Abstract
Heterogeneous expression of several antigens on the three currently defined tonsil dendritic cell (DC) subsets encouraged us to re-examine tonsil DCs using a new method that minimized DC differentiation and activation during their preparation. Three-color flow cytometry and dual-color immunohistology was used in conjunction with an extensive panel of antibodies to relevant DC-related antigens to analyze lin− HLA-DR+ tonsil DCs. Here we identify, quantify, and locate five tonsil DC subsets based on their relative expression of the HLA-DR, CD11c, CD13, and CD123 antigens. In situ localization identified four of these DC subsets as distinct interdigitating DC populations. These included three new interdigitating DC subsets defined as HLA-DRhi CD11c+ DCs, HLA-DRmod CD11c+ CD13+ DCs, and HLA-DRmod CD11c− CD123− DCs, as well as the plasmacytoid DCs (HLA-DRmod CD11c− CD123+). These subsets differed in their expression of DC-associated differentiation/activation antigens and co-stimulator molecules including CD83, CMRF-44, CMRF-56, 2-7, CD86, and 4-1BB ligand. The fifth HLA-DRmod CD11c+ DC subset was identified as germinal center DCs, but contrary to previous reports they are redefined as lacking the CD13 antigen. The definition and extensive phenotypic analysis of these five DC subsets in human tonsil extends our understanding of the complexity of DC biology.
Dendritic cells (DCs) have a central role in the initiation and regulation of immune responses in both lymphoid and nonlymphoid tissues. They share a number of common features, notably MHC class II expression, in combination with an absence of the lineage-specific markers CD3, CD14, CD16, and CD19 (lin−). 1-3 There is however considerable intra- and intertissue variation in the phenotype, morphology, function, and tissue localization of different DC populations. 1-7 After the identification of distinct myeloid DC and lymphoid DC subsets in mice 3 there has been increasing interest in the characterization of human DC subsets. These have been categorized on the basis of differential expression of myeloid and lymphoid lineage-associated markers, as well as their responsiveness to maturation or differentiation stimuli. 8-13 There is evidence that myeloid DCs and lymphoid DCs direct immunogenic and tolerogenic responses, respectively. 13 It is unclear whether human DC subsets represent distinct cell lineages 10,12 or differing maturation states. 9,14 As a result, the ontogeny and interrelationship of human DC subsets requires further investigation.
Tonsils have been used as a readily available source of lymphoid tissue to characterize human DCs. 15 Three tonsil DC subsets have been identified: interdigitating DCs (IDCs), 16 plasmacytoid DCs, 17 and germinal center DCs (GCDCs). 18 These DC subsets were isolated after a period of in vitro culture, which is likely to alter cell phenotype and morphology. 19 They were also positively selected from lin− cells based on their expression of the CD4, CD11c, or CD40 antigens, which would exclude any DC subsets lacking these antigens and would incorporate all DC subsets expressing those antigens into one population. The observation that both IDCs and GCDCs were heterogeneous with regard to CD11c, CD83, HLA-DR, and CD13 intensity raised the possibility that these tonsil DC populations might contain additional subsets.
This is the first study to analyze the composition of tonsil DC subsets within the entire lin− HLA-DR+ DC population isolated using a new method that maintains the cells at 4°C to minimize changes in cellular differentiation/activation induced by the isolation procedure. We report the presence of five distinct DC subsets within human tonsils. The phenotype of each tonsil DC subset was analyzed by three-color flow cytometry and two-color immunohistochemistry using an extensive panel of antigens relevant to DC lineage, activation state, and function.
Materials and Methods
Patients and Samples
After approval by the Canterbury Ethics Committee and appropriate informed consent, tonsils were obtained from 68 patients undergoing routine tonsillectomies, which were excised in a noninflamed state. They were transported in sterile saline and processed immediately after excision. Tissue specimens for immunohistochemistry were snap-frozen in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA) and stored at −80°C until required. The remaining fresh tissue was used for DC isolation.
Monoclonal Antibodies (mAbs) and Soluble Ligands
The mAbs and recombinant proteins used in this study are listed in Table 1 ▶ and were used at optimal working concentration established by staining relevant control cell populations.
Table 1.
List of mAbs and Recombinant Proteins Used in This Study
| Antigen/molecule | Clone | Source |
|---|---|---|
| CD1a | Na1/34 | A. McMichael (Oxford, UK) |
| CD3 | OKT3 | ATCC* (Rockville, MD) |
| CD4 | OKT4 | ATCC |
| CD11a | TS1/22 | ATCC |
| CD11b | OKM1 | ATCC |
| CD11c | 3.9 KB90 | Caltag (Burlingame, CA) DAKO (Carpenteria, CA) |
| CD13 | L138 WM-15 | Becton Dickinson (Sydney, Australia) K. Bradstock (Westmead, Australia) |
| CD14 | CMRF31 | Our laboratory |
| CD16 | HuNK2 | I. McKenzie (Melbourne, Australia) |
| CD18 | TS1/18 | ATCC |
| CD19 | FMC63 | H. Zola (Adelaide, Australia) |
| CD20 | L27 | Becton Dickinson (Sydney, Australia) |
| CD21 | THB5 | ATCC |
| CD24 | ALB9 BA1 Vibe-3 | Serotec (Oxford, UK) T. LeBien (MN, USA) W. Knapp (Vienna, Austria) |
| CD25 | 7G7 | ATCC |
| CD33 | WM-54 My9 LIB2 L4F3 | K. Bradstock (Westmead, Australia) 3rd LDAW† 3rd LDAW 3rd LDAW |
| CD38 | OKT10 | ATCC |
| CD40 | G28-5 | ATCC |
| CD45pan | CMRF12 | Our laboratory |
| CD45RA | CMRF11 | Our laboratory |
| CD45RB | MT3 | 6th LDAW |
| CD45RC | 11G8 | 6th LDAW |
| CD45RO | UCHL1 | ATCC |
| CD50 | CG106 | D. Simmons (Oxford, UK) |
| CD54 | WCAM1 | A. Boyd (Melbourne, Australia) |
| CD58 | TS/2914 | ATCC |
| CD68 | KiM7 | 3rd LDAW |
| CD70 | BU69 | D. Hardie (Birmingham, UK) |
| CD72 | BU41 | D. Hardie (Birmingham, UK) |
| CD79a | JCB117 | D. Mason (Oxford, UK) |
| CD79a peptide | Hm57 | D. Mason (Oxford, UK) |
| CD79b | B29/127 | D. Mason (Oxford, UK) |
| CD80 | L307 BB1 | Becton Dickinson (Sydney, Australia) P. Linsley (Seattle, WA) |
| CD83 | HB15a | Immunotech (Marseille Cedex, Fr) T. Tedder (Durham, NC) |
| CD86 | BU63 | Serotec (Oxford, UK) |
| CD95 | APO1 | DAKO (Carpenteria, CA) |
| CD123 | Ancell (Bayport, MN) | |
| HLA-ABC | W6/32 | ATCC |
| HLA-DP | B7/21 | F. Brodsky (San Francisco, CA) |
| HLA-DQ | L227 | ATCC |
| HLA-DR | L243 L243 HK14 | ATCC Becton Dickinson (Sydney, Australia) Sigma (St Louis, MO) |
| B cell antigen | FMC1 | H. Zola (Adelaide, Australia) |
| Plasma cell antigen | VS38 | D. Mason (Oxford, UK) |
| Bcl-2 | D. Mason (Oxford, UK) | |
| Fascin/p55 | E. Langhoff (Hershey, PENN) | |
| 2–7 | 3rd LDAW | |
| 41BB | Ancell | |
| CMRF-44 | Our laboratory | |
| CMRF-56 | Our laboratory | |
| CTLA-4Ig | P. Linsley (Seattle, WA) | |
| IgA,D,G,M,κ,λ | Coulter (Sydney, Australia) | |
| IgG1 | X63 | ATCC |
| IgG2a | Sal5 UPC10 | H. Zola (Adelaide, Australia) Sigma (St Louis, MO) |
| IgG2b | Sal4 | H. Zola (Adelaide, Australia) |
| IgM | CMRF50 | Our laboratory |
*ATCC, American Type Culture Collection.
†Leukocyte Differentiation Antigen Workshop.
