Abstract
The third isoform (MT-3) of the metallothionein gene family is unique in that it has a limited tissue distribution, is not induced by metals, has a neuronal growth inhibitory activity, and sequesters zinc more effectively under zinc-depleted conditions. The goal of the present study was to determine whether MT-3 was absent in normal breast tissue, was overexpressed in breast cancers, and if MT-3 overexpression would be associated with disease outcome. A combination of immunohistochemistry and reverse-transcription polymerase chain reaction was used to demonstrate that the normal breast had no detectable expression of MT-3 mRNA or protein. Using immunohistochemistry, it was shown that MT-3 was overexpressed in 25 of 34 cases of breast cancer. In all cases of positive staining, MT-3 was diffusely localized to the cytoplasm. The tumors from these 34 cases were divided as to outcome based on known 5-year survival, with 20 patients being disease free at 5 years (good outcome) and the other 14 having recurring disease within 5 years (bad outcome). When analyzed for MT-3 staining, it was shown that there was a trend for increased MT-3 immunoreactivity in the group having bad outcomes. However, when the tumor subgrouping was further defined on the basis of carcinoma in situ (CIS), there was a marked significant difference in MT-3 staining between patients with good and bad outcomes. Limited to DCIS, MT-3 staining was significantly increased in patients with bad outcomes compared to those with good outcomes. Thus, these studies demonstrate that MT-3 is overexpressed in selected breast cancers and that overexpression is associated with tumors having a poor prognosis.
The metallothioneins (MTs) are a family of cysteine-rich, low molecular weight, intracellular proteins that bind transition metals. 1 In both mice and humans, there are four classes of very similar MT proteins, designated MT-1 through -4, defined on the basis of small differences in sequence and charge characteristics. 1,2 The MT-1 and MT-2 isoforms have been extensively studied, and are believed to serve an important role in the homeostasis of essential metals such as Zn2+ or Cu2+ during growth and development, as well as in the detoxification of heavy metals such as Cd2+ and Hg2+, rendering the MTs important mediators and attenuators of heavy metal-induced toxicity, particularly hepato- and nephrotoxicity. 1-3 The MT-1 and MT-2 isoforms exhibit a ubiquitous pattern of tissue expression and are highly inducible by any number of stimuli. 1,3 The MT-3 isoform of the human MT gene family appears unique in contrast to the highly studied and ubiquitous MT-1 and MT-2 family members. The gene for the MT-3 protein was first isolated in 1992, is encoded by a single gene with no pseudogenes, and initial studies suggested its expression was highly restricted, being limited to that of neural tissues. 4 The MT-3 isoform is also unique in that, in the neural system and derived cell cultures, MT-3 has been shown to possess a neuronal cell growth inhibitory activity that is not duplicated by the other human MT classes. 5,6 This nonduplication of function occurs despite a 63 to 69% homology in amino acid sequence among MT-3 and the other human MT isoforms. The MT-3 isoform also possesses a unique sequence of 8 additional amino acids that is not present in any other member of the MT gene family. 4,5
The existence of this unique amino acid sequence has allowed the generation of an antibody specific for MT-3. 7 Using this antibody, non-neural expression of MT-3 has been shown to occur in the human kidney, with appreciable MT-3 immunoreactivity localized to epithelial cells of both glomerular and tubular origin. 7 It has also been shown that there was no expression of MT-3 in any of the various cell types comprising the normal human bladder, but expression was found in all bladder cancers, and degree of staining correlated to tumor grade. 8 In the normal human prostate, MT-3 staining was very limited in distribution, with only very weak staining in the basal cells and epithelial cells of the prostatic ducts. 9 In prostate cancer, MT-3 immunoreactivity was variable in both tumors and prostatic intraepithelial neoplasia lesions, with some tumors having strong reactivity similar to nerve, whereas others were totally devoid of MT-3 immunoreactivity. 9 In general, expression of MT-3 appeared to correlate with the Gleason score. The finding that MT-3 expression was altered in both bladder and prostate cancer led to the current examination of MT-3 expression in human breast cancer. The initial goal was to determine, using diagnostic samples from a small population of patients with known outcomes, if MT-3 immunoreactivity might be a candidate prognostic marker for this disease.
