Abstract
Renal malformations are the commonest cause of chronic renal failure in children and they are often associated with urinary tract abnormalities that impair fetal urine flow. Up-regulation of transforming growth factor-β1 (TGF-β1) occurs after experimental postnatal urinary tract obstruction and we recently reported increased levels of TGF-β1 in human renal malformations (Yang SP et al, Am J Pathol 2000, 157:1633–1647). These findings led us to propose that obstruction-induced stretch of developing renal epithelia causes up-regulation of TGF-β1, which then perturbs renal development. In this study, therefore, we examined expression of components of the TGF-β1 signaling axis in a previously characterized ovine model of fetal short-term urine flow impairment in which complete unilateral ureteric obstruction was induced at 90 days when a few layers of glomeruli had formed. Up-regulation of TGF-β1 mRNA and protein was observed in obstructed kidneys, compared to sham-operated control organs, after only 10 days. Increased levels of TGF-β1 receptors I (TGF-βR1) and II (TGF-βR2) were also detected on Western blot, and the cytokine and TGF-βR1 co-localized in disrupted epithelia on immunohistochemistry. De novo expression of α-smooth muscle actin, a structural protein up-regulated during TGF-β1-induced phenotypic switching between human renal dysplastic epithelial and mesenchymal lineages in vitro, was also observed in these aberrant epithelia. These findings implicate increased TGF-β1 signaling in the early biological changes generated by fetal urinary tract obstruction.
Renal malformations, such as dysplastic and hypoplastic kidneys, are the commonest cause of end-stage renal failure in early childhood 1,2 and they are often associated with anatomical urinary tract abnormalities that impair urine flow. 3,4 The spectrum of associated lower tract abnormalities ranges from complete obstruction, as observed in multicystic dysplastic kidneys attached to atretic ureters, to partial urinary flow impairment in conditions such as posterior urethral valves. 3,4 Bilateral fetal obstructive nephropathy is associated with oligohydramnios with lung hypoplasia, and newborns often die from respiratory or renal failure. Although prenatal surgical intervention to re-establish urine flow is feasible, controlled studies to assess potential long-term clinical benefits are lacking. 5
Experimental disruption of urine flow during renal development causes renal malformations in several animal species. 6-8 Sheep constitute a particularly good experimental model because the urinary tract can be manipulated relatively early in gestation in vivo, hence mimicking potential events in utero in humans. The ovine metanephros appears at 27 to 30 days of gestation, urine flows at an estimated 5 to 6 ml/hour by 75 days, 9 and full term is 145 days. Interruption of urine flow by either ureteral or urinary bladder outlet obstruction generates a spectrum of anatomical abnormalities, which are dependent on the timing of the surgery, ranging from growth failure and dysplasia to hydronephrosis and subcapsular cysts. 6,10,11 Moreover, our previous studies have demonstrated both morphological and molecular changes after a relatively short period of fetal urine flow impairment: complete unilateral ureteric obstruction was performed at 90 days and, after 10 days, we observed disruption of nephrogenesis with diminished formation of nephrons and cystic dilatation of maturing glomeruli. 12 These anatomical changes were accompanied by dysregulation of cell proliferation and apoptosis, and aberrant expression of molecules critical for normal nephrogenesis such as the transcription factor PAX2, molecular aberrations similar to those observed in human renal malformations. 13-15
Transforming growth factor-β1 (TGF-β1) is a multifunctional cytokine, which is widely expressed during normal development and up-regulated in diverse adult pathological conditions, including kidney diseases. 16 TGF-β1 signaling is transduced via cell surface type I and type II receptors (TGF-βR1 and TGF-βR2): TGF-βR2 binds the ligand and forms a complex with TGF-βR1 that activates intracellular signaling cascades. 17 Exogenous TGF-β1 blocks branching morphogenesis in metanephric cell and organ culture, 18,19 hence reproducing in vitro one of the classic features of human dysplastic kidneys. 4 We therefore recently studied the expression of components of the TGF-β1 axis in human dysplastic kidneys, a subset of which were associated with impaired urine flow, and demonstrated up-regulation of the cytokine and persistent expression of its receptors. 20 In addition, exogenous TGF-β1 caused dysplastic epithelial cells in culture to lose epithelial characteristics (eg, ZO1) and gain mesenchymal markers such as α-smooth muscle actin (SMA). Based on these findings, we postulated that up-regulated TGF-β1 signaling, potentially induced by urinary tract obstruction, could have a number of effects in human dysplastic kidneys including inhibitory effects on PAX2-mediated cyst growth and induction of epithelial to mesenchymal transformation with consequent loss of potential nephrons. 20
We therefore hypothesized that surgical ureteric obstruction of mid-gestation sheep would lead to up-regulation of components of the TGF-β1 axis in the ipsilateral kidney. Our results confirm that a relatively short period of obstruction is sufficient to up-regulate TGF-β1 expression during nephrogenesis.
Materials and Methods
General chemicals and materials were obtained from Sigma (Poole, Dorset, UK) unless otherwise stated.
Surgery and Collection of Samples
Sheep samples were obtained from a tissue bank established from previous operations. 12 In brief, pregnant Mule cross-breed sheep (R. White, Oxfordshire, UK) were fasted for 1 day, at 90 days of gestation, and anesthesia was induced with sodium pentothal, then maintained with halothane/oxygen. The abdomen was incised in the midline, the uterus entered with diathermy and the fetal hindquarters delivered. Diathermy was used to make a lumbotomy incision through skin and muscle and the fetal ureter was identified and ligated. The muscle layers, uterus, and skin were closed with vicryl or silk sutures, anesthesia was stopped, and animals were allowed to recover. Gentamicin (80 mg) and penicillin (600 mg) were injected into the uterine cavity at operation, and intramuscular streptomycin (1 g) was administered for 5 days postoperatively. Obstructed kidneys from six animals were harvested 10 days after surgery at 100 days of gestation (obstructed group). Contralateral kidneys were not examined in this project, because no significant difference in weight or gross morphology was observed in our previous study of short-term obstruction, 12 although longer term unilateral fetal obstruction or abnormal kidney development is associated with contralateral overgrowth and changes in gene expression. 10,21 Control, or sham-operated, kidneys (n = 5) were obtained from the twin animals to the operated group, which underwent the same operative anesthesia but did not have ureteral ligation. Half of each freshly-removed fetal sheep kidney was fixed in 2% paraformaldehyde and embedded in paraffin wax and the symmetrical other half was snap-frozen in liquid nitrogen and stored at −70°C.
