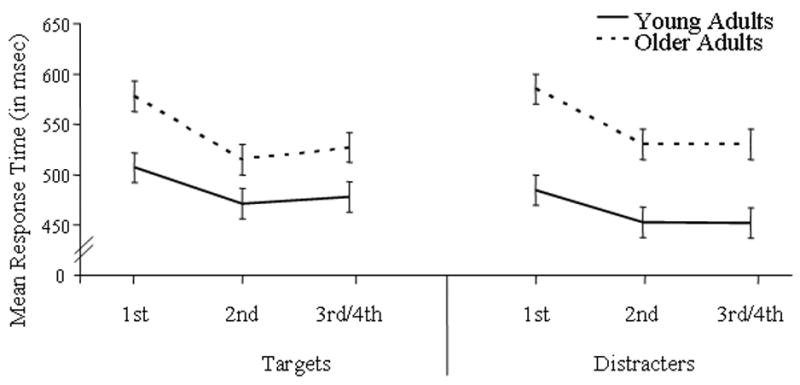
Mean response times for younger and older adults as a function of repetition.
ERP Results
For both target and distractor objects, the N250 component was centered frontally at site Fz and the LPC (late positive component) was centered over parietal site CPz from 300 – 700 msec and centered over FCz from 700 – 850 msec. ERP waveforms at representative electrodes for each age group are presented in Figure 3 to illustrate general repetition effects for targets and in Figure 4 to illustrate general repetition effects for distractors. For target activation, an early repetition effect occurred from 200 – 400 msec and a late repetition effect occurred from 400 – 850 msec. For distractor activation, an early repetition effect lasted from 200 – 550 msec and a late repetition effect occurred from 550 – 850 msec. To clearly disseminate results, significant ERP findings were categorized into general age effects and repetition effects, and further subdivided into early repetition effects and late repetition effects.
Figure 3.
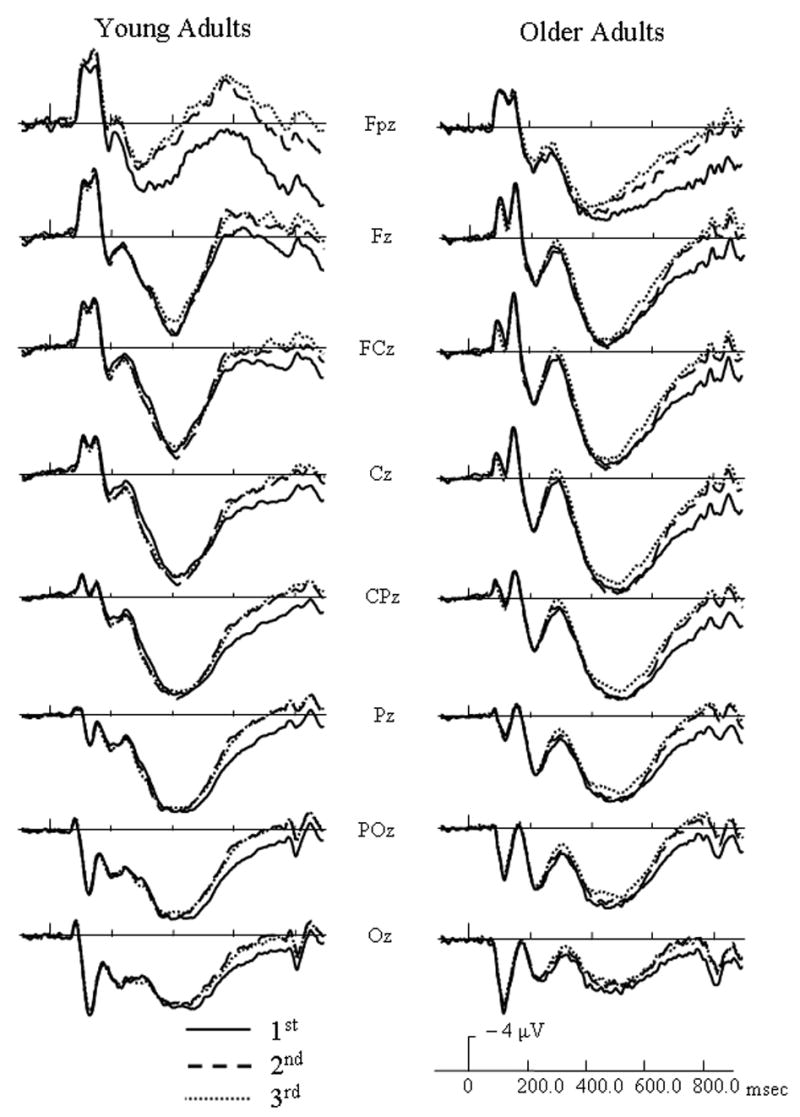
Event-related potentials (ERPs) of distractor (non-match) responses for each age group at initial (solid lines), 2nd (dashed line), and 3rd/4th (dotted line) presentations of distractors, and collapsed across study type.