Isolation of Fresh Tonsil DCs
Enzymatic digestion was avoided as it subjects the cells to a period of incubation at 37°C and has been shown previously to release follicular DCs. 15 Tonsil tissue was carefully minced using scissors and dissociated using a syringe plunger. Released cells were passed through a 40-grade mesh sieve into cold 10% fetal calf serum/RPMI media (RPMI 1640 supplemented with 100 U/ml penicillin, 1 mmol/L glutamine, and 100 U/ml streptomycin). Cellular debris was allowed to settle for 5 minutes. Remaining supernatant was filtered through a 70-μm nylon cell strainer (Falcon, Becton Dickinson Labware, NJ) and spun over a Ficoll-Hypaque density gradient (d = 1.077) to obtain mononuclear cells. The DC isolation was continued as previously described for blood DCs 20 and synovial fluid DCs. 21 Briefly, T cells were depleted by rosetting with neuraminidase-treated sheep erythrocytes for 1.5 to 16 hours at 4°C. Non-T cells were isolated over a Ficoll-Hypaque density gradient and labeled with a mixture of mAbs (OKT3, CMRF-31, HuNK2, FMC-63) against the mature lineage-specific markers CD3 (T cells), CD14 (monocytes), CD16 (NK cells), and CD19 (B cells), respectively. In some cases, CD13 (WM-15) was added to the mAb mixture to isolate CD13− DCs or CD11c (KB90) was added to the mAb mixture to isolate CD11c− DC subsets. Labeled cells were depleted by positive selection using BioMag goat anti-mouse IgG-coated immunomagnetic beads (PerSeptive Biosystems, Framingham, MA) on a Dynamagnet (Dynal, Oslo, Norway) followed by sorting on a flow cytometer (FACS Vantage; Becton Dickinson, Australia), which resulted in >97% pure unlabeled cells. The resulting cells lacked lineage-specific markers (lin−) and comprised the final DC-enriched populations. DCs were identified within the lin− populations as CD45+, HLA-DR+ cells. May-Grunswald Giemsa stain was used to analyze cell morphology on cytospins.
Isolation of T Cells
T cells were obtained from tonsils and normal blood by hypotonic lysis of Er+ cells after rosetting. Contaminating non-T cells were labeled with a mAb mixture against CD14, CD16, CD19, and HLA-DR and removed using BioMag immunomagnetic beads and a Dynamagnet. This resulted in >97% pure CD3+ T cells.
Mixed Leukocyte Reaction
Tonsil lin− cells were sorted into CD11c+ and CD11c− DC subsets and added at the indicated numbers to 2 × 10 5 purified allogeneic CD3+ blood T cells in triplicate wells. 3H-Thymidine (0.5 μCi) was added in the last 18 hours of a 6-day culture and uptake measured on a scintillation counter.
Cell Immunofluorescence Staining
Freshly sorted tonsil lin− cells were labeled with an extensive panel of mAbs using standard two- and three-color immunofluorescence staining. Intracytoplasmic staining using mAbs against p55, CD68, and Ig was performed using a Perm & Fix kit (Caltag Laboratories, Burlingame, CA). In the former case, the primary mAb was detected with fluorescein isothiocyanate (FITC)-conjugated sheep anti-mouse (SAM) IgG F(ab′)2 (Silenus Laboratories, Hawthorne, Australia), whereas the second mAb was HLA-DR-PE-conjugated. Triple-color staining was used to analyze DC subsets on lin− cells and CD13−/lin− cells using FITC-conjugated CD11c, PE/Cy5-conjugated HLA-DR and PE-SAM to detect the third antigen; or on CD11c−/lin− cells using CD123 or CD4 plus FITC-SAM, PE/Cy5-conjugated HLA-DR and a PE-conjugated third mAb. Isotype-matched controls were used for each mAb. In all phenotypic analyses HLA-DR was used as a marker to identify the DC population. A total of 10,000 events was collected for each sample. Cells were either analyzed on the FACS immediately or were fixed in 1% paraformaldehyde and stored at 4°C for next day analysis. The staining pattern and intensity appeared unaltered by cell fixation.
Tissue Immunohistochemistry
Cryostat sections of fresh tonsil tissue were dual-labeled as previously described. 22 Alkaline phosphatase staining with a Fast Blue substrate (Sigma, St Louis, MO) detected the primary mAb (blue color), whereas immunoperoxidase staining with diaminobenzidine (DAKO Corporation, Carpinteria, CA) detected the second mAb (brown color). Double-stained cells were a grayish-green color. Stained sections were analyzed by light microscopy (BX50; Olympus Optical Co. Ltd., Tokyo, Japan) and photographed using the Olympus PM30 photomicrography system.
Tissue Immunofluorescence
Sections of fresh tonsil tissue were dual-labeled as described above for cell immunofluorescence staining. Briefly, after labeling the tissue for 30 minutes with an unconjugated mAb, excess mAb was removed using three 5 minutes washes with phosphate-buffered saline (PBS) and bound mAb detected with FITC-SAM. After another wash to remove excess FITC-SAM, 10% mouse serum/PBS was added to the tissue for 20 minutes to block nonspecific staining of a PE-conjugated second mAb. Tissue sections were analyzed using a fluorescent microscope (BX50, Olympus) and photographed using the Olympus PM30 photomicrography system.
Results
Characterization of lin− Tonsil Cells Based on HLA-DR Density
Depletion of lin+ cells (CD3, CD14, CD16, and CD19+) from the tonsil mononuclear cell fraction resulted in lin− preparations that were highly enriched for DCs, as defined by HLA-DR expression (74.9% ± 11.5 SD, n = 18). Based on the surface density of the HLA-DR molecule, tonsil DCs could be clearly subdivided into distinct HLA-DRhi- and HLA-DRmod-expressing populations (Figure 1A) ▶ . HLA-DRhi DCs represented a minor subset, accounting for only 4.1% ± 1.8 SD (n = 18) of the total HLA-DR+ lin− DC population. All tonsil DCs expressed HLA-DQ and HLA-ABC at a similar density (Table 2) ▶ and showed weaker nonspecific esterase activity than autologous tonsil macrophages (data not shown).
Figure 1.
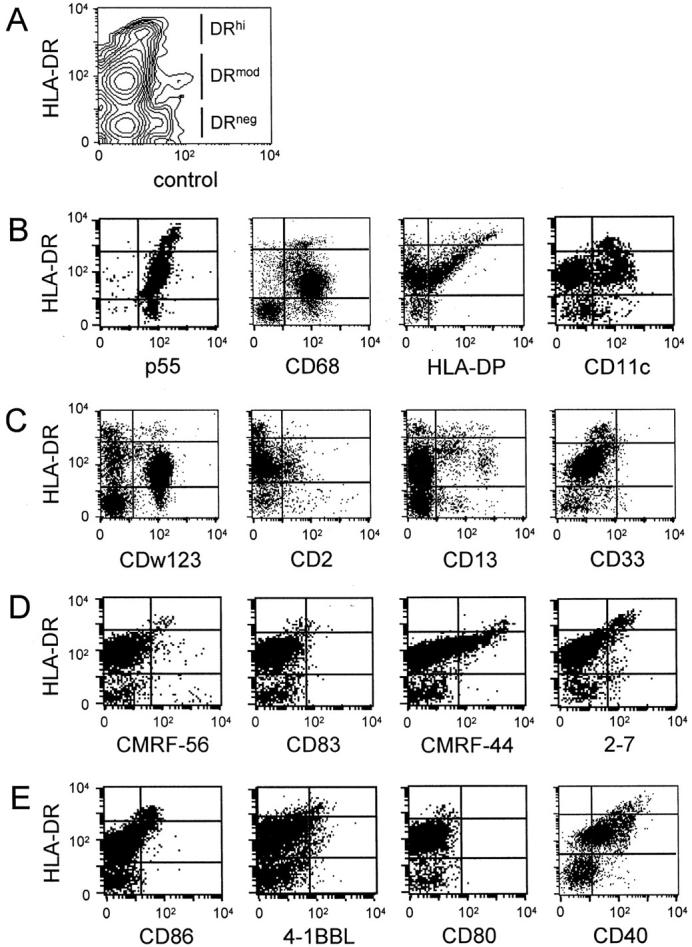
Expression of selected antigens on HLA-DRhi and HLA-DRmod tonsil DC subsets. Flow cytometric analyses of fresh lin− tonsil cells using test-FITC mAb and HLA-DR-PE. A: Intensity of HLA-DR divided tonsil lin− cells into HLA-DRhi, HLA-DRmod, and HLA-DRneg cells. Dot plots of DC-associated markers (B), lineage markers (C), DC-associated activation markers (D), and co-stimulator molecules (E). Quadrant markers are set on the basis of isotype-matched control and HLA-DR staining to define the HLA-DRhi, HLA-DRmod, and HLA-DRneg populations. Data are from representative experiments of 18 performed.