Materials and Methods
Specimens for Immunohistochemical Analysis of MT-3 Expression
The study used formalin-fixed, paraffin-embedded diagnostic samples from 34 patients with outcome follow up data of at least 5 years. Within these 34 patients, there were a total of 72 surgical accessions related to the breast carcinomas in this patient study group. Each patient had between one and five separate surgical accessions, each surgical accession had between one and three separately identified parts. Of these 34 patients, 20 patients had no evidence of recurring disease at a follow-up of at least 5 years. These 20 patients were classified as good outcome and included 17 patients alive and with no evidence of disease, one patient that died with no evidence of disease and two patients lost to follow-up but without evidence of disease at 5 and 6 years, respectively. Fourteen patients were classified as having bad outcomes. These include 10 patients that died of the disease and 4 patients with currently active disease.
The ages of the patients from which specimens were used ranged from 29 to 80 years of age at initial diagnosis, with a mean age of 53.8 years (SD, ± 12.4 years). There were 33 females and one male in the study. Thirty-three33 of the patients were white and one patient was black, which is reflective of the racial demographic distribution in the general population of the region. Initial detection of the tumors was most commonly by a breast mass (14 patients, 56%), followed by mammography without a palpable mass (7 patients, 28%), axillary node masses (2 patients, 8%), and changes in the skin or nipple (2 patients, 8%). In 9 patients, the initial clinical presentation was not recorded. The most common diagnostic assessment was an excisional biopsy of the breast mass (20 patients, 59%). Five patients (15%) had needle biopsies, two patients had incisional biopsies (6%), and four patients were diagnosed by frozen section at the time of modified radical mastectomy. The majority of patients underwent modified radical mastectomy (30 patients, 88%). Three patients (9%) were treated with lumpectomy and node dissection and one patient (3%) had a simple mastectomy performed without nodal dissection. The values for ploidy, estrogen receptor, progesterone receptor, and HER-2/neu were taken from the clinical charts. Values for estrogen receptor (ER) and progesterone receptor (PR) that used biochemical analysis were re-run using immunoperoxidase staining with confirmation of the biochemical analysis in all cases.
Immunohistochemical Localization of MT-3
The preparation of the affinity purified antibody against MT-3 and its use on formalin-fixed, paraffin-embedded tissue has been described previously. 7-9 Archival specimens were routinely fixed in 10% neutral buffered formalin for 16–18 hours. All tissues were transferred to 70% ethanol and dehydrated in 100% ethanol. Dehydrated tissues were cleared in xylene, infiltrated, and embedded in paraffin. Serial sections were cut at 3–5 μm for use in immunohistochemical protocols. Before immunostaining, sections were pretreated in a microwave at 700W in 10 mmol/L citrate buffer (pH 6.0) for 5 minutes. Sections were allowed to cool for 5 minutes at room temperature, microwaved again for 5 minutes, and immersed into distilled water. The affinity purified primary anti-MT-3 antibody was localized using the avidin-biotin-peroxidase complex procedure (BioGenex Optimax Immunostainer, BioGenex Inc, San Ramon, CA) using diaminobenzidine for visualization (Stable DAB, Research Genetics, Huntsville, AL). Slides were rinsed in distilled water, dehydrated in solutions containing graded ethanol concentrations, cleared in xylene, and coverslips placed on the slides. The positive control used sections of human kidney and brain with known staining for MT-3. The negative controls consisted of omission of primary antibody from the immunohistochemical avidin-biotin-peroxidase complex sequence as well as cell types of the positive control with negative staining for MT-3. In sections with nerve twigs or ganglia, these served as internal positive controls, and in all cases were appropriately positive. The semiquantitative assessment of immunohistochemical staining of MT-3 was analyzed using the multiplicative quickscore method, 10 which accounts for both the intensity and the percentage of cells staining, yielding values from 0 to 18. Intensity of staining was categorized as negative = 0, weak = 1, moderate = 2 or intense = 3. This number was multiplied by a number based on the percentage of tissue showing positive staining (0 to 4% = 1; 5 to 19% = 2; 20 to 39% = 3; 40 to 59% = 4; 60 to79% = 5; 80 to 100% = 6). Following assessment of the quickscore, the cutoff for positive staining was determined by K-means cluster analysis and corresponded to staining in at least 5 to 20% of tumor cells. The scoring of tumors was done by a single pathologist, however, scoring of repeat blinded serial sections of ∼ 20% of the tumor slides on different days did not disclose discrepancies in assessment. At the time of scoring and tabulation, the patient outcome data were not known to the scoring pathologist.