In Situ Hybridization
For TGF-β1 in situ hybridization we used a human TGF-β1 cDNA plasmid, as described. 20 Other groups have previously confirmed the use of human probes to detect sheep TGF-β1 mRNA. 22 Plasmids were linearized with restriction enzymes and sense and anti-sense uridine triphosphate-digoxigenin-labeled riboprobes were prepared using an RNA polymerase kit (Digoxigenin RNA labeling kit; Boehringer Mannheim, Lewes, UK). In situ hybridization was performed on 7-μm paraffin sections of formation-fixed tissue, as described. 20 After dewaxing, sections were digested for 10 minutes with proteinase K (20 μg/ml) at 37°C and then postfixed in 4% paraformaldehyde. Sections were then washed in 2× standard saline citrate, prehybridized, and then hybridized with digoxigenin-labeled sense or anti-sense TGF-β1 probes at 60°C overnight in 100 μl of hybridization buffer containing 50% v/v formamide, 5× standard saline citrate, 1× Denhardt’s reagent, heat-denatured salmon sperm DNA (0.1 mg/ml), and 10% w/v dextran sulfate. Sections were finally washed at 60°C in 2× standard saline citrate with 25% formamide, followed by 1× standard saline citrate and 0.1% sodium dodecyl sulfate. Hybridized probe was detected by incubation with anti-digoxigenin antibody conjugated to alkaline phosphatase and color development with NBT/BCIP substrate (Promega, Southampton, UK). Slides were washed and mounted with Citifluor (Chemical Labs, University of Kent, UK).
Immunohistochemistry
For TGF-β1 immunohistochemistry we used a rabbit polyclonal antibody (sc146; Santa Cruz Biotechnology, Santa Cruz, CA) raised against a peptide corresponding to amino acid residues 328 to 353 within the carboxy-terminal region of the human TGF-β1. This antibody shows no cross-reactivity with TGF-β2 or TGF-β3 and, despite being directed against the human protein, has confirmed specific staining patterns in sheep. 22 For TGF-β receptor immunohistochemistry we used specific anti-TGF-βR1 (sc402) and TGF-βR2 (sc220) antibodies raised against the carboxy terminus of the human proteins. We have previously validated these antibodies in human studies 20 but found in this study that, although both gave specific results on Western blotting, only the anti-TGF-βR1 antibody worked for immunohistochemistry on sheep tissue sections. A mouse monoclonal antibody was used for α-SMA (A5691). Immunohistochemistry was performed as described. 20 Four-μm sections were dewaxed through Histo-Clear (National Diagnostics, Atlanta, GA) and rehydrated through 100 to 30% alcohols. After washing in tap water and phosphate-buffered saline (PBS) (pH 7.4) for 5 minutes, they were treated with trypsin (1 mg/ml) for 10 to 15 minutes at 37°C. Endogenous peroxidase was quenched with 3% hydrogen peroxide for 30 minutes at room temperature. Sections were washed with blocking buffer (PBS, 10% goat serum, 0.1% Tween-20) and then incubated with the primary antibody (TGF-β1 antibody at 1:150 to 1:300 dilution, TGF-βR1 and TGF-βR2 antibody at 1:500 to 1:1000, α-SMA antibody at 1:150 to 1:300) in blocking buffer overnight at 4°C. After thorough washing in PBS/0.1% Tween-20, primary antibodies were detected by the sensitive DAKO EnVision+ System (DAKO, Ely, UK). Controls were omission of primary antibody or preincubation with a 10-fold excess of the appropriate peptide for 2 hours at room temperature. Sections were counterstained with hematoxylin, mounted in dextropropoxyphene (BDH, Poole, UK), examined on a Zeiss Axioplan microscope (Carl Zeiss, Oberkochen, Germany), and photographed under oil immersion with a ×63 magnification lens.
Western Blotting
Kidney samples were homogenized in RIPA buffer (PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) plus protease inhibitors (30 μg/ml aprotinin, 100 mmol/L sodium orthovanadate, 100 mmol/L phenylmethyl sulfonyl fluoride) and supernatants were collected by centrifugation at 13,000 rpm for 15 minutes. Protein concentration was determined using the bicinchoninic acid protein assay (Pierce, Rockford, IL). Samples were boiled for 5 minutes, then 50 μg of total protein was loaded per well and electrophoresed through an 8 or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel (8% for TGF-β1 analysis or 12% for the other proteins). Equality of loading was determined by staining representative gels with Coomassie blue (data not shown). After electrophoresis, proteins were transferred to nitrocellulose membranes (Amersham Life Science Ltd., Little Chalfont, UK) by electroblotting (Bio-Rad, Hemel Hempstead, UK) and left overnight at 4°C in blocking solution [5% (w/v) fat-free milk powder, 0.3% (v/v) Tween-20 in PBS]. They were then incubated with primary antibodies, as above, including anti-TGF-β1, TGF-βR1, and TGF-βR2 at 1:200 to 500 dilution, or α-SMA (A2547) at 1:1000 dilution, at 4°C for 1 hour. After washing in blocking solution, blots were incubated for 30 minutes with appropriate horseradish peroxidase-conjugated second antibodies diluted 1:1000 to 1500 in blocking solution. Blots were washed three times with blocking solution and once with PBS. The blot was then developed using the ECL detection kit (Amersham Life Science Ltd.), and intensity of staining was determined by optical densitometry after scanning on a Hewlett-Packard ScanJet 5100C (Hewlett-Packard Ltd., Bracknell, UK). Results were standardized so that protein levels in the sham-operated kidneys were nominally designated as 100% and statistical assessment of staining intensity was performed using a Student’s t-test. Rainbow markers were used to determine protein size. In some experiments, after visualizing the immobilized proteins the antibody complex was stripped by rocking the blot in stripping buffer (Chemistrip; Chemicon, Harrow, UK) for 1 hour at room temperature. The blot was then reprobed with other antibodies, as above. This procedure allowed direct comparison of staining intensity on the same samples with different antibodies.