Table 2.
Selected Phenotype of Tonsil DC Subsets*
| DC subsets | GCDC | IDC | |||||
|---|---|---|---|---|---|---|---|
| HLA-DRmod CD11c+ CD13− | HLA-DRhi CD11c+ | HLA-DRmod CD11c+ | HLA-DRmod CD11c− CD123+ | HLA-DRmod CD11c− CD123− | |||
| CD13hi | CD13lo | ||||||
| p55 | +++ | +++ | +++ | +++ | +++ | +++ | |
| CD1a | − | − | − | − | − | − | |
| HLA-DP | ++ | +++ | ++ | ++ | + | − | |
| CD68 | + | + | ++ | + | +++ | +++/− | |
| CMRF-44 | − | +++ | − | + | − | − | |
| CMRF-56 | − | ++ | − | − | − | − | |
| CD83 | − | + | − | − | − | − | |
| 2–7 | − | +++ | + | ++ | − | − | |
| CD45RA | + | +/− | − | + | ++ | +/− | |
| CD45RB | + | +/− | ++ | ++ | ++ | +/− | |
| CD45RO | − | +/− | +++ | ++ | − | − | |
| CD2 | − | − | − | + | − | − | |
| CD4 | ++ | ++ | ++ | ++ | +++ | − | |
| CD13 | − | +/− | +++ | + | − | − | |
| CD24 (vib-e3) | − | +/− | − | − | + | − | |
| CD38 | ++ | +/− | ++ | ++ | + | +/− | |
| CD40 | ++ | +++ | ++ | ++ | + | + | |
| CD86 | − | ++ | − | − | − | − | |
| 4-1BB ligand | − | ++ | − | − | − | − | |
| CD123 | − | +/− | − | − | + | − | |
| % of total DC | 5.1± 2.1 | 4.1± 1.8 | 13.7± 4.6 | 61.4± 11.6 | 15.8± 6.0 | ||
*Reactivity defined as percent positive relative to an isotype-matched negative control: +++, ++, +, 100% positivity with decreasing density; −, negative; +/−, heterogeneous expression. Antigens expressed at equal density on all DC subsets were HLA-DQ, HLA-ABC, p55, CD11a, CD18, CD50, CD54, CD95. Antigens absent from all DC subsets were CD1a, CD3, CD8, CD10, CD11b, CD14, CD16, CD19, CD20, CD21, CD24 (ALB-9, BA-1), CD25, CD33, CD45RC, CD58, CD70, CD72, CD79, CD80, IgA, IgG, IgD, IgM, Igκ, Igλ, bcl2, plasma cell marker (VS38).
HLA-DRneg lin− cells were also isolated, which comprised 24.2% ± 11.7 SD (n = 18) of the total viable lin− tonsil cells. These were lymphoid-sized cells (data not shown) of which only a proportion expressed CD45RA, RB, and RO isoforms. They were also heterogeneous in their expression of CD2, CD11a/b/c, CD13, CD18, CD24 (vib-e3), CD54, and CD68. All HLA-DRneg lin− cells expressed p55/fascin, HLA-ABC, and CD95. They lacked expression of the MHC class II molecules HLA-DQ and HLA-DP, DC-associated activation markers, co-stimulator molecules, and the CD1a, CD4, CD8, CD25, CD33, CD123, bcl2, CD45RC, and CD58 antigens. MHC class II molecules could not be induced on these cells with overnight culture in media (data not shown). Thus HLA-DRneg lin− cells did not seem to represent DC precursors and were therefore excluded from further analyses.
Phenotype of All Tonsil DCs (lin− HLA-DR+ Cells)
DC-Associated Markers
All tonsil DCs constitutively expressed the intracytoplasmic p55/fascin protein but lacked the CD1a antigen. The majority of tonsil DCs expressed the cytoplasmic CD68 antigen (Figure 1B ▶ , Table 2 ▶ ).
Leukocyte Common Antigen
All tonsil DCs expressed the CD45 antigen but lacked its CD45RC isoform. There was differential expression of the CD45RA, RB, and RO isoforms between and within DC subsets (Table 2) ▶ .
T Cell, B Cell, and Myeloid Antigens
Because of the high number of B cells in the non-T cell fraction of tonsil, an extensive panel of B-cell-specific antigens was examined to confirm that DCs were not subsets of B cells or plasma cells. Antigens expressed on tonsil B cells that were absent from DCs included CD10, CD20, CD21, CD24 (using ALB9 and BA-1 mAb), CD70, CD72, CD79a, CD79a peptide, and CD79b. In addition the unclustered B cell antigen, FMC-1, and the plasma cell marker, VS38, 23 were absent from all tonsil DC subsets. Furthermore neither cytoplasmic nor membrane bound IgA, IgD, IgG, IgM, κ, or λ light chains were detected. The carbohydrate-specific CD24 epitope detected by the mAb vib-e3, the CD123, CD2, CD4, and the myeloid CD13 antigen showed heterogeneous expression on DCs. The CD8 and CD33 antigens were absent on all DC subsets, as confirmed by the lack of staining using four different CD33 mAbs (Figure 1C ▶ , Table 2 ▶ ). Culture in media for 16 hours, dramatically reduced CD4 expression and induced CD13 expression on DCs (lin− HLA-DR+ cells) (data not shown). This may reflect the differential survival of distinct DC subsets in culture.
CD95 and bcl2 Antigens
The CD95 (Fas) antigen was constitutively expressed on all tonsil DC subsets, and cytoplasmic bcl2 was absent (Table 2) ▶ .
Adhesion, Activation, and Co-Stimulator Molecules
The CD11a (LFA-1β), CD18 (LFA-1β), CD50 (ICAM-3), CD54 (ICAM-1), and CD40 molecules were expressed constitutively on all DC subsets. In contrast the CD11b, CD25, CD58 (LFA-3), and CD80 antigens were not expressed on any DC subsets. CD11c had heterogeneous expression on HLA-DRmod DCs. The DC activation antigens CMRF-44 and 2-7 showed heterogeneous expression on DCs. Expression of 4-1BB ligand, CD86, CMRF-56, and CD83 was restricted to HLA-DRhi DCs (Figure 1; B, D, and E ▶ , and Table 2 ▶ ). Expression of co-stimulator (CD40, CD80, CD86) and activation (CMRF-44, CMRF-56, CD83) antigens was up-regulated on HLA-DRhi DCs and a significant number of HLA-DRmod DCs after in vitro culture in media (data not shown). CMRF-44+ lin− cells formed more stable clusters and were twice (mean, 2.3×; n = 5) as effective at stimulating allogeneic blood T cells than CMRF-44− lin− tonsil cells (data not shown).
Identification of Three Tonsil DC Subsets Based on HLA-DR and CD11c Expression
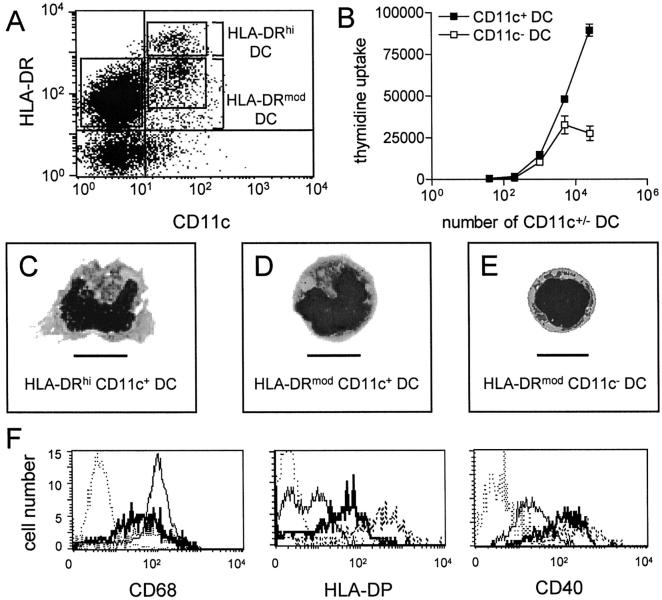
The above phenotypic analyses demonstrated that the CD11c antigen was constitutively expressed on HLA-DRhi DCs, but subdivided HLA-DRmod DCs into CD11c+ (17.7% ± 6.2 SD, n = 7) and CD11c− (81.4% ± 6.0 SD, n = 7) subsets. CD11c− DCs had the lowest density expression of HLA-DR (Figure 2A) ▶ . Therefore three tonsil DC subsets could be clearly defined as 1) HLA-DRhi CD11c+ DCs; 2) HLA-DRmod CD11c+ DCs; and 3) HLA-DRmod CD11c− DCs.