Figure 4.
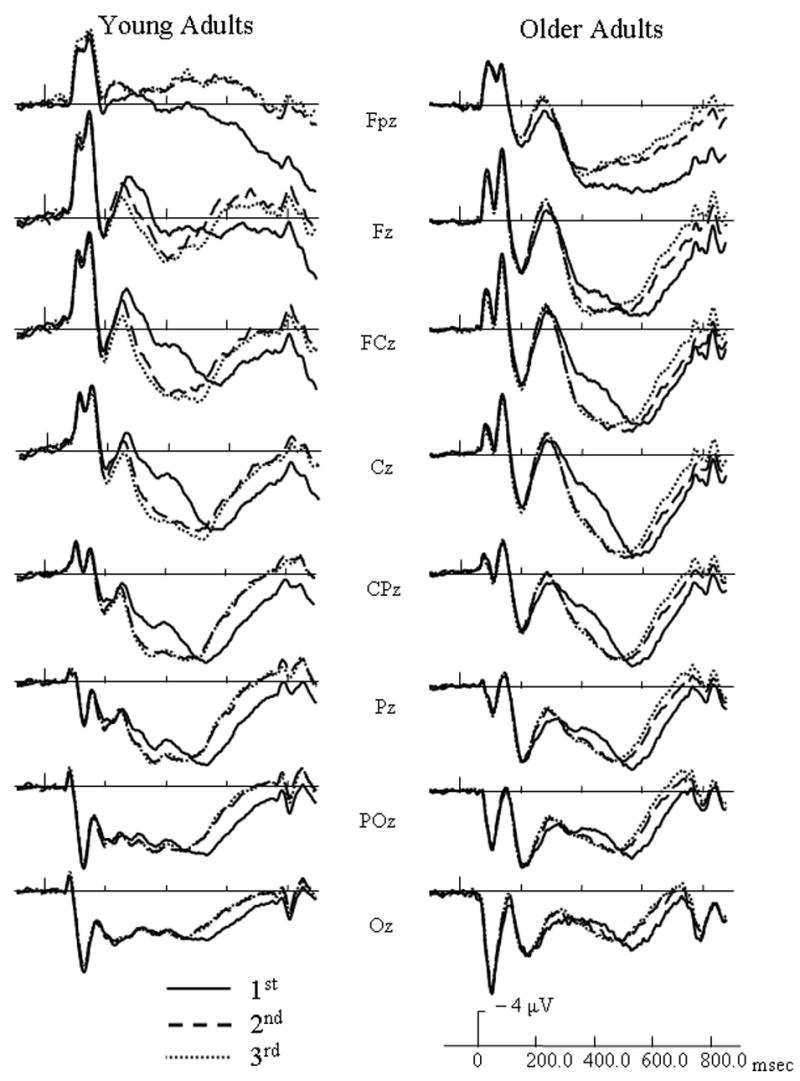
Event-related potentials (ERPs) of target (match) responses for each age group at initial (solid lines), 2nd (dashed line), and 3rd/4th (dotted line) presentations of distractors, and collapsed across study type.
General ERP age effects
Mean amplitude data during the 200 – 300 msec interval indicated no age effects for target responses. Distractors during the 200 – 300 msec interval, however, revealed an age group by electrode site interaction [F(7, 182) = 3.96, p = .023]. At frontal sites Fpz, Fz, and FCz, younger adults had more negative going activation than older adults.