Results
At 100 days of gestation, sham-operated sheep kidneys had a clearly defined nephrogenic zone (Figure 1A) ▶ , containing ureteric bud branch tips, mesenchymal condensates and early nephron precursors, such as S-shaped bodies, as previously described. 12 In contrast, in organs obstructed from 90 to 100 days of gestation, the superficial cortex was grossly abnormal with dilated ureteric bud branches, sparse mesenchymal condensates, and numerous cysts (Figure 1B) ▶ . Normal S-shaped bodies were not detected and cystic dilatation of developing glomeruli was frequently observed (Figure 1B) ▶ . Obstruction of urine flow also perturbed development of the medulla, with marked dilatation of collecting ducts (Figure 1; C, D, and E ▶ ).
Figure 1.
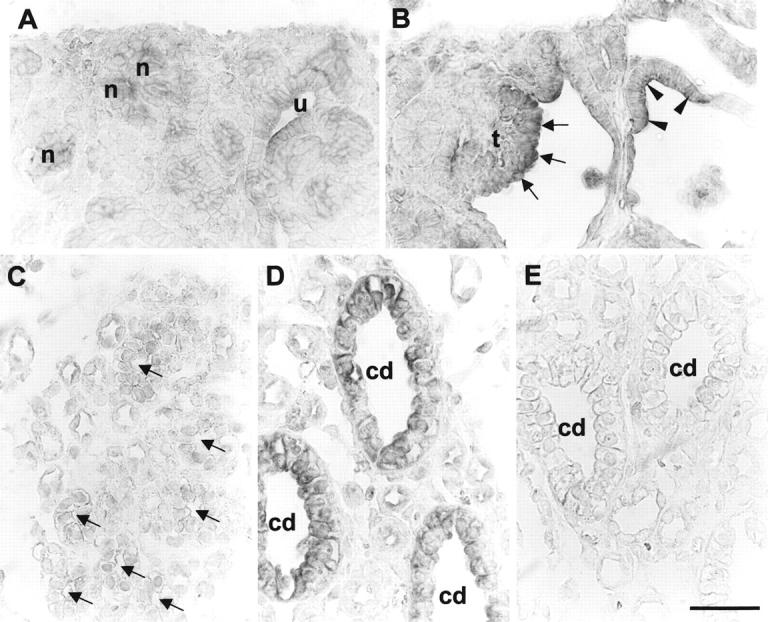
TGF-β 1 in situ hybridization in sham-operated and obstructed ovine fetal kidneys. A and C are sections of sham-operated developing kidneys, whereas B, D, and E are from obstructed kidneys. All panels represent the results of hybridization with TGF-β1 antisense probes, apart from E in which a control sense probe was used. A: TGF-β1 transcripts were detected in ureteric bud (u) and forming nephrons (n) in the normal superficial cortex. B: Prominent signal for TGF-β1 was observed in dilated tubule epithelia (arrowheads) and glomerular tufts (t; arrows) in the obstructed cortex. C: Faint TGF-β1 signal was detected in sham-operated developing medullary collecting ducts (arrows). D: In contrast, there was TGF-β1 transcript up-regulation in the larger dilated collecting ducts (cd) in the obstructed kidneys. E: Only background signal was detected using the sense probe. Scale bar, 15 μm.
In situ hybridization for TGF-β1 in sham-operated kidneys demonstrated transcripts in the ureteric bud and early nephron precursors (Figure 1A) ▶ . Weak signals were also observed in some glomeruli in the deeper cortex, particularly over endothelial cells and podocytes but not in the parietal epithelium of Bowman’s capsule (data not shown). Using the same antisense probe, prominent TGF-β1 signal was detected in the superficial cortex of obstructed kidneys at 100 days (Figure 1B) ▶ : main sites of expression were dilated ureteric bud branches, dilated tubules, and both the tufts and parietal epithelia of cystic glomeruli. Normal medullary collecting ducts in sham-operated organs expressed low levels of TGF-β1 transcripts (Figure 1C) ▶ , whereas strong signals were detected in dilated medullary collecting ducts in obstructed kidneys (Figure 1D) ▶ . Significant signals were not detected in any of these sites in control hybridizations with a TGF-β1 sense probe (Figure 1E ▶ and data not shown).