Figure 2.
CD11c defines three tonsil DC subsets. A: Flow cytometric analyses of fresh lin− tonsil cells using CD11c-FITC and HLA-DR-PE. CD11c was present on all HLA-DRhi DCs and divided HLA-DRmod DCs into CD11c+ and CD11c− subsets. Boxes indicate these three DC subsets. B: Allogeneic MLR performed using sorted CD11c+ DCs (HLA-DRhi/mod DCs) and CD11c− (HLA-DRmod/neg) cells. Data are shown as mean ± SEM and are from a representative experiment of four performed. C, D, and E: May-Grunswald Giemsa stain of the three CD11c DC subsets. Original magnification, ×1197. Scale bars, 10 mm. F: Flow cytometric analyses of fresh lin− tonsil cells triple-labeled with CD11c-FITC, HLA-DR-PE/Cy5, and test-PE mAb. Expression of CD68, HLA-DP, and CD40 was examined on HLA-DRhi CD11c+ DCs (dashed line), HLA-DRmod CD11c+ DCs (thick straight line), and HLA-DRmod CD11c− DCs (thin straight line). Isotype-matched controls are shown as dotted lines. Data are from representative experiments of four performed.
Morphologically, fresh HLA-DRhi CD11c+ DCs were large cells with irregular-shaped nuclei, lightly basophilic cytoplasm containing vacuoles and dendritic processes (Figure 2C) ▶ . HLA-DRmod CD11c+ DCs were medium-sized cells with an irregular shaped nucleus, cytoplasmic vacuoles, and lacking dendrites (Figure 2D) ▶ . HLA-DRmod CD11c− DCs were small cells resembling lymphocytes with a rounded nucleus, little cytoplasm and no dendritic processes (Figure 2E) ▶ . The combined CD11c+ DC subsets stimulated a stronger (1.4 to 3.2×) allogeneic T cell response than CD11c− DCs (Figure 2B) ▶ .
The phenotypes of these three tonsil DC subsets were analyzed further by triple labeling for CD68/HLA-DP/CD40, CD11c, and HLA-DR on purified lin− cells. The density of CD68 differed between the DC subsets with the highest expression on HLA-DRmod CD11c− DCs, whereas HLA-DRhi CD11c+ DCs and HLA-DRmod CD11c+ DCs expressed CD68 at an equally lower density (Figure 2F ▶ , left). The highest density of HLA-DP was found on HLA-DRhi CD11c+ DCs with decreasing levels on HLA-DRmod CD11c+ DCs and HLA-DRmod CD11c− DCs, respectively. HLA-DP was absent on a subset of CD11c− DCs (Figure 2F ▶ , middle). CD40 was present on all tonsil DC subsets with the highest density on HLA-DRhi CD11c+ DCs followed by decreasing levels on HLA-DRmod CD11c+ DCs and HLA-DRmod CD11c− DCs, respectively (Figure 2F ▶ , right, and Table 2 ▶ ).
Identification of Five Tonsil DC Subsets Based on HLA-DR, CD11c, CD13, and CD123 Expression
A more extensive phenotypic analysis was undertaken using triple labeling on sorted lin− cells. The resultant phenotypes and relative proportions of the five DC subsets identified are outlined in Table 2 ▶ .
Phenotype of the HLA-DRhi CD11c+ DC Subset
Expression of the DC-associated activation markers CMRF-56 and CD83 and the co-stimulator molecules CD86 and 4-1BB ligand were restricted to the HLA-DRhi CD11c+ DC subset, with CD83 being expressed only at low density. This DC subset expressed the highest density of HLA-DP, the DC-activation markers CMRF-44 and 2-7 and the CD40 co-stimulator molecule. HLA-DRhi CD11c+ DCs expressed CD4 at moderate density and CD68 at low density, whereas CD2 was not expressed. Differential expression of the CD123, CD45 isoforms, CD24 (vib-e3), CD38, and CD13 antigens may subdivide this population, however as the total number of HLA-DRhi CD11c+ DCs was already low (<10% of the total DC population) it was difficult to further characterize these subsets separately (Figure 1; B, D, and E ▶ , and Table 2 ▶ ).
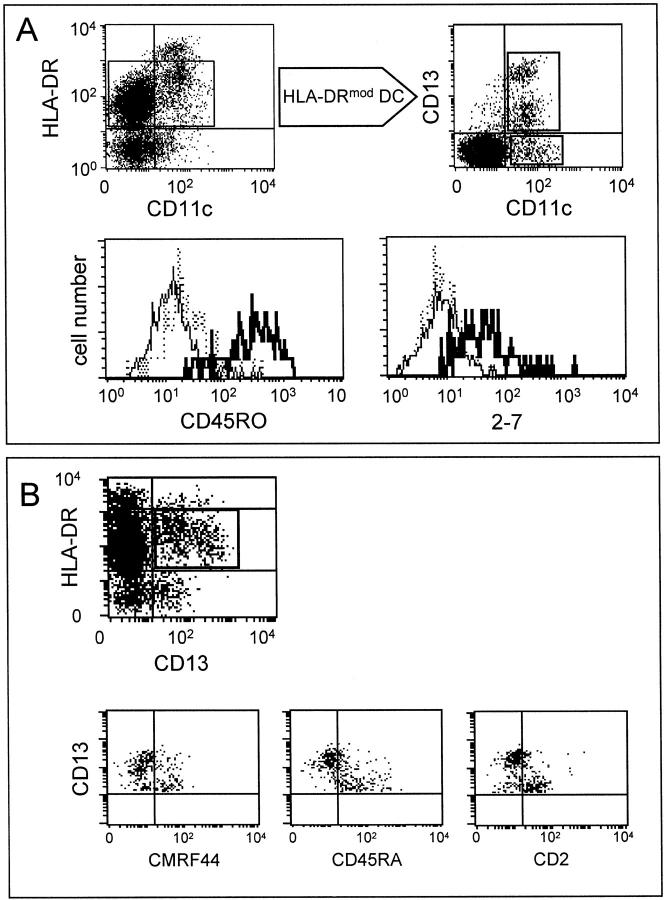
Phenotyping of HLA-DRmod CD11c+ DCs Reveals CD13+ and CD13− Subsets
As shown in Figure 1C ▶ , HLA-DRmod DCs could be subdivided based on their expression of CD13. Triple labeling of sorted lin− cells with CD13, CD11c, and HLA-DR confirmed that CD13 expression was restricted to a subset of HLA-DRmod CD11c+ DCs (Figure 3A ▶ , top, and Table 2 ▶ ). CD13+ DCs were the predominant subset comprising 69.3% ± 5.5 SD (n = 3) of the total HLA-DRmod CD11c+ DC population. The phenotype of HLA-DRmod CD11c+ CD13+ DCs was analyzed by triple labeling sorted lin− cells with HLA-DR, CD13, and test mAbs. HLA-DRmod CD11c+ CD13− DCs were analyzed by triple labeling sorted CD13− lin− cells with HLA-DR, CD11c, and test mAbs. Both CD13+ and CD13− DC subsets expressed the HLA-DP, CD68, CD45RB, CD4, CD38, and CD40 antigens. Neither subset expressed the CMRF-56, CD83, CD24 (vib-e3), CD86, 4-1BB ligand, or CD123 antigens.
Figure 3.