All data during the 300 – 400, 400 – 550, and 550 – 700 msec intervals had age by electrode site interactions with F(7, 182) values ranging between 8.39 (p. = .001) and 18.88 (p. < .0005). For the 300 – 400 and 400 – 550 msec intervals, older adults had more positive activation at anterior sites than younger adults, whereas younger adults had more positive activation than older adults at posterior sites. From 550 – 700 msec, older adults had more positive activation than younger adults at frontal and central sites, and no age group differences occurred at posterior sites. No age effect was found with targets during the 700 – 850 msec interval. The 700 – 850 msec interval for distractors, however, indicated a main effect of age group [F(1, 26) = 4.42, p = .045]. Overall, older adults had more positive activation than younger adults.
Target early repetition effects
From 200 – 300 msec, a repetition by electrode site interaction [F(14, 364) = 6.31, p < .0005] indicated that 3rd/4th presentations had less positive activation than their initial presentations at site Fpz, and the 2nd presentation was in between the two. A three-way interaction involving age group, repetition, and electrode site [F(14, 364) = 5.17, p = .002] was found. For younger adults, repeated targets had more positive activation than their initial presentations at central sites FCz, Cz, and CPz. At these same electrode sites, however, older adults had incremental decreases in positive activation with additional presentations of targets.
From 300 – 400 msec, a three-way interaction including age group, repetition, and electrode [F(14, 364) = 4.81, p = .002] indicated that repeated targets had less positive activation than when initially presented at site Fpz, and this repetition effect was more robust for younger than older adults.
Distractor Early Repetition Effects
From 200 – 300 msec., several interactions were found. Similar to targets, a repetition by electrode site interaction [F(14, 364) = 8.56, p < .0005] indicated that repeated distractors had less positive activation than their initial presentations at Fpz. At sites Fz and FCz, however, repeated presentations had incrementally more positive-going activation than their initial presentations. Also, a three-way interaction involving age group, repetition, and electrode site [F(14, 364) = 4.52, p = .006] was found. Similar to targets, younger adults indexed repeated distractors by more positive activation than their initial presentations at sites Fz, FCz, Cz, CPz, and Pz. At these same electrode sites, however, older adults had incremental decreases in positive activation with additional presentations of distractors. A four-way interaction of age group, repetition, prior study, and electrode site [F(14, 364) = 2.49, p = .047] was found. As Figure 5 shows, younger adults had more positive-going activation for repeated new distractors than to their initial presentations at frontal and central sites, but no repetition effect occurred with studied distractors. Repetition effects for older adults were not affected by study type.
Figure 5.
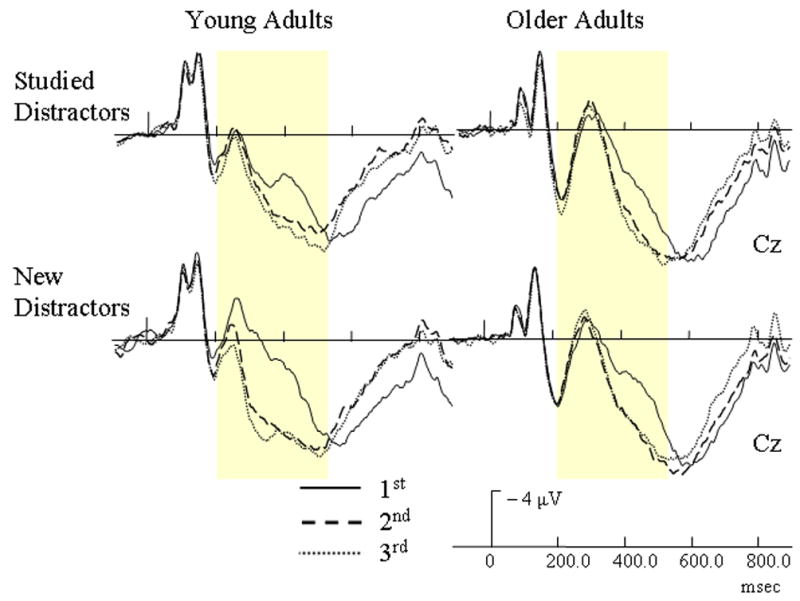
Event-related potentials (ERPs) of repetition effects with studied and non-studied distractors for each age group. The yellow bars highlight the early repetition effect (200 – 550 msec).