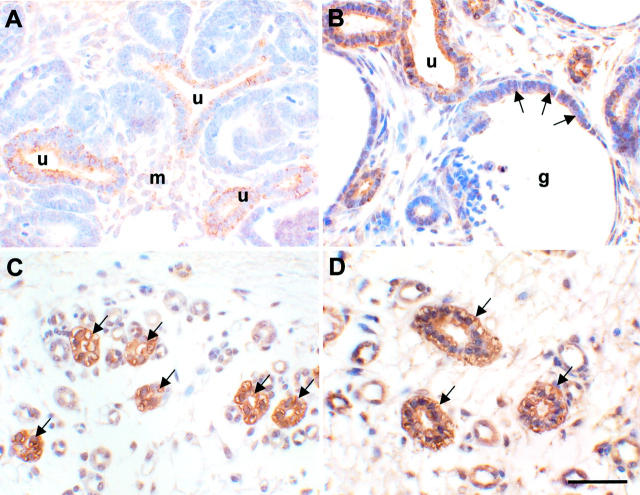
Histochemistry demonstrated faint immunoreactivity for TGF-β1 in ureteric bud branch tips in the sham-operated nephrogenic cortex (Figure 2A) ▶ , whereas strong immunostaining was observed in epithelia of dilated ureteric buds and developing tubules from obstructed organs (Figure 2B) ▶ . Deeper in the cortex of sham-operated kidneys, TGF-β1 immunostaining was weakly positive in normal glomeruli and maturing distal tubules (Figure 2C) ▶ . Prominent TGF-β1 immunoreactivity was observed, however, in obstructed kidneys, particularly in the epithelia of glomerular cysts and dilated tubules (Figure 2D) ▶ . Occasional cells around these distorted epithelia were also positive. TGF-β1 was also detected in vessels in both sham-operated and obstructed kidneys (Figure 2C ▶ and data not shown). In the medulla, faint TGF-β1 immunoreactivity was detected in collecting ducts in control kidneys whereas, in parallel with our in situ hybridization findings, more intense TGF-β1 immunostaining was recorded in large, dilated collecting ducts in obstructed kidneys (data not shown). Significant TGF-β1 immunostaining was not detected in sham-operated or obstructed kidney sections when the primary antibody was either omitted or preabsorbed with TGF-β1 peptide (Figure 2, E and F ▶ , respectively).
Figure 2.
Localization of TGF-β1 protein in sham-operated and obstructed ovine fetal kidneys. A–D and F were reacted with anti-TGF-β1 antibodies, whereas the antibody was omitted in E. A, C, and E are sections of sham-operated kidneys whereas B, D, and F are from obstructed kidneys. A and B show the outer cortex; C–F show areas deeper in the cortex. A: TGF-β1 immunostaining was detected in ureteric bud branches (u) in sham-operated kidneys. Significant staining, greater than background levels, was not detected in developing proximal tubules (p) or glomeruli (g). B: Strong TGF-β1 immunoreactivity was detected in dilated ureteric bud branches (u) and tubules (t) in obstructed kidneys. C: TGF-β1 staining was prominent in vessels (v) in sham-operated kidneys, with weak signals in glomeruli (g) and distal tubules (d). Vessels were also positive in obstructed kidneys (data not shown). D: Glomerular (g) and tubular cyst (t) epithelia stained strongly for TGF-β1 deeper in the cortex of the obstructed kidneys. E: No immunoreactivity was detected in vessels or glomeruli in normal kidneys when the primary anti-TGF-β1 antibody was omitted. F: Similarly, positive immunostaining was not detected in cystic glomeruli or dilated tubules in obstructed kidneys after preincubation of the primary antibody with excess TGF-β1 peptide. Scale bar, 15 μm.
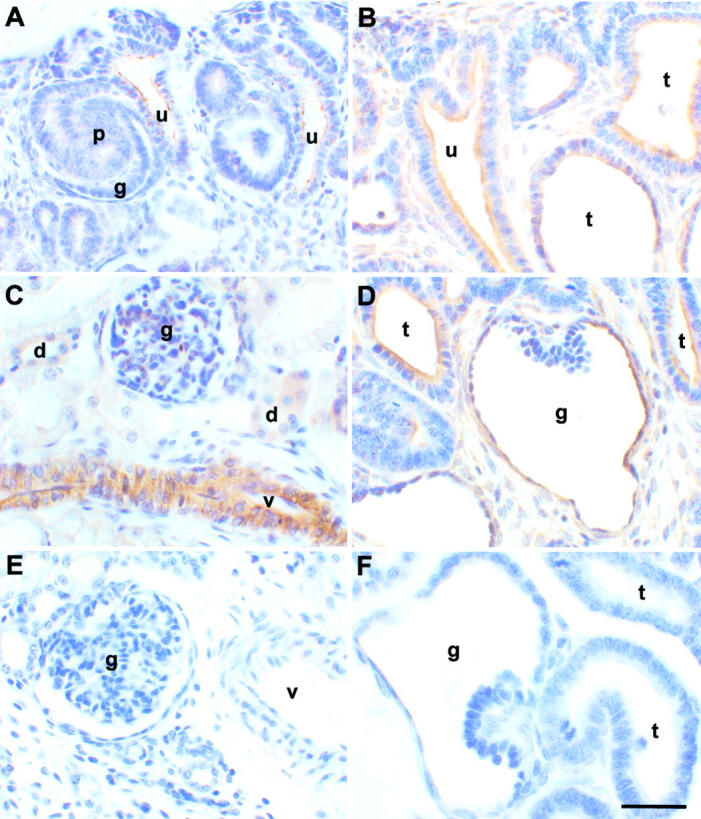
TGF-βR1 immunostaining was observed in ureteric bud tips in nonobstructed organs (Figure 3A) ▶ . Low levels of receptor protein were also detected in normal loosely-arranged uninduced mesenchyme (Figure 3A) ▶ . In the obstructed kidneys, prominent TGF-βR1 immunostaining was detected in both normal-caliber and dilated tubules, and in glomerular cysts (Figure 3B) ▶ . Weak receptor immunoreactivity was also noted in a subset of surrounding cells (Figure 3B) ▶ . Large collecting ducts were positive for TGF-βR1 in the medulla of sham-operated animals (Figure 3C) ▶ and this receptor was strongly expressed in abnormal dilated collecting ducts in obstructed kidneys (Figure 3D) ▶ .
Figure 3.