Analysis of Data
The data gathered for this study were stored in Microsoft Access 97 format. The pathological assessment and review of the cases were standardized with synoptic report formats, with standard choices, designed by the pathologist, in a format that would ease database entry. The standard reviewed elements concerning histological grade, assessment and histological features known or proposed to be of prognostic and diagnostic significance were done for each surgical accession. All elements of the report had the ability for free text entry if deemed appropriate for pathological assessment.
All statistical analysis was performed with Systat9 software, which allowed direct access from either the Access database or intermediate MS Excel 97 files. For each pathological observation report element, one-way frequency tables were calculated. For groups outcome, two-way tables were constructed for each of the pathological assessments. Significance was assessed using Pearson’s χ 2 with Yates corrected χ2, and the two-tailed Fisher exact test when multiple variables were evaluated. For ordered variables, the coefficient and asymptomatic standard errors were additionally determined for Goodman-Kruskal γ, Kendall τ-B, Stuart τ-C, Spearman ρ, and Somers D. These were used to approximate a χ estimation accounting for variable ordering. For 2 × 2 tables, additional testing included Odds Ratio, Yule Q, and Yule Y for testing of significance.
The numeric variables (age, tumor size, quickscore of immunostaining, and the maximum intensity of immunostaining) were evaluated with standard statistical measurements, including mean, median, SD, and SEM. For the numeric variables of age and tumor size, separate and pooled variance t-tests were run for groupings of outcome using the Dunn-Sidak and Bonferroni adjusted probability to test for significance. The semiquantitative assessment of immunostaining for CIS lesions and invasive carcinoma was assessed using analysis of variance for groupings and outcome. Power analysis for assessment of the estimated unmatched cohort sample size needed for assessment of the MT-3 immunoreactivity and outcome was calculated with EpiStat 6 software. Results for all statistical measurements were considered significant at the 95% level of confidence (α ≤ 0.050) unless otherwise stated.
Specimens for Analysis of MT-3 mRNA and Protein Expression
For the determination of MT-3 expression in normal breast, total RNA and protein samples were prepared from normal breast tissue obtained from five cases of elective reduction surgeries. The tissue source was anonymous and obtained following completion of diagnostic protocols. For the determination of MT-3 mRNA expression in in situ breast cancers, total RNA was isolated from full-thickness (5-μm) sections by laser capture microdissection from the paraffin-embedded tissues used above to determine the immunohistochemical expression of MT-3. The procedures for total RNA isolation, laser capture microdissection, reverse-transcription polymerase chain reaction (RT-PCR) analysis of MT-3 mRNA, and immunoblot analysis of MT-3 protein have been described previously. 8,9,11
Results and Discussion
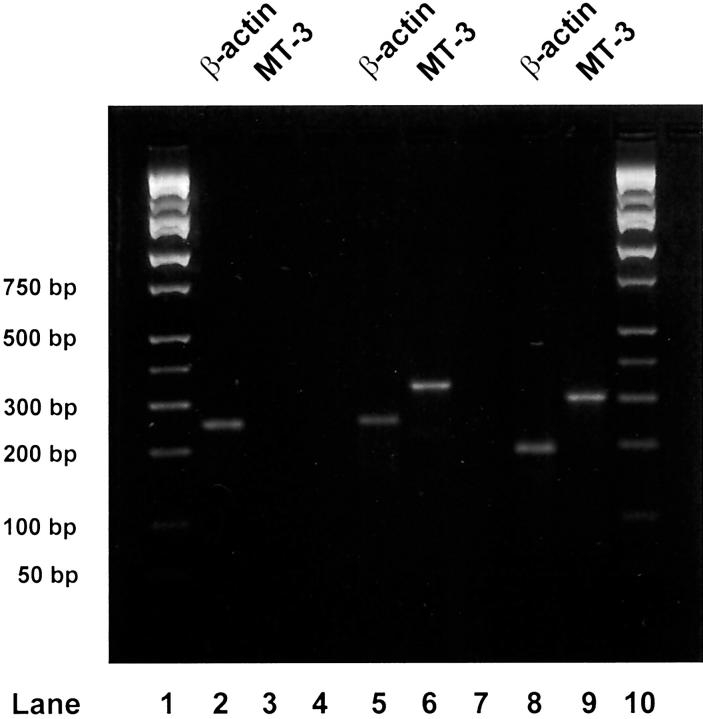
From the 34 patients in the study, there were 63 surgical specimens that contained areas of benign breast tissue in which there were a variety of proliferative and fibrocystic changes. From six of these patients, there were seven specimens that had substantial areas of normal breast tissue and these were assessed immunohistochemically for MT-3 immunoreactivity. All seven of these specimens were shown to have no staining for MT-3 (Figure 1, A and B) ▶ . To further confirm the low level or absence of MT-3 immunoreactivity, MT-3 mRNA was determined by RT-PCR on total RNA isolated from 5 independent specimens of normal breast tissue. In all 5 samples, no reaction product for MT-3 mRNA was obtained at total RNA inputs of 500 ng and 40 cycles of PCR; however, strong reaction products for the β-actin housekeeping gene were obtained from the identical total RNA samples (Figure 2) ▶ . As an additional control, it was demonstrated that positive reaction products for both MT-3 and β-actin mRNA could be obtained using total RNA from the human kidney (Figure 2) ▶ . A similar study was done to assess the presence of MT-3 protein, and no MT-3 protein was found in total protein samples prepared from normal breast tissue, but was found in control samples from human kidney (data not shown; detection limit, 0.5 pg of MT-3/μg protein). The presence of MT-3 mRNA and protein in the human kidney has been reported previously by this laboratory. 12 Together, these determinations demonstrated that there was no appreciable expression of the MT-3 gene in the normal human breast.