From 300 – 400 msec, a repetition by electrode interaction [F(14, 364) = 29.47, p < .0005] indicated that all subjects had increased positive activation for repeated distractors in comparison to their initial presentations, and this repetition effect occurred over frontal and central sites. An age group by repetition by electrode interaction [F(14, 364) = 2.93, p = .03] also indicated that younger adults had larger repetition effects than older adults, and this group by repetition effect was most apparent over frontal and central sites. A four-way interaction of age group, repetition, prior study, and electrode site [F(14, 364) = 4.34, p < .002] was also found. As Figure 5 illustrates, younger adults had larger increases in positive activation for repeated new distractors at frontal and central sites than for studied distractors. This repetition effect for older adults, however, was not modulated by study type.
During the 400 – 550 msec interval, a repetition by electrode interaction [F(14, 364) = 39.42, p < .0005] indicated that repeated objects had more positive activation than their initial presentations at sites FCz, Cz, and CPz. A four-way interaction involving age group, repetition, study type, and electrode [F(14, 364) = 2.58, p = .04] was also found. For older adults, the repetition effect was not affected by whether a distractor was prior studied or new (Figure 5). Younger adults, however, showed larger repetition effects for new distractors than studied distractors.
Target Late Repetition Effects
For the time intervals reflecting activation from 400 – 850 msec, repetition by electrode interactions [F(14, 364) ranging from 8.1 to 8.9, p < .0005] indicated that repeated targets had less positive activation than their initial presentations at central and posterior sites between 400 – 700 msec and at frontal and central sites between 700 – 850 msec.
Distractor Late Repetition Effects
During the 550 – 700 msec interval, a repetition by electrode interaction [F(14, 364) = 9.43, p < .0005] indicated that repeated objects had less positive activation than their initial presentations, and this effect was most robust at posterior sites.
From 700 – 850 msec, a main effect of repetition [F(2, 52) = 51.67, p < .0005] indicated that repeated distractors had less positive activation than their initial presentations. Also, a marginal age group by repetition interaction [F(2, 52) = 3.32, p = .055] was found. As Figure 6 illustrates, older adults appeared to distinguish between 2nd and 3rd/4th presentations at this time interval while younger adults did not.
Figure 6.
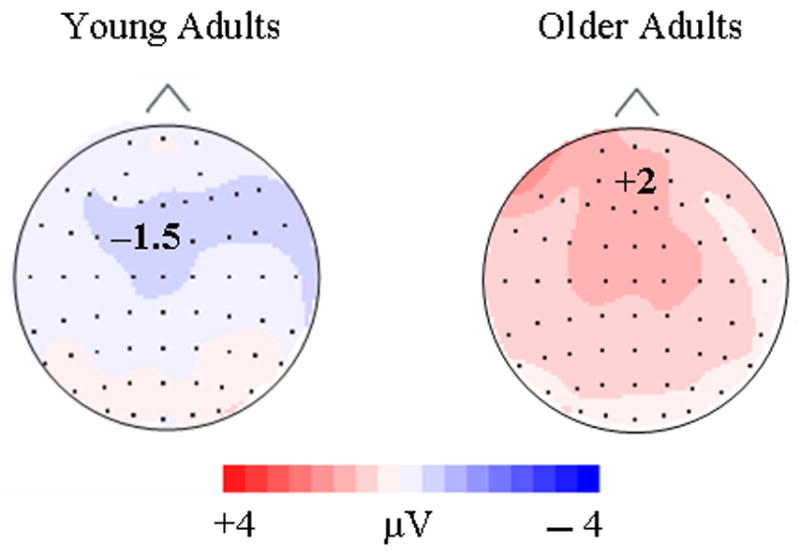
Topographic maps were formed by subtracting the 3rd/4th presentation condition from the 2nd presentation condition during the 550 – 850 interval (late repetition effect) for young and old adults.
Discussion
Age-related changes in priming have typically been viewed as relatively weak in comparison to explicit memory declines and recent reports suggest that explicit memory contamination may account for these implicit age effects (e.g., Mitchell & Bruss, 2003). Based on these studies, we hypothesized that prior intentional learning would have an effect on age-related differences in repetition. While, our behavioral results did not indicate such an interaction, our hypothesis was confirmed by virtue of age-related differences in our early ERP repetition component.