Localization of TGF-βR1 protein in sham-operated and obstructed ovine fetal kidneys. All sections were reacted with anti-TGF-βR1 antibodies. A and C are sections of sham-operated kidneys whereas B and D are from obstructed kidneys. A and B show the outer cortex; C and D show the medulla. Positive immunostaining was not observed in control sections when the primary antibody was omitted (data not shown). A: TGF-βR1 immunoreactivity was detected in ureteric bud tips (u), with low levels in loosely arranged uninduced mesenchyme (m) in sham-operated kidneys. B: Prominent expression of the receptor was detected in the epithelia of dilated ureteric bud branches (u), tubules, and glomerular cysts (g; arrows), and in a subset of surrounding cells, in obstructed kidneys. C: Normal medullary collecting ducts (arrows) expressed TGF-βR1. D: Prominent expression of the receptor was also detected in large abnormally dilated collecting ducts (arrows) in obstructed kidneys. Scale bar, 15 μm.
Significant α-SMA staining was not detected in the sham-operated nephrogenic cortex (Figure 4A) ▶ . In contrast, there was marked up-regulation of this mesenchymal marker in the superficial cortex of obstructed kidneys, both within dilated epithelia and in cells around distorted glomeruli and tubules (Figure 4B) ▶ . Deeper in the normal cortex, α-SMA staining was observed in the mesangium of normal maturing glomeruli (Figure 4C) ▶ , but there was stronger staining in obstructed kidneys, particularly within the tufts in cystic glomeruli (Figure 4D) ▶ . In the medulla, very little α-SMA immunoreactivity was detected in normal collecting ducts (Figure 4E) ▶ , whereas there was prominent staining in the epithelia of collecting ducts in obstructed kidneys (Figure 4F) ▶ . Strong α-SMA staining was also observed in vessels in both normal and abnormal kidneys (Figure 4 ▶ ; C, E, and F).
Figure 4.
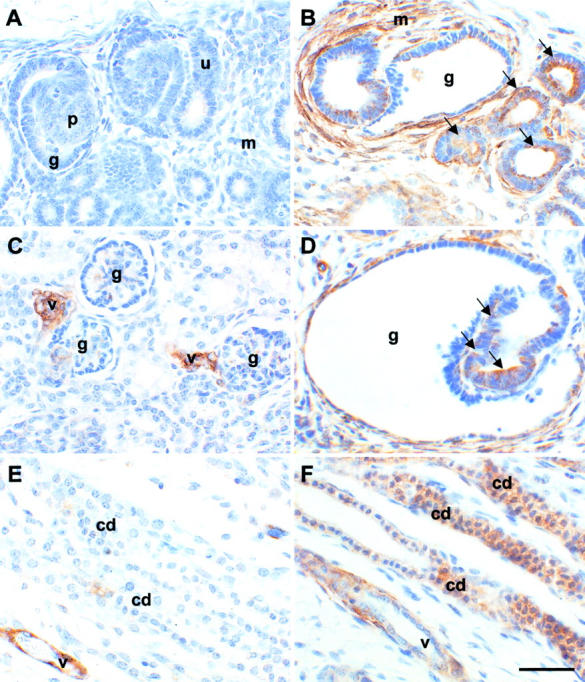
Localization of α-SMA protein in sham-operated and obstructed ovine fetal kidneys. All sections were stained with anti-α-SMA antibodies. A, C, and E are sections of sham-operated kidneys whereas B, D, and F are from obstructed kidneys. A and B show the outer cortex; C and D show areas deeper in the cortex; E and F show the medulla. No staining was observed in control experiments when the primary antibody was omitted (data not shown). A: Significant α-SMA immunoreactivity was not detected in ureteric bud tips (u), developing proximal tubules (p), or glomeruli (g), or in mesenchymal areas (m) in the outer cortex of sham-operated kidneys. B: Strong expression of this protein was detected, however, in the mesenchyme-like tissues (m) around dilated epithelia in obstructed kidneys, and in some tubule epithelia (arrows) and glomerular cysts (g). C: Deeper in the cortex, α-SMA was detected in normal glomeruli (g) and in vessels (v). D: There was, however, striking up-regulation of α-SMA around dilated glomeruli (g) and in aberrant glomerular tuft epithelia (arrows) in obstructed kidneys. E: In the normal medulla, minimal α-SMA immunoreactivity was detected in collecting ducts (cd), whereas vessels were strongly positive. F: In obstructed kidneys, up-regulated α-SMA expression was observed in dilated collecting ducts; staining intensity of these dilated ducts was comparable to the vessels. Scale bar, 15 μm.