Figure 1.
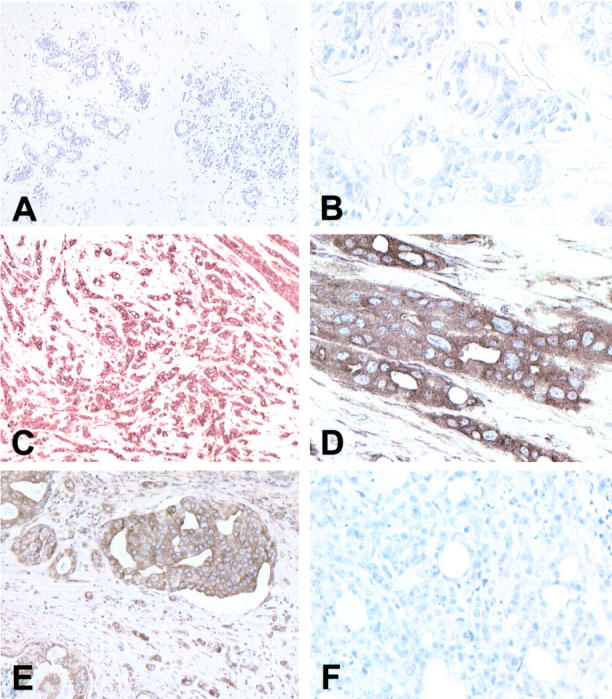
MT-3 staining in the human breast. MT-3 antibody was used at a 1:200 dilution and all slides were counterstained with hematoxylin. A: Normal breast epithelium, both ducts and lobules, does not stain for MT-3. Original magnification, ×100. B: Higher power of normal breast ducts reveals absence of staining for MT-3. Original magnification, ×400. C: Case of invasive duct carcinoma which stains strongly for MT-3. Original magnification, ×100. D: Higher power of that in C demonstrating the strong cytoplasmic MT-3 staining. Original magnification, ×400. E: Area of DCIS and adjacent invasive duct carcinoma, which stains strongly for MT-3. Original magnification, ×200. F: Case of invasive ductal carcinoma that does not stain for MT-3. Original magnification, ×200
Figure 2.
MT-3 mRNA expression in human breast tissue. Lanes 1 and 10: DNA ladder. Lanes 2 and 3: RT-PCR was performed on total RNA (500 ng) isolated from normal breast tissue. A band representing the PCR product for β-actin (249 bp) at 35 PCR cycles is shown (lane 2). No PCR product was detected for MT-3 in normal breast tissue at an RNA input of 500 ng and 40 PCR cycles (lane 3). Lanes 5 and 6: RT-PCR was performed on total RNA (500 ng) isolated from human kidney tissue. Bands representing PCR products for β-actin (249 bp) at 35 PCR cycles (lane 5) and MT-3 (325 bp) at 40 PCR cycles (lane 6) are shown. Lanes 8 and 9: RT-PCR was performed on total RNA isolated from an MT-3 positive microdissected breast tumor. Bands representing nested PCR products for β-actin (194 bp) and MT-3 (296 bp) at 30 PCR cycles are shown in lane 8 and 9, respectively. Lanes 4 and 7: Blank.