Behaviorally, response times decreased as a function of repetition for both younger and older adults. Defining repetition priming as the improved processing of a repeated stimulus according to a behavioral measure, these reductions in response time confirm that repetition priming did occur in the present study. Unexpected though interesting, the decreases in reaction time with repetition were larger for older than younger adults, indicating more enhanced repetition benefits for older individuals (Figure 2). Such age findings confirm a recent report on age-related differences in repetition priming using familiar faces in a similar WM task (Caggiano, Jiang, & Parasuraman, 2006). Furthermore, our ERP evidence points to the presence of age differences with two functionally distinct repetition effects. That is, our early and late ERP repetition effects were differentially affected by age.
An early ERP repetition effect ranging from 200 – 550 msec over central sites was found with both target and distractor objects and is composed of a N2 component and the incline of the LPC (Figures 3 & 4). The increased neural activation of repeated items during this early effect (Figure 4) is consistent with recent reports indicating that repetition can evoke increases in neural activity (Henson et al, 2000). Repetition induced increases in neural activation could reflect the focusing of neural activity as indicated in sharpening models of repetition or the earlier onset of the LPC predicted by facilitation models (Grill-Spector et al., 2006). Importantly, the early repetition effect for distractors was affected by prior explicit learning for younger, but not older adults (Figure 5). The early component for young adults disappeared (200 – 300 msec) or diminished (300 – 550 msec) when an object was previously studied or served as a target. Repetition effects for older adults, however, were never affected by prior study at any time interval.
The finding that prior intentional study can affect incidental repetition effects in a subsequent task supports previous ERP (e.g., Joyce et al., 1998) and behavioral (e.g., Mitchell & Bruss, 2003) findings indicating that explicit memory can modulate repetition effects. Although our behavioral results did not show an age difference in the interaction between explicit prior learning and repetition, other researchers have found such effects. Mitchell and Bruss (2003) found behavioral age differences in explicit memory but not in implicit memory when novel geometric figures were used. Such findings led the authors to conclude that age effects in implicit memory were likely due to explicit memory contamination, since reducing the likelihood of explicit learning resulted in no age effects.
Indeed, age differences revealed by our early component may have been due to explicit memory modulations. For young adults, explicit modulation seems plausible since prior intentional study had a similar effect on ERP amplitudes as repetition (i.e., more positive-going activation). For older adults, the lack of differences found in processing non-studied versus studied objects could simply reflect the well-established age declines in explicit and working memory, as shown by the consistently slower RTs for older adults in the present study. Although speculative, the lack of influence of prior study on the early repetition effect for targets may be due to explicit WM processes involved in holding targets in mind throughout each trial. In contrast to the early repetition effect, such explicit modulation is missing for the late ERP repetition effect.
A late repetition ERP effect ranging from 400 – 850 msec was found with both targets and distractors and was preserved for both younger and older adults (Figures 3 & 4). Importantly, the ERP effect was indifferent to prior intentional learning. Similar ERP repetition effects between 400 and 900 msec have been found with young adults using both novel and familiar objects (Rugg et al., 1995; Penney, Mecklinger, & Nessler, 2001). Rugg and colleagues suggested that reduced stimulus analysis processes most likely accounted for their late repetition effect (see also Bentin & McCarthy, 1994). Also, Penney et al. (2001) suggested that a reduction in ERP positive activation for repeated objects may be due to more efficient identification of those objects. Repetition suppression accounts proposed by fMRI studies also correspond to this repetition induced decline in LPC activation.
Using a similar matching task with repeated familiar faces, Jiang et al. (2000) showed fMRI repetition response reductions to targets and distractors at occipital, temporal, and parietal lobes. Such response reductions have been consistently reported in repetition priming tasks (Buckner et al 1998; Gabrieli, 1998). Consistent with such posterior mechanisms, the lack of influence from intentional prior learning processes may indicate that this late ERP effect is a “universal” effect, and is a consequence of automatic processes (Wiggs & Martin, 1998; Fiebach, Gruber, & Supp, 2005). The substantial posterior activation found with our late repetition component for both young and old adults is consistent with these previous findings (Figure 6).