Phenotype of HLA-DRmod CD11c+ tonsil DCs. A: Gating on the HLA-DRmod lin− tonsil cells shows heterogeneous expression of CD13 on CD11c+ DCs (top). Triple labeling of sorted lin− tonsil cells with HLA-DR-PE/Cy5, CD13-PE, and test-FITC mAb determined antigen expression on HLA-DRmod CD11c+ CD13+ DCs (thick straight line). Triple labeling of sorted CD13− lin− tonsil cells with HLA-DR-PE/Cy5, CD11c-FITC, and test-PE mAb determined antigen expression on HLA-DRmod CD11c+ CD13− DCs (thin straight line). Isotype-matched controls were identical on both populations and are shown as dotted lines. B: HLA-DRmod CD11c+ CD13+ DCs could be subdivided into CD13hi- and CD13lo-expressing DCs. Triple labeling of sorted lin− tonsil cells with HLA-DR-PE/Cy5, CD13-PE, and test-FITC mAb determined antigen expression on HLA-DRmod CD11c+ CD13hi and CD13lo DCs. Quadrants are set on isotype-matched controls. Data are from representative experiments of four performed.
In contrast to the CD13+ DC subset, HLA-DRmod CD11c+ CD13− DCs lacked expression of the CD45RO and 2-7 antigens (Figure 3A ▶ , Table 2 ▶ ). HLA-DRmod CD11c+ CD13− DCs expressed CD45RA but lacked CMRF-44 and CD2 expression. HLA-DRmod CD11c+ CD13+ DCs could be further subdivided into CD13hi and CD13lo subsets, whereby only the CD13lo DCs expressed CMRF-44 and CD45RA. Interestingly HLA-DRmod CD11c+ CD13lo DCs were the only tonsil DC subset to express the CD2 antigen (Figure 3B ▶ , Table 2 ▶ ).
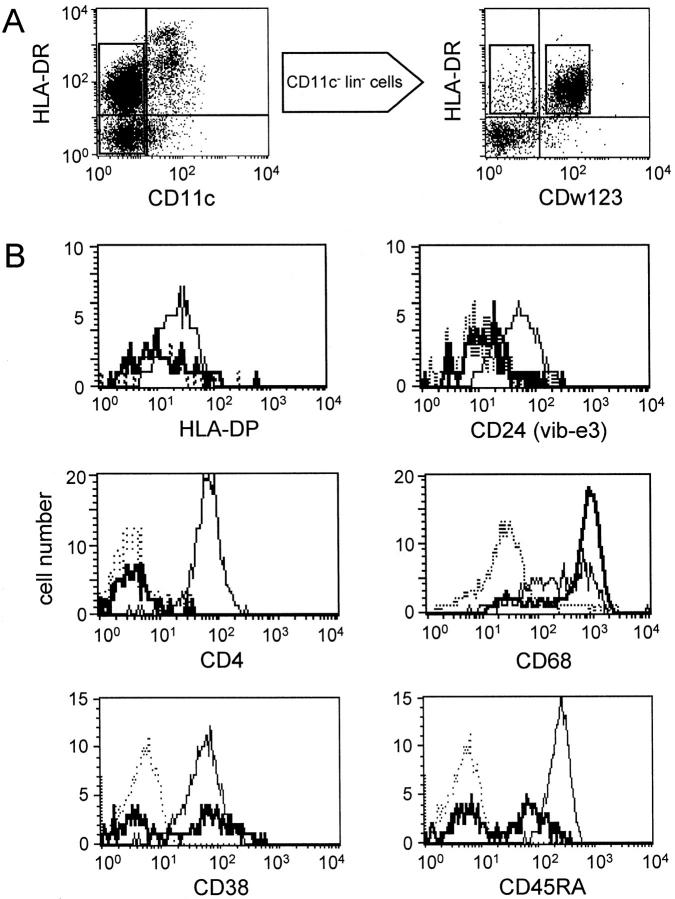
Phenotyping of HLA-DRmod CD11c− DCs Reveals CD123+ and CD123− Subsets
Triple labeling of sorted lin− cells with CD11c, HLA-DR, and CD123 confirmed that HLA-DRmod CD11c− DCs were a heterogeneous population based on CD123 expression (Figures 1C and 4A) ▶ ▶ . HLA-DRmod CD11c− CD123+ DCs comprised the majority (78.5% ± 9.2 SD, n = 4) of the total HLA-DRmod CD11c− DC population.
Figure 4.
Phenotype of HLA-DRmod CD11c− DCs. A: Flow cytometric analyses of fresh lin− tonsil cells triple labeled with CD11c-FITC, HLA-DR-PE/Cy5, and CD123-PE. Gating on the CD11c− DCs showed heterogeneous expression of CD123. B: Flow cytometric analyses of CD11c− lin− tonsil cells triple labeled with HLA-DR-PE/Cy5, CD123-FITC, and test-PE mAb determined antigen expression on gated CD11c− CD123+ DCs (thin straight line) and CD11c− CD123− DCs (thick straight line). Isotype-matched controls are shown as dotted lines.
The two CD11c− DC subsets were analyzed by triple labeling sorted CD11c− lin− tonsil cells with CD123, HLA-DR, and test mAbs. Both CD11c− DC subsets expressed low levels of CD40, whereas neither subset expressed CMRF-44, CMRF-56, CD83, 2-7, CD86, 4-1BB ligand, CD2, CD13, or CD45RO. Only the HLA-DRmod CD11c− CD123+ DCs expressed the CD4, HLA-DP, and CD24 (vib-e3) antigens. The CD68, CD45RA/RB, and CD38 antigens were expressed by CD123+ DCs, but showed heterogeneous expression on the HLA-DRmod CD11c− CD123− DCs (Figure 4B ▶ , Table 2 ▶ ).
Identification of Tonsil DC Subsets in Situ
HLA-DR staining was not useful as a single marker to identify all tissue DCs or discriminate DC subsets because of its extensive expression in tonsil tissue and the difficulty of quantitative analyses in situ, respectively. However based on flow cytometric analyses, the entire tonsil DC population (including all DC subsets) could be best identified in situ as either CD68+/CD14− cells (Figure 5A) ▶ or p55+ cells (data not shown). There were limitations to both methods however as the former combination excluded the minor HLA-DRmod CD11c− CD123− CD68− DC subset, whereas p55 overrepresented the number of DCs because of its expression on endothelial cells and HLA-DRneg lin− tonsil cells. Both methods revealed similar findings in that DCs formed an extensive network of interconnecting cells throughout the T-cell-enriched areas of tonsils. An additional population of DCs was sparsely scattered within the follicles.
Figure 5.
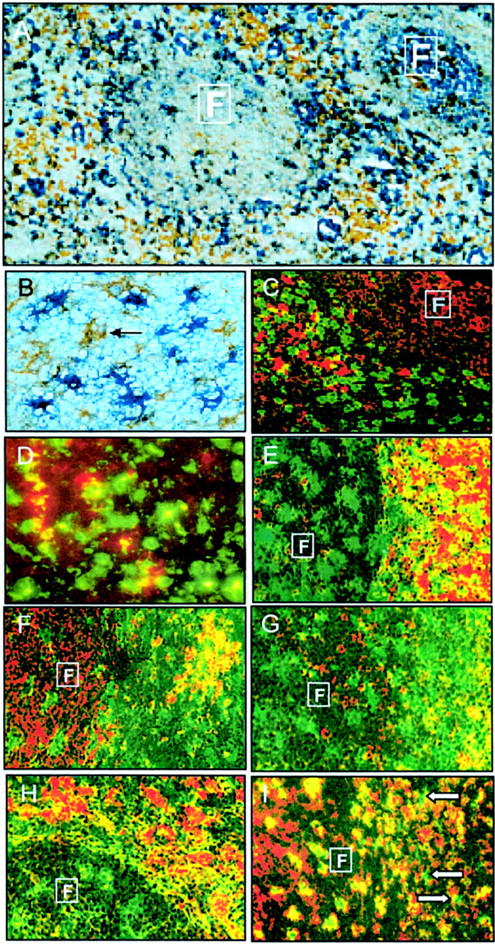
Identification of five tonsil DC subsets in situ. A: Majority of DCs (brown cells) were identified in the follicles and T cell areas as CD68+ (brown)/CD14− (blue) cells. B: Subset 1: The HLA-DRhi CD11c+ DC subset (brown cells, arrow) could be identified as CD83+ (brown)/CD3− (blue) cells. C: CD83+ DCs (red cells) were located near CD8+ (green) T cells. D: A small number of the total CD68+ (green) DC population were CD83+ (red) DCs (yellow cells). E: CD11c+ DCs (green cells) were located near CD3+ (red) T cells in both the follicles and T cell areas. F: CD11c+ (green) CMRF-44+ (red) DCs (yellow cells) were located in the T cell areas. G: Subset 2: CD11c+ (green) CD13+ (red) DCs (yellow cells) were located in the T cell areas. Subset 3: CD11c+ CD13− DCs (green cells) were located in the follicle. H: Subset 4: CD11c− (green) CD123+ DCs (red cells) and (I) subset 5: CD11c− CD123− CD14− (red) CD68+ DCs (green cells, arrows) were located in the T cell areas. Boxed F defines follicular areas. Original magnifications: ×240 (A), ×573 (B and D), ×286 (C, E–I).