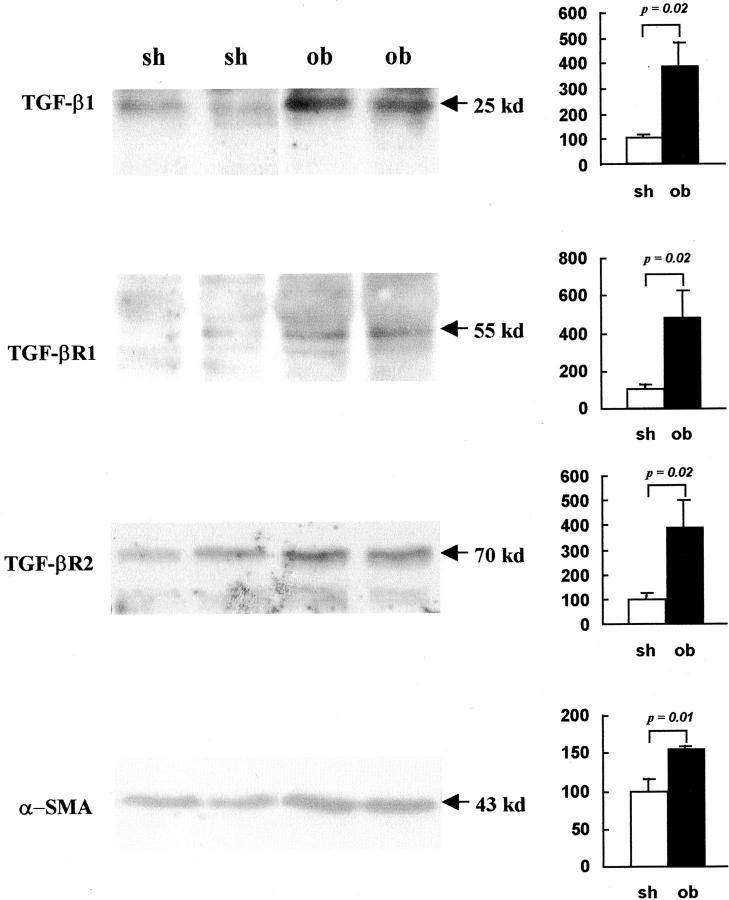
Specificity of the anti-TGF-β1, TGF-βR1, TGF-βR2, and α-SMA antibodies was confirmed by Western blotting (Figure 5) ▶ , because these antibodies were originally intended for human studies. Major bands, of the expected size, 17,20 were detected in both sham-operated and obstructed kidneys as follows: 25 kd corresponding to TGF-β1 dimers, 55 kd for TGF-βR1, 70 kd for TGF-βR2, and 43 kd for α-SMA (Figure 5) ▶ . Compared to sham-operated kidneys, where protein levels were designated as 100%, there were increases of 281% in TGF-β1 (P = 0.02), 379% in TGF-βR1 (P = 0.02), 291% in TGF-βR2 (P = 0.02), and 54% in α-SMA (P = 0.01).
Figure 5.
Western blot of TGF-β1, its receptors and α-SMA protein in sham-operated and obstructed ovine fetal kidneys. Representative Western blots showing protein extracts from two sham-operated (sh) and two obstructed (ob) kidneys probed with antibodies to TGF-β1, TGF-βR1, TGF-βR2, and α-SMA. Bands at the expected sizes were detected in each Western blot (left). Signal intensity, measured by densitometry, was compared in sham-operated and obstructed samples (n = 5) using a Student’s t-test (right). This analysis demonstrated that obstruction was associated with significant up-regulation of protein expression as follows: TGF-β1 (281% greater than control, P = 0.02), TGF-βR1 (379% greater than control, P = 0.02), TGF-βR2 (291% greater than control, P = 0.02), and α-SMA (54% greater than control, P = 0.01).
Discussion
Our results are the first demonstration of deregulated expression of the renal TGF-β1 axis after short-term fetal urinary flow impairment in sheep. These findings lend support to our proposed model linking prenatal urinary flow impairment with increased TGF-β1 levels in the pathogenesis of renal malformations. 20
We recorded up-regulation of TGF-β1 mRNA and protein in the malformed kidneys attached to obstructed ureters, particularly in aberrant epithelia. These changes were observed after only 10 days of obstruction, suggesting that TGF-β1 may be important in the early biological changes generated by impairment of urinary flow. A potential explanation for these findings could be that the increased hydrostatic pressure generated by impaired urinary flow causes direct stretch of the epithelia with resultant epithelial up-regulation of TGF-β1, as reported for other renal cells subjected to cyclical stretch in culture. 23 This is the first time that up-regulation of TGF-β1 has been reported after such short-term fetal obstruction, although the results are consistent with our observations of up-regulated TGF-β1 in human dysplastic kidneys, 20 some of which were attached to obstructed lower urinary tracts. Up-regulation of TGF-β1 has also been described by Medjebeur and colleagues 22 in longer term urinary tract obstruction in fetal sheep: they reported that bladder obstruction, generated by combined ligation of the urethra and urachus between 60 and 80 days of gestation, caused increased TGF-β1 mRNA and protein expression when assessed at 120 days of gestation, after nephrogenesis was complete. 22 Increased TGF-β1 protein expression was observed in both tubular epithelia and the renal interstitium in this model 22 and it would have been intriguing to examine either protein distribution at earlier time points or mRNA expression by in situ hybridization to determine whether the cytokine was produced within the epithelia, as we observed. Other reports relevant to the current study describe increased TGF-β1 in rats with congenital hydronephrosis, 24 and angiotensin II-mediated up-regulation of TGF-β1 with perturbed renal growth after neonatal ureteric ligation in the rat. 8 TGF-β1 has also been implicated in the progressive fibrosis induced by obstruction of the adult kidney. 25
TGF-β1 signaling is transduced via TGF-βR1 and TGF-βR2, but the distribution of these receptors has not previously been reported in fetal urinary tract obstruction. In this study, we detected up-regulation of both receptors on Western blot, and TGF-βR1 protein immunolocalized to abnormally dilated tubule and glomerular epithelia in the cortex, sites in which we detected its ligand. Moreover, there was persistent expression of TGF-βR1 in abnormally dilated medullary collecting ducts. These expression sites would be consistent with an autocrine TGF-β1 signaling system within epithelia, although we were unable to confirm co-localization with TGF-βR2, which is necessary for initial TGF-β1 binding, because the anti-human TGF-βR2 antibody did not work on sheep sections in this study. Up-regulation of TGF-βR1 has also been reported after postnatal ureteric obstruction in the rat 26 but was not observed in ureteric tumor-associated obstruction in humans. 27
What are the likely biological effects of elevated TGF-β1 signaling within developing renal epithelia? We postulate that there would be at least two distinct effects: firstly, TGF-β1 could perturb epithelial cell turnover by restricting proliferation and promoting apoptosis, as described in other epithelia. 20,28,29 In this context, it is of note that apoptosis was detected in cyst walls and glomerular tuft epithelia in our previous experiments using this short-term obstruction model (see Figures 5C and D ▶ in Attar et al 12 ), sites where we observed TGF-β1 expression in the present study. Secondly, TGF-β1 may divert potential functional renal epithelia toward a mesenchymal lineage, as reported in vitro in mature rat kidney tubular epithelial cells. 30 Evidence supporting this second mechanism comes from our current observation that α-SMA was up-regulated in malformed epithelia in obstructed kidneys. We suggest that this is unlikely to be a nonspecific stress response in view of our previous findings that excess TGF-β1 directly causes increased α-SMA in human renal epithelial cells in vitro. 20 Hence, the α-SMA-positive epithelia observed in obstructed kidneys may represent a first step of transitional cells that are undergoing TGF-β1-induced phenotypic transformation toward a mesenchyme-like lineage.