During the late ERP repetition effect, young and old adults had similar repetition related decreases in positive activation with the probable exception of the 700 – 850 msec time interval. The marginal age by repetition effect (p = .055) for distractors at this interval suggests that older adults had repetition related declines in positive activation from the 2nd to 3rd/4th presentations that were not apparent for younger adults (Figure 6). Friedman and colleagues (1993) found a similar age-related ERP repetition effect late in the recording epoch (600 – 900 msec), which they attributed to enhanced processing by older adults and characteristic of increased LPC and slow wave activation. Future research should be conducted to clarify this probable age-related mechanism of repetition.
The current study provides evidence that cognitive aging influences mechanisms of repetition priming, and such age effects can not be attributed entirely to explicit processes. Prior repetition studies of cognitive aging typically have only relied on behavioral measures which reflect the accumulated processes leading up to participants’ responses, or PET/MRI measures that have relatively poor temporal resolution. The present results provide evidence that ERP measures with high temporal resolution are more sensitive in detecting differential brain mechanisms related to repetition. The influence of prior intentional learning on the early ERP repetition effect demonstrates the co-existence of these memory processes for younger adults. Why these memory processes do not persist in later life, however, remains unclear. Explicit contamination may account for this early age effect, but the marginal age difference with the late repetition effect suggests that implicit memory is affected by age. Future research will need to reexamine the effects of age on brain mechanisms accounting for repetition effects. It is clear from these findings, however, that a greater understanding of aging effects on implicit memory will rely on our ability to distinguish the differential brain mechanisms affected by repetition.
Acknowledgments
The present study was supported by a pilot grant as part of NIH grant P50 AG05144-21, and NIH grant AG00986 to Y.J., Chinese Ministry of Education grant 20040028001, Ministry of Science and Technology grant 95-special-09, and National Natural Science Foundation of China (No. 30170322, 30570603) to C.G. Reprint requests should be sent to: Yang Jiang, Department of Behavioral Science, University of Kentucky College of Medicine, Lexington, KY 40536-0086, USA or via email: yjiang@email.uky.edu
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bentin S, McCarthy G. The effects of immediate stimulus repetition on reaction time and event-related potentials in tasks of different complexity. J of Exp Psych: Learn, Mem, & Cogn. 1994;20:130 – 149. [Google Scholar]
- Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ. Effects of attention and emotion on repetition priming and their modulation by cholinergic enhancement. J of Neurophysio. 2003;90:1171 – 81. doi: 10.1152/jn.00776.2002. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen B, Dale AM. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Caggiano DM, Jiang Y, Parasuraman R. Aging and repetition priming for targets and distractors in a working memory task. Aging, Neuropsych & Cogn. 2006;13:552–573. doi: 10.1080/138255890969555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani M, Gratton G. Electrophysiological and optical measures of cognitive aging. In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neurosci of Aging: Linking Cognitive & Cerebral Aging. New York, NY: Oxford Univ. Press; 2005. pp. 85–106. [Google Scholar]
- Fiebach CJ, Gruber T, Supp GG. Neuronal mechanisms of repetition priming in occipitotemporal cortex: Spatiotemporal evidence from functional magnetic resonance imaging and electroencephalography. J of Neurosci. 2005;25:3414 – 3422. doi: 10.1523/JNEUROSCI.4107-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman DA, Gabrieli JDE. Repetition priming in normal aging and Alzheimer’s disease: a review of findings and theories. Psych & Aging. 1998;13:88 – 119. doi: 10.1037//0882-7974.13.1.88. [DOI] [PubMed] [Google Scholar]