HLA-DRhi CD11c+ Tonsil DC Subset
HLA-DRhi CD11c+ DCs could be identified in fresh tonsil tissue as either CD83+/CD14−/CD19− cells (Figure 5B) ▶ or CMRF-56+/CD19− cells (data not shown). This DC subset was situated in close contact with CD8+ (and CD4+) T cells in the T cell areas of tonsils (Figure 5C) ▶ . Although CD4+ T cells dominate in paracortical areas, it was difficult to decipher their interaction with adjacent CD4+ DCs. To determine the percentage of HLA-DRhi CD11c+ DCs within the total DC population accurately, triple labeling immunohistochemistry techniques would be necessary. As this was not technically possible, double labeling of serial sections was used instead and sequential sections analyzed. The CD68+/CD14− phenotype was used to define tonsil DCs, CD83+/CD14−/CD19− to define HLA-DRhi CD11c+ DCs and CD68+/CD83+ to examine these activated DCs within the total DC population. The number of activated HLA-DRhi CD83+ DCs comprised a minority of the total number of CD68+ paracortical DCs (Figure 5D) ▶ , which is in similar proportions found in isolated DC preparations.
Double labeling using the CMRF-44 antigen plus the CD80 or CD86 co-stimulator molecules confirmed flow cytometric findings that all CMRF-44+ DCs (HLA-DRhi CD11c+ DCs and HLA-DRmod CD11c+ CD13lo DCs) lacked CD80 and only some CMRF-44+ DCs (presumably the HLA-DRhi CD11c+ DCs) expressed the CD86 antigen (data not shown).
HLA-DRmod CD11c+ Tonsil DC Subsets
The CD11c+ DCs (HLA-DRmod and HLA-DRhi DC subsets) were located in both the germinal center and T cell areas of tonsils (Figure 5E) ▶ . CD11c+ GCDCs appeared to be larger in size than CD11c+ paracortical DCs and, as opposed to the latter population, GCDCs lacked expression of the CMRF-44 antigen (Figure 5F) ▶ .
HLA-DRmod CD11c+ CD13+ DCs were located in the T cell areas, whereas HLA-DRmod CD11c+ CD13− DCs were located in tonsil follicles (Figure 5G) ▶ . Thus HLA-DRmod CD11c+ CD13− DCs clearly represented GCDCs. Because of technical limitations with triple-color analyses in situ, it remained unclear whether some HLA-DRmod CD11c+ CD13− DCs were also located in the T cell areas.
HLA-DRmod CD11c− Tonsil DC Subsets
The vast majority of CD123+ cells lacked CD11c expression and were located near high endothelial venules in the T cell areas of tonsil (Figure 5H) ▶ . Therefore GCDCs do not express the CD123 antigen. Using a combination of the CD68, CD11c, and CD123 antigens, CD11c− CD123− DCs could be identified as a minor population within the T cell areas of tonsil tissue (Figure 5I) ▶ . It was not technically possible to identify the CD11c− CD123− CD68− DC subset in situ because of the lack of defining antigens on these cells.
Discussion
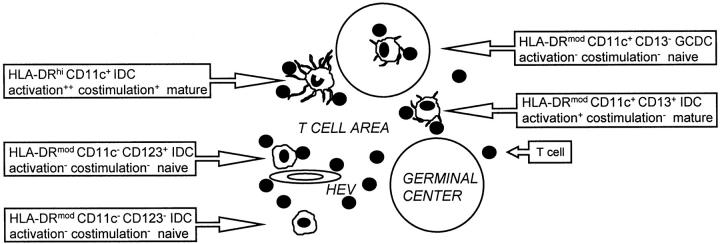
In this study we demonstrate using a new method of DC preparation that five distinct DC subsets are present in human tonsils (Figure 6) ▶ . Extensive analysis of a large number of lin− HLA-DR+ tonsil DC preparations revealed the presence of HLA-DRhi CD11c+ DCs, HLA-DRmod CD11c+ CD13+ DCs, HLA-DRmod CD11c+ CD13− DCs, HLA-DRmod CD11c− CD123+ DCs, and HLA-DRmod CD11c− CD123− DCs subsets. In situ localization identified four of these subsets as distinct IDC populations, which included plasmacytoid DCs 17 and three new DC subsets. We also confirmed the presence of GCDCs 18 but clarified their phenotype as a fifth HLA-DRmod CD11c+ CD13− DC subset.
Figure 6.
Five distinct subsets of DCs in human tonsils. In situ localization identified four of these subsets as distinct IDC populations, which included: 1) plasmacytoid DCs (HLA-DRmod CD11c− CD123+ DCs) and three new DC subsets; 2) HLA-DRhi CD11c+ DCs; 3) HLA-DRmod CD11c+ CD13+ DCs; and 4) HLA-DRmod CD11c− CD123− DCs. GCDCs were also clarified as 5) HLA-DRmod CD11c+ CD13− DCs. The three CD11c+ DC subsets are likely to represent different stages of DC maturation/activation. The CD11c+ DCs and CD11c− DCs are thought to arise from separate myeloid and lymphoid developmental pathways, respectively. IDC, interdigitating DCs; GCDC, germinal center DCs; HEV, high endothelial venule.
A number of DC subsets with differing functional and phenotypic characteristics have been identified in human blood and tissues. 7-10,14 Three tonsil DC subsets consisting of GCDCs, 18 plasmacytoid DCs, 17 and IDCs 16 have been described previously. Our new methodology has the advantage of avoiding potential problems of antigenic changes associated with in vitro culture 19 plus the biases induced by positive selection. This combined with a very extensive phenotypic analysis established that the apparent heterogeneity in markers noted in these other studies 16,18 could be extended into a more defined set of five DC subsets.
These five tonsil DC subsets each shared a number of phenotypic features including expression of HLA-DQ, HLA-ABC, the DC-associated p55 marker, 24 and CD95 antigen. They also expressed HLA-DR, CD40, and CD45RB albeit at varying density between DC subsets, but lacked the CD33, CD45RC, and CD1a antigens. The absence of CD33 contradicts previous reports of low-level expression on GCDCs, which may relate to the absence of cell culture as CD33 is inducible. In contrast to previous reports, we did not detect CD80 16 or bcl2 25 within any tonsil DC subsets. This may again reflect a difference in isolation procedures because CD80 is induced by in vitro culture, which was used in previous studies on tonsil DCs. Similar to blood DCs, 8 high-density expression of cytoplasmic CD68 was associated with CD11c− tonsil DCs. A separate population of HLA-DRneg lin− tonsil cells was also identified, as described previously in normal blood 26 and chronic arthritic synovial fluid. 27 Although these cells expressed p55 and showed heterogeneous expression of CD45, CD13, and adhesion antigens, they did not express MHC class II antigens either before or after in vitro culture. They therefore did not seem to represent DC precursor cells or a separate DC subset.
The majority (∼80%) of tonsil DCs are HLA-DRmod lin− cells, which can be subdivided into morphologically distinct smaller CD11c+ and more substantial CD11c− DC subsets. These are found in similar proportions to normal blood. 9 The CD11c+ DCs can be further subdivided into a small CD13− subset and a CD13+ subset that are localized in the germinal center and T cell areas, respectively. The CD13− and CD13+ DC subsets comprised 5.1% ± 2.1 SD and 13.7 ± 4.6 SD (n = 4) of the total DC population, respectively. HLA-DRmod CD11c+ CD13− and CD13+ DCs shared a similar phenotype with the exception that only the CD13+ DCs expressed the 2-7 and CD45RO activation antigens. The CD13+ DCs could be further subdivided based on CD13 intensity into CD13hi and CD13lo subsets. These CD13+ DC subsets only differed in their expression of the CMRF-44, CD2, and CD45RA antigens, which were present only on the CD13lo DC subset. HLA-DRmod CD11c+ CD13− DCs were the only subset to express the CD2 antigen.