TGF-β1 is not the only molecule that is known to be dysregulated in urinary tract maldevelopment, however, and other potential conflicting or modifying factors may also affect the final renal phenotype. The transcription factor PAX2, for example, is up-regulated in both human cystic epithelia 14 and in this sheep obstruction model. 12 PAX2 has been implicated in the survival and expansion of metanephric precursor cells during development and cyst growth in transgenic mice. 31 One would therefore predict that excess PAX2 alone should promote cyst growth, which is consistent with our earlier observation of increased proliferating cell nuclear antigen expression in dilated epithelia in the obstructed sheep kidneys (see Figures 3 and 4 ▶ ▶ in Attar et al 12 ). In this location, therefore, it is likely that the biological results of the up-regulation of TGF-β1 reported in our present study would be to limit the rate of cyst growth by antagonizing the effects of PAX2. One potential mechanism for this would be a TGF-β1-mediated reduction in the stability of PAX2 mRNA, as demonstrated in cultured renal epithelial cells. 32 Moreover, we have demonstrated that expression of PAX2 protein is reduced after TGF-β1 treatment of human dysplastic epithelial cells. 20 These complex potential interactions comprise part of the working model, linking up-regulation of TGF-β1 and Pax2 to fetal urinary tract obstruction in the pathogenesis of kidney malformations, recently proposed by our group (see Figure 10 in Yang et al 20 ). Other potential modifying factors described in obstructed kidneys include angiotensin II, the survival factor BCL2, and epidermal growth factor. 8
In summary, in the present study we observed up-regulation of the multifunction cytokine TGF-β1 and its receptors TGF-βR1 and TGF-βR2, co-localization of the cytokine with TGF-βR1 in aberrant epithelia and elevated levels of α-SMA in a sheep model of short-term mid-gestation unilateral ureteric obstruction. These observations raise the possibility that therapeutic interventions to reduce the activity of the TGF-β1 signaling axis (eg, using substances such as decorin 33 ) may ameliorate the severity of human renal malformations. Some of the effects of excess TGF-β1 may, however, be beneficial by preventing or restricting the actions of other factors, such as PAX2. Further clarification of the biological effects of TGF-β1 blockade will therefore be required in animal models such as this one before such therapies could be contemplated in humans.
Acknowledgments
We thank Peta Foxall, Pierre Mouriquand, and Mark Hanson for their initial input and practical help with this study.
Footnotes
Address reprint requests to Dr. Su Ping Yang, Division of Experimental Medicine, Harvard Institutes of Medicine, HIM Building, Room 318, 4 Blackfan Circle, Boston, MA 02115. E-mail: pwinyard@ich.ucl.ac.uk.
Supported by project grants from Action Research (S/P/3178), the National Kidney Research Fund (R11/2/1996 and R18/1/2000), and the Kidney Research Aid Fund.
References
- 1.Warady BA, Hebert D, Sullivan EK, Alexander SR, Tejani A: Renal transplantation, chronic dialysis, and chronic renal insufficiency in children and adolescents. The 1995 Annual Report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 1997, 11:49-64 [DOI] [PubMed] [Google Scholar]
- 2.Lewis M: Report of the Paediatric Renal Registry 1999. UK Renal Registry. The Second Annual Report. Edited by D Ansell, T Feest, E Will, M Smith, M Lewis, C Burton, C Dudley. Bristol, UK, UK Renal Registry, 1999, pp 175–188
- 3.Woolf AS, Winyard PJD: Gene expression and cell turnover in human renal dysplasia. Histol Histopath 2000, 15:159-166 [DOI] [PubMed] [Google Scholar]
- 4.Potter EL: Normal and abnormal development of the kidney. 1972. Year Book Medical Publishers Inc., Chicago
- 5.Coplen DE: Prenatal intervention for hydronephrosis. J Urol 1997, 157:2270-2277 [PubMed] [Google Scholar]
- 6.Beck AD: The effect of intra-uterine urinary obstruction upon the development of the fetal kidney. J Urol 1971, 105:784-789 [DOI] [PubMed] [Google Scholar]
- 7.Liapis H, Yu H, Steinhardt GF: Cell proliferation, apoptosis, Bcl-2 and Bax expression in obstructed opossum early metanephroi. J Urol 2000, 164:511-517 [PubMed] [Google Scholar]
- 8.Chevalier RL: Pathophysiology of obstructive nephropathy in the newborn. Semin Nephrol 1998, 18:585-593 [PubMed] [Google Scholar]
- 9.Moritz KM, Wintour EM: Functional development of the meso- and metanephros. Pediatr Nephrol 1999, 13:171-178 [DOI] [PubMed] [Google Scholar]
- 10.Peters CA, Carr MC, Lais A, Retik AB, Mandell J: The response of the fetal kidney to obstruction. J Urol 1992, 148:503-509 [DOI] [PubMed] [Google Scholar]
- 11.Kitagawa H, Pringle KC, Zuccolo J, Stone P, Nakada K, Kawaguchi F, Nakada M, Wakisaka M, Furuta S, Koike J, Seki Y: The pathogenesis of dysplastic kidney in a urinary tract obstruction in the female fetal lamb. J Pediatr Surg 1999, 34:1678-1683 [DOI] [PubMed] [Google Scholar]