- Friedman D. Event-related brain potential investigations of memory and aging. Biological Psych. 2000;54:175 – 206. doi: 10.1016/s0301-0511(00)00056-9. [DOI] [PubMed] [Google Scholar]
- Friedman D, Hamberger M, Ritter W. Event-related potentials as indicators of repetition priming in young and older adults: amplitude, duration, and scalp distribution. Psych & Aging. 1993;8:120 – 125. doi: 10.1037//0882-7974.8.1.120. [DOI] [PubMed] [Google Scholar]
- Friedman D, Hamberger M, Stern Y, Marder K. Event-related potentials (ERPs) during repetition priming in Alzheimer’s patients and young and older adults. J of Clin & Exp Neuropsych. 1992;14:448 – 462. doi: 10.1080/01688639208402837. [DOI] [PubMed] [Google Scholar]
- Friedman D, Ritter W, Snodgrass JG. ERPs during study as a function of subsequent direct and indirect memory testing in young and old adults. Cognitive Brain Res. 1996;4:1 – 13. doi: 10.1016/0926-6410(95)00041-0. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE. Cognitive neuroscience of human memory. Annu Rev Psych. 1998;49:87 – 115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: Neural models of stimulus-specific effects. Trends in Cognitive Sci. 2006;10(1):14 – 23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMR-adaptation: A tool for studying the functional properties of human cortical neurons. Acta Psych. 2001;107:293 – 321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Dolan R. Neuroimaging evidence for dissociable forms of repetition priming. Science. 2000;287:1269–1272. doi: 10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rylands A, Ross E, Vuilleumeir P, Rugg MD. The effect of repetition lag on electrophysiological and haemodynamic correlates of visual object priming. NeuroImage. 2004;21:1674 – 1689. doi: 10.1016/j.neuroimage.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Ishai A, Pessoa L, Bikle PC, Ungerleider LG. Repetition suppression of faces is modulated by emotion. Proc Natl Acad Sci USA. 2004;101:9827 – 32. doi: 10.1073/pnas.0403559101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Haxby JV, Martin A, Ungerleider LG, Parasuraman R. Complementary neural mechanisms for tracking items in human working memory. Science. 2000;287:643 – 646. doi: 10.1126/science.287.5453.643. [DOI] [PubMed] [Google Scholar]
- Joyce CA, Paller KA, McIsaac HK, Kutas M. Memory changes with normal aging: Behavioral and electrophysiological measures. Psychophysiology. 1998;35:669 – 678. [PubMed] [Google Scholar]
- Karayanidis F, Andrews S, Ward PB, McConaghy N. Event-related potentials and repetition priming in young, middle-aged, and elderly normal subjects. Cognitive Brain Res. 1993;1:123 – 134. doi: 10.1016/0926-6410(93)90017-y. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557 – 577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- La Voie D, Light LL. Adult age differences in repetition priming: A meta-analysis. Psych & Aging. 1994;9:539 – 553. doi: 10.1037//0882-7974.9.4.539. [DOI] [PubMed] [Google Scholar]
- Mitchell DB, Bruss PJ. Age differences in implicit memory: Conceptual, perceptual, or methodological? Psych & Aging. 2003;18:807 – 822. doi: 10.1037/0882-7974.18.4.807. [DOI] [PubMed] [Google Scholar]
- Nagy ME, Rugg MD. Modulation of event-related brain potentials by word repetition: The effects of inter-item lag. Psychophysiology. 1989;26:431 – 436. doi: 10.1111/j.1469-8986.1989.tb01946.x. [DOI] [PubMed] [Google Scholar]
- Penney TB, Mecklinger A, Nessler D. Repetition related ERP effects in a visual object target detection task. Brain Res Cogn Brain Res. 2001;10:239 – 250. doi: 10.1016/s0926-6410(00)00041-0. [DOI] [PubMed] [Google Scholar]
- Pfutze EM, Sommer W, Schweinberger SR. Age-related slowing in face and name recognition: Evidence from event-related brain potentials. Psych & Aging. 2002;17:140 – 160. doi: 10.1037//0882-7974.17.1.140. [DOI] [PubMed] [Google Scholar]
- Pickering EC, Schweinberger SR. N200, N250r, and N400 event-related brain potentials reveal three loci of repetition priming for familiar faces. J of Exp Psych: Learn, Mem, & Cogn. 2003;29:1298 – 1311. doi: 10.1037/0278-7393.29.6.1298. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA. Cognitive neuropsychology of the aging brain. In: Park DC, Schwarz N, editors. Cognitive Aging: A Primer. New York, NY: Psychology Press; 2000. pp. 93–114. [Google Scholar]