In addition to location, the HLA-DRmod CD11c+ CD13− DC subset phenotypically resembled GCDCs as they lacked DC-associated activation and co-stimulator molecules. The previously described GCDCs were isolated based on a CD11c+ CD4+ lin− cell phenotype 18 but, as outlined in this study, this phenotype is common to three distinct DC subsets located in both the T cell areas and germinal centers, one of which expresses CD13. This fact, plus the observation that CD13 can be induced on a subpopulation of tonsil DCs after culture (data not shown), could explain the heterogeneous expression of CD13 originally reported on GCDCs. Our study further extends their phenotype and shows that GCDCs have many phenotypic similarities to mature/myeloid blood DCs 9,14 including low-density CD68 and lack of CD123 expression. However, unlike CD11c+ blood DCs, 9 the GCDCs identified in this study did not express the CD13 or CD33 antigens.
Consistent with a low activation state, the HLA-DRmod CD11c− DC subset lacked expression of DC activation antigens, CD45RO, and co-stimulator molecules, although CD40 was expressed at low levels. The CD2 and CD13 antigens were also absent. Heterogeneous expression of CD123 and CD4 enabled us to define two distinct HLA-DRmod CD11c− DC subsets. The predominant HLA-DRmod CD11c− DC subset (comprising 61.4% ± 11.6 SD of the total DC population) was CD123+, CD4+, CD68hi, and closely resembled in phenotype, morphology, and localization near paracortical high endothelial venules 28 the plasmacytoid tonsil DCs 17 and immature CD11c− DCs described in blood, 9,10 bone marrow, and thymus. 10 As a result, it has been suggested that this tonsil DC subset may be derived from CD11c− immature/lymphoid blood DCs. 12,17 Our study further extends the phenotype of plasmacytoid DCs and demonstrates that they express p55, CD45RB, CD24 (vib-e3), low levels of HLA-DP, but lack DC activation markers and 4-1BB ligand.
HLA-DRmod CD11c− CD123− DCs were the only subset that lacked CD4 and HLA-DP expression. A small number of these cells also lacked cytoplasmic CD68, CD45RA, CD45RB, and CD38. Interestingly a minor CD68− CD4− lin− cell population had also been observed in a study of blood DCs. 8 Unfortunately it was not possible to identify these cells in tonsil sections because of their lack of suitable discriminatory markers.
The HLA-DRhi CD11c+ DC subset was the only subset that expressed the DC activation antigens, CD83 29 and CMRF-56 30 and the co-stimulator molecules, CD86 31 and 4-1BB ligand. 32 This tonsil DC subset also expressed the highest levels of the DC-associated activation marker CMRF-44, 26,33 HLA-DP, CD40, and 2-7, 19 which we newly identify as a DC-associated activation marker. Morphologically this DC subset resembled activated blood DCs. 2 In tissue sections these activated DCs comprised only a minority of the total paracortical DC population, comparable to their low numbers present in DC isolates (4.1% ± 1.8 SD, n = 18, of the total DC population. The close association of HLA-DRhi CD11c+ DCs to paracortical T cells and their low frequency resembles the characteristics of antigen-bearing DCs in lymph nodes that have a rapid turnover after antigen presentation events. 34-36 The phenotypes of the HLA-DRhi CD11c+ DCs and HLA-DRmod CD11c+ CD13− DC subsets are broadly similar, differing mainly in their expression of activation-associated antigens. As in vitro activation of blood DCs induces similar changes, 29-31,33 this raises the possibility that HLA-DRhi CD11c+ DCs represent a more activated form of HLA-DRmod CD11c+ CD13− DCs.
IDCs have been identified in tonsils as CD40hi lin− cells, however the constitutive presence of CD40 on all five DC subsets described in our study indicates that these IDCs incorporated several DC subsets. This is verified by the heterogeneous expression of the CD11c and CD83 antigens described on the former IDC population. 16 Here we report that, in addition to plasmacytoid DCs (HLA-DRmod CD11c− CD123+ DCs), three other newly described DC subsets are located in the T cell areas of tonsils and can be defined as 1) HLA-DRhi CD11c+ DCs, 2) HLA-DRmod CD11c+ CD13− DCs, and 3) HLA-DRmod CD11c− CD123− DCs (Figure 6) ▶ . Interestingly IDCs have been reported to stimulate activated B cells 16,37 suggesting that at least one DC subset may be involved in primary B cell responses.
There is accumulating evidence in mice that DC subsets arise from different lymphoid and myeloid developmental pathways. 4,38 However, although the immature DC subsets (ie, CD11c−, CD33lo, CD68hi, and CD123hi) and mature DC subsets (ie, CD11c+, CD33hi, CD68lo, and CD123lo) within human blood display a number of lymphoid and myeloid features, respectively 4,8-10,14,38 , it remains unclear whether they develop from distinct cell lineages. A recent report provides evidence that, as opposed to different maturation states, CD11c+ blood DCs represent multipotent myeloid precursor cells, whereas CD11c− blood DCs represent lymphoid progenitors. 12 The tonsil CD11c+ DC subsets described in this study share a number of features with CD11c+ mature/myeloid blood DCs being CD68lo and CD123lo/neg. They do however, differ from each other in their expression of CD13, CD33, activation, and co-stimulator molecules. As each of these antigens can be up-regulated after culture on tonsil DCs, one could speculate that the CD11c+ DC subsets represent different maturation stages, ie, CD11c+ CD13− DRmod (early), CD11c+ CD13+ DRmod (intermediate), and CD11c+ CD13+ DRhi (late). However, functional studies would be necessary to establish the relationship of these five tonsil DC subsets both to each other and to other tissue DCs. Consistent with previous studies on blood DC subsets, CD11c+ DCs, 9 as well as activated DCs, 33,39 were the most potent stimulators of allogeneic T cell responses in the tonsil.
The HLA-DRhi CD11c+ DCs and GCDCs seem to represent highly immunogenic DCs that are actively presenting antigens to paracortical and germinal center T and/or B cells, respectively. The other less-activated IDC subsets may represent DCs that have migrated to the draining lymph node (tonsil) either without receiving up-regulatory signals or else receiving negative regulatory signals from interacting self T cells. 40 These distinct DC subsets may play different roles in regulating immunity or tolerance, although it remains unclear whether the type of T cell response is driven by intrinsic properties of the DC subset 13 or by local environmental factors. 41 Ultimately, targeting a specific DC subset may provide more effective therapeutic regulation of immunological disorders.
Acknowledgments
We thank the ENT surgeons and nursing staff from Christchurch, St George’s and Southern Cross Hospitals for providing us with tonsil specimens, Dr. Axel Heiser for helpful suggestions, and Lisa Haring for providing assistance with flow cytometry.
Footnotes
Address reprint requests to Professor Derek N. J. Hart, Director, Mater Medical Research Institute, Aubigny Place, Raymond Terrace, South Brisbane 4101, QLD, Australia. E-mail: dhart@mmri.mater.org.au.
Supported by New Zealand Lotteries Grant Board (to D. H.) and Canterbury Medical Research Foundation (to K. S.).