- 12.Attar R, Quinn F, Winyard PJ, Mouriquand PD, Foxall P, Hanson MA, Woolf AS: Short-term urinary flow impairment deregulates PAX2 and PCNA expression and cell survival in fetal sheep kidneys. Am J Pathol 1998, 152:1225-1235 [PMC free article] [PubMed] [Google Scholar]
- 13.Winyard PJD, Nauta J, Lirenman DS, Hardman P, Sams VR, Risdon RA, Woolf AS: Deregulation of cell survival in cystic and dysplastic renal development. Kidney Int 1996, 49:135-146 [DOI] [PubMed] [Google Scholar]
- 14.Winyard PJD, Risdon RA, Sams VR, Dressler G, Woolf AS: The PAX2 transcription factor is expressed in cystic and hyperproliferative dysplastic epithelia in human kidney malformations. J Clin Invest 1996, 98:451-459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granata C, Wang Y, Puri P, Tanaka K, O’Briain DS: Decreased bcl-2 expression in segmental renal dysplasia suggests a role in its morphogenesis. Br J Urol 1997, 80:140-144 [DOI] [PubMed] [Google Scholar]
- 16.Border WA, Ruoslahti E: Transforming growth factor-β in disease: the dark side of tissue repair. J Clin Invest 1992, 92:1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wrana JL: TGF-β receptors and signaling mechanisms. Miner Electrolyte Metab 1998, 24:120-130 [DOI] [PubMed] [Google Scholar]
- 18.Rogers SA, Ryan G, Purchio AF, Hammerman MR: Metanephric transforming growth factor-β1 regulates nephrogenesis in vitro. Am J Physiol 1993, 264:F996-F1002 [DOI] [PubMed] [Google Scholar]
- 19.Sakurai H, Barros EJ, Tsukamoto T, Barasch J, Nigam SK: An in vitro tubulogenesis system using cell lines derived from the embryonic kidney shows dependence on multiple soluble growth factors. Proc Natl Acad Sci USA 1997, 94:6279-6284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang SP, Woolf AS, Yuan HT, Scott RJ, Risdon RA, O’Hare MJ, Winyard PJD: Biological role of transforming growth factor-β1 in human congenital kidney malformations. Am J Pathol 2000, 157:1633-1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandell J, Peters CA, Estroff JA, Allred EN, Benacerraf BR: Human fetal compensatory renal growth. J Urol 1993, 150:790-792 [DOI] [PubMed] [Google Scholar]
- 22.Medjebeur AA, Bussieres L, Gasser B, Gimonet V, Laborde K: Experimental bilateral urinary obstruction in fetal sheep: transforming growth factor-β1 expression. Am J Physiol 1997, 273:F372-F379 [DOI] [PubMed] [Google Scholar]
- 23.Riser BL, Cortes P, Heilig C, Grondin J, Ladson-Wofford S, Patterson D, Narins RG: Cyclic stretching force selectively up-regulates transforming growth factor-β isoforms in cultured rat mesangial cells. Am J Pathol 1996, 148:1915-1923 [PMC free article] [PubMed] [Google Scholar]
- 24.Seseke F, Thelen P, Hemmerlein B, Kliese D, Zoller G, Ringert RH: Histologic and molecular evidence of obstructive uropathy in rats with hereditary congenital hydronephrosis. Urol Res 2000, 28:104-109 [DOI] [PubMed] [Google Scholar]
- 25.Kaneto H, Morrissey J, Klahr S: Increased expression of TGF-β mRNA in the obstructed kidneys of rats with unilateral ureteral ligation. Kidney Int 1993, 44:313-321 [DOI] [PubMed] [Google Scholar]
- 26.Sutaria PM, Ohebshalom M, McCaffrey TA, Vaughan ED, Jr, Felsen D: Transforming growth factor-β receptor types I and II are expressed in renal tubules and are increased after chronic unilateral ureteral obstruction. Life Sci 1998, 62:1965-1972 [DOI] [PubMed] [Google Scholar]
- 27.Kaneto H, Ohtani H, Fukuzaki A, Ishidoya S, Takeda A, Ogata Y, Nagura H, Orikasa S: Increased expression of TGF-β1 but not of its receptors contributes to human obstructive nephropathy. Kidney Int 1999, 56:2137-2146 [DOI] [PubMed] [Google Scholar]
- 28.Moses HL, Yang EY, Pietenpol JA: TGF-β stimulation and inhibition of cell proliferation: new mechanistic insights. Cell 1990, 63:245-247 [DOI] [PubMed] [Google Scholar]
- 29.Bursch W, Oberhammer F, Jirtle RL, Askari M, Sedivy R, Grasl-Kraupp B, Purchio AF, Schulte-Hermann R: Transforming growth factor-β1 as a signal for induction of cell death by apoptosis. Br J Cancer 1993, 67:531-536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan JM, Ng YY, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY: Transforming growth factor-β regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int 1999, 56:1455-1467 [DOI] [PubMed] [Google Scholar]
- 31.Dressler GR, Woolf AS: Pax2 in development and renal disease. Int J Dev Biol 1999, 43:463-468 [PubMed] [Google Scholar]
- 32.Liu S, Cieslinski DA, Funke AJ, Humes HD: Transforming growth factor-β1 regulates the expression of Pax-2, a developmental control gene, in renal tubule cells. Exp Nephrol 1997, 5:295-300 [PubMed] [Google Scholar]
- 33.Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E: Natural inhibitor of transforming growth factor-β protects against scarring in experimental kidney disease. Nature 1992, 360:361-364 [DOI] [PubMed] [Google Scholar]