- Rugg MD. Dissociation of semantic priming, word and non-word repetition effects by event-related potentials. Quart J of Exp Psych: Human Exp Psych. 1987;39:123 – 148. [Google Scholar]
- Rugg MD. Event-related potentials dissociate repetition effects of high and low frequency words. Mem & Cogn. 1990;18:367 – 379. doi: 10.3758/bf03197126. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Doyle MC. Event-related potentials and stimulus repetition in direct and indirect tests of memory. In: Heinze H, Munte T, Mangun GR, editors. Cognitive Electrophysiology. Boston: Birkhauser; 1994. pp. 124–148. [Google Scholar]
- Rugg MD, Nagy ME. Lexical contribution to nonword-repetition effects: Evidence from event-related potentials. Mem and Cogn. 1987;15:473 – 481. doi: 10.3758/bf03198381. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Ruth ME, Gilchrist J, Roberts RC. ERP repetition effects in indirect and direct tasks: Effects of age and interitem lag. Psychophysiology. 1997;34:572 – 586. doi: 10.1111/j.1469-8986.1997.tb01744.x. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Pearl S, Walker P, Roberts RC, Holdstock JS. Word repetition effects on event-related potentials in healthy young and old subjects, and in patients with Alzheimer-type dementia. Neuropsychologia. 1994;32:381 – 398. doi: 10.1016/0028-3932(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Soardi M, Doyle MC. Modulation of event-related potentials by the repetition of drawings of novel objects. Brain Res Cogn Brain Res. 1995;3:17 – 24. doi: 10.1016/0926-6410(95)00014-3. [DOI] [PubMed] [Google Scholar]
- Sawamura H, Orban GA, Vogels R. Selectivity of neuronal adaptation does not match response selectivity: A single-cell study of the fMRI adaptation paradigm. Neuron. 2006;49:307–318. doi: 10.1016/j.neuron.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Sayres R, Grill-Spector K. Object-selective cortex exhibits performance-independent repetition suppression. J Neurophysiol. 2006;95:995 – 1007. doi: 10.1152/jn.00500.2005. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL. On the relations among priming, conscious recollection, and intentional retrieval: Evidence from neuroimaging research. Neurobiology of Learn & Mem. 1998;70:284 – 303. doi: 10.1006/nlme.1998.3854. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psych. 1980;6:174 – 215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Swick D, Knight RT. Contributions of prefrontal cortex to recognition memory: Electrophysiological and behavioral evidence. Neuropsychol. 1999;13:155 – 170. doi: 10.1037//0894-4105.13.2.155. [DOI] [PubMed] [Google Scholar]
- Van Turennout MV, Ellmore T, Martin A. Long-lasting cortical plasticity in the object naming system. Nature Neurosci. 2000;3:1329 – 1334. doi: 10.1038/81873. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Henson RN, Driver J, Dolan RJ. Multiple levels of visual object constancy revealed by event-related fMRI of repetition priming. Nature Neurosci. 2002;5:491 – 499. doi: 10.1038/nn839. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Schwartz S, Duhoux S, Dolan RJ, Driver J. Selective attention modulates neural substrates of repetition priming and “implicit” visual memory: Suppressions and enhancements revealed by fMRI. J of Cognitive Neurosci. 2005;17(8):1245 – 1260. doi: 10.1162/0898929055002409. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Schacter DL. Interactions between forms of memory: When priming hinders new episodic learning. J of Cognitive Neurosci. 2000;12(Suppl 2):52 – 60. doi: 10.1162/089892900564064. [DOI] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Kelley WM. Reductions in neural activity underlie behavioral components of repetition priming. Nature Neurosci. 2005;9(8):1228 – 1233. doi: 10.1038/nn1515. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Current Opinion in Neurobiol. 1998;8:227 – 233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- Yi DJ, Chun MM. Attentional modulation of learning-related repetition attenuation effects in human parahippocampal cortex. J Neurosci. 2005;25:3593 – 3600. doi: 10.1523/JNEUROSCI.4677-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zago L, Fenske MJ, Aminoff E, Bar M. The rise and fall of priming: How visual exposure shapes cortical representations of objects. Cereb Cortex. 2005;15:1655 – 1666. doi: 10.1093/cercor/bhi060. [DOI] [PMC free article] [PubMed] [Google Scholar]