References
- 1.Steinman RM: The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 1991, 9:271-296 [DOI] [PubMed] [Google Scholar]
- 2.Hart DNJ: Dendritic cells: unique leucocyte populations which control the primary immune response. Blood 1997, 90:3245-3287 [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM: Dendritic cells and the control of immunity. Nature 1998, 392:245-252 [DOI] [PubMed] [Google Scholar]
- 4.Suss G, Shortman K: A subclass of dendritic cells kills CD4 T cells via Fas/fas-ligand-induced apoptosis. J Exp Med 1996, 183:1789-1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill S, Coates JP, Kimber I, Knight SC: Differential function of dendritic cells isolated from blood and lymph nodes. Immunology 1994, 83:295-301 [PMC free article] [PubMed] [Google Scholar]
- 6.Pulendran B, Lingapappa J, Kennedy MK, Smith J, Teepe M, Rudensky A, Maliszewski CR, Maraskovsky E: Developmental pathways of dendritic cells in vivo. J Immunol 1997, 159:2222-2231 [PubMed] [Google Scholar]
- 7.Nestle FO, Zheng X-G, Thompson CB, Turka LA, Nickoloff BJ: Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol 1993, 151:6535-6545 [PubMed] [Google Scholar]
- 8.Strobl H, Scheinecker C, Riedl E, Csmarits B, Bello-Fernandez C, Pickl WF, Majdic O, Knapp W: Identification of CD68+lin− peripheral blood cells with dendritic precursor characteristics. J Immunol 1998, 161:740-748 [PubMed] [Google Scholar]
- 9.O’Doherty U, Peng M, Gezelter S, Swiggard WJ, Betjes M, Bhardwaj N, Steinman RM: Human blood contained two subsets of dendritic cells, one immunologically mature and the other immature. Immunology 1994, 82:487-493 [PMC free article] [PubMed] [Google Scholar]
- 10.Olweus J, BitMansour A, Warnke R, Thompson PA, Carballido J, Picker LJ, Lund-Johansen F: Dendritic cell ontogeny: a human dendritic cell lineage of myeloid origin. Proc Natl Acad Sci USA 1997, 94:12551-12556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohrgruber N, Halanek N, Groger M, Winter D, Rappersberger K, Schmitt-Egenolf M, Stingl G, Maurer D: Survival, maturation, and function of CD11c− and CD11c+ peripheral blood dendritic cells are differentially regulated by cytokines. J Immunol 1999, 163:3250-3259 [PubMed] [Google Scholar]
- 12.Robinson S, Patterson S, English N, Davies S, Knight S, Reid C: Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol 1999, 29:2769-2778 [DOI] [PubMed] [Google Scholar]
- 13.Rissoan M, Soumelis V, Kadowaki N, Grouard G, Brieree F, de Waal Malefy R, Liu YJ: Reciprocal control of T helper cell and dendritic cell differentiation. Science 1999, 283:1183-1186 [DOI] [PubMed] [Google Scholar]
- 14.Thomas R, Lipsky PE: Human peripheral blood dendritic cell subsets: isolation and characterization of precursor and mature antigen presenting cells. J Immunol 1994, 153:4016-4028 [PubMed] [Google Scholar]
- 15.Hart DNJ, McKenzie JL: Isolation and characterisation of human tonsil dendritic cells. J Exp Med 1988, 168:157-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjorck P, Flores-Romo L, Liu Y-J: Human interdigitating dendritic cells directly stimulate CD40-activated naive B cells. Eur J Immunol 1997, 27:1266-1274 [DOI] [PubMed] [Google Scholar]
- 17.Grouard G, Rissoan M-C, Filgueira L, Durand I, Banchereau J, Liu Y-J: The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med 1997, 185:1101-1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grouard G, Durand I, Filgueira L, Banchereau J, Liu Y-J: Dendritic cells capable of stimulating T cells in germinal centres. Nature 1996, 384:364-367 [DOI] [PubMed] [Google Scholar]
- 19.Teunissen MBM, Wormmeester DJ, Kreig SR, Peters PJ, Vogels IMC, Kapsenberg ML, Bos JD: Human epidermal Langerhans cells undergo profound morphologic and phenotypic changes during in vitro culture. J Invest Dermatol 1990, 94:166-173 [DOI] [PubMed] [Google Scholar]
- 20.Egner W, Andreesen R, Hart DNJ: Allostimulatory cells in fresh human blood: heterogeneity in antigen presenting cell populations. Transplantation 1993, 56:945-950 [DOI] [PubMed] [Google Scholar]
- 21.Summers K, O’Donnell J, Williams LA, Hart DNJ: Expression and function of CD80 and CD86 costimulator molecules on synovial dendritic cells in chronic arthritic disease. Arthritis Rheum 1996, 39:1287-1291 [DOI] [PubMed] [Google Scholar]
- 22.Troy AJ, Summers KL, Davidson PJT, Atkinson CA, Hart DNJ: Minimal recruitment and activation of dendritic cells within renal cell carcinoma. Clin Cancer Res 1998, 4:585-593 [PubMed] [Google Scholar]
- 23.Turley H, Jones M, Erber W, Mayne K, de Waele M, Gatter K: VS38: a new monoclonal antibody for detecting plasma cell differentiation in routine sections. J Clin Pathol 1994, 47:418-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosialos G, Birkenbach M, Ayehunie S, Matsumura F, Pinkus GS, Kieff E, Langhoff E: Circulating human dendritic cells differentially express high levels of a 55-kd actin-bundling protein. Am J Pathol 1996, 148:593-600 [PMC free article] [PubMed] [Google Scholar]
- 25.Bjorck P, Banchereau J, Flores-Romo L: CD40 ligation counteracts Fas-induced apoptosis of human dendritic cells. Int Immunol 1997, 9:365-372 [DOI] [PubMed] [Google Scholar]
- 26.Fearnley DB, McLellan AD, Mannering SI, Hock BD, Hart DNJ: Isolation of human blood dendritic cells using the CMRF-44 monoclonal antibody: implications for studies on antigen presenting cell function and immunotherapy. Blood 1997, 89:3708-3716 [PubMed] [Google Scholar]
- 27.Summers KL, Daniel PB, O’Donnell J, Hart DNJ: Dendritic cells in synovial fluid chronic inflammatory arthritis lack CD80 surface expression. Clin Exp Immunol 1995, 100:81-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cella M, Jarrossay D, Facchietti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M: Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med 1999, 8:919-929 [DOI] [PubMed] [Google Scholar]
- 29.Zhou LJ, Tedder TF: Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol 1995, 154:3821-3835 [PubMed] [Google Scholar]
- 30.Hock BD, Fearnley DB, Boyce A, McLellan AD, Sorg RV, Summers KL, Hart DNJ: Human dendritic cells express a 95kDa activation/differentiation antigen defined by CMRF-56. Tissue Antigens 1999, 53:320-334 [DOI] [PubMed] [Google Scholar]
- 31.McLellan AD, Starling GC, Williams LA, Hock BD, Hart DNJ: Activation of human peripheral blood dendritic cells induces the CD86 costimulatory molecule. Eur J Immunol 1995, 25:2064-2068 [DOI] [PubMed] [Google Scholar]
- 32.Zhou Z, Kim S, Hurtado J, Lee ZH, Kim KK, Pollok KE, Kwon BS: Characterization of human homologue of 4-1BB and its ligand. Immunol Lett 1995, 45:67-73 [DOI] [PubMed] [Google Scholar]
- 33.Hock BD, Starling GC, Daniel PB, Hart DNJ: Characterisation of CMRF-44, a novel monoclonal antibody to an activation antigen expressed by the allostimulatory cells within peripheral blood, including dendritic cells. Immunology 1994, 83:573-581 [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuno K, Ezaki T, Kudo S, Uehara Y: A life stage of particle-laden rat dendritic cells in vivo: their terminal division, active phagocytosis and translocation from the liver to the draining lymph. J Exp Med 1996, 183:1865-1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill S, Edwards AJ, Kimber I, Knight SC: Systemic migration of dendritic cells during contract sensitization. Immunology 1990, 71:277-281 [PMC free article] [PubMed] [Google Scholar]
- 36.Ingulli E, Mondino A, Khoruts A, Jenkins MK: In vivo detection of dendritic cell antigen presentation to CD4+ T cells. J Exp Med 1997, 185:2133-2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johansson B, Ingvarsson S, Bjorck P, Borrebaeck CA: Human interdigitating dendritic cells induce isotype switching and IL-13-dependent IgM production in CD40-activated naive B cells. J Immunol 2000, 164:1847-1854 [DOI] [PubMed] [Google Scholar]
- 38.Steinman RM, Pack M, Inaba K: Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev 1997, 156:25-37 [DOI] [PubMed] [Google Scholar]
- 39.Zhou L, Schwarting R, Smith HM, Tedder TF: A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J Immunol 1992, 149:735-742 [PubMed] [Google Scholar]
- 40.McLellan AD, Heiser A, Hart DNJ: Induction of dendritic cell costimulator molecule expression is suppressed by T cells in the absence of antigen-specific signalling: role of cluster formation, CD40 and HLA-class II for DC activation. Immunology 1999, 98:171-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P: Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol 2000, 164:4507-4512 [DOI] [PubMed] [Google Scholar]