The histological tumor types examined in this study included infiltrating ductal, not otherwise specified (NOS), 22 infiltrating lobular, 6 comedocarcinoma, 2 medullary carcinoma, 2 mucinous carcinoma, 1 and adenosquamous carcinoma. 1 In 12 tumors, a different minor histological components of either infiltrating ductal 7 or infiltrating lobular 5 was noted. Immunohistochemical examination of the tumors from the 34 patients showed 25 tumors to be positive for MT-3 and 9 tumors to be negative, corresponding to at least 5% of the tumor cells exhibiting definite staining as discussed in methods. Representative examples of both positive (C, D, and E) and negative (F) MT-3 staining of the tumors are shown in Figure 1 ▶ . The MT-3 staining was diffuse and localized to the cytoplasm in all MT-3 positive tumors. Repeatability was examined, and the MT-3 staining characteristics were reproducible between individual immunostaining runs, between different sections of the same tumor, and between portions of the primary tumors from the same patient resected at different times, ie, biopsy and mastectomy with residual tumors (data not shown). Because the colocalized MT-1 and MT-2 proteins have been reported to be overexpressed in breast cancer, 13 this staining was also performed in the present study to confirm that MT-3 staining was independent of MT-1 and MT-2 staining. It was shown that MT-3 staining was independent of MT-1 and MT-2 staining pattern visualized using the commonly used E-9 antibody (data not shown). The presence of MT-3 mRNA was also confirmed in the tumors by the isolation of total RNA from an MT-3 positive staining tumor using laser capture microdissection technology. This allows tumor tissue to be selectively isolated from surrounding non-tumor tissue using the same formalin-fixed, paraffin-embedded samples that were used in the immunohistochemical determinations. The results of this analysis demonstrated MT-3 mRNA to be present in the MT-3 immunoreactive tumor at a relative level similar to that of the β-actin housekeeping gene (Figure 2) ▶ . In addition, the MT-3 reaction product was cleaved by the FokI restriction endonuclease and it has been shown previously that MT-3 is the only MT family member having a FokI site. 8,9,12 These findings demonstrated that MT-3 was overexpressed in a subset of human breast cancers.
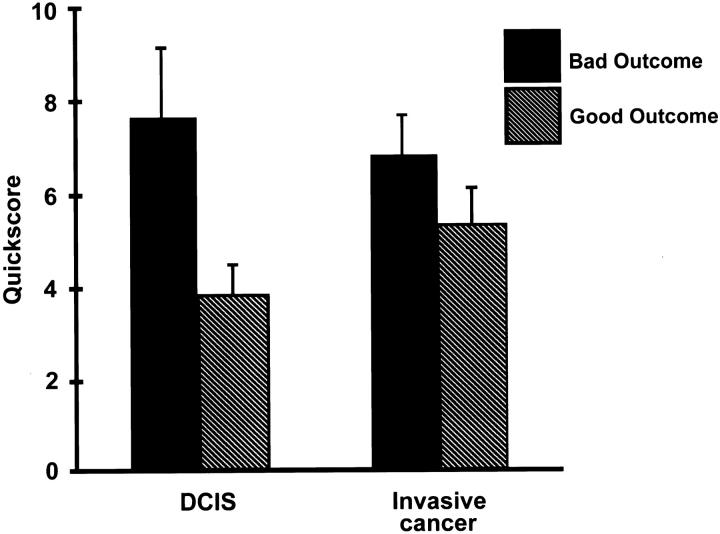
The goal of this study was to determine whether MT-3 overexpression in breast cancer was associated with disease outcome in a small, but well-characterized, set of diagnostic specimens. For these comparisons, the staining of MT-3 was semiquantified using the quickscore method, a procedure that accounts for both the intensity and the percentage of the cells that stain. When the value of MT-3 staining was compared against outcome for patients with breast cancer in general, there was a trend for stronger MT-3 staining in patients with bad outcomes (Figure 3) ▶ . Two-way tables were constructed to determine whether histological features of the tumors or prognostic markers would increase the association of MT-3 staining with outcome. The histological features examined included tumor size, ploidy, presence of multiple primary tumor foci, histological tumor grade, nuclear grade, presence of lymphatic/vascular invasion, perineural invasion, histological type, presence of microcalcifications, tumor immune response, nodal histocytic response, nodal germinal centers, tumor necrosis, and stromal fibrosis or angiogenesis. The prognostic markers examined were ER, PR, ploidy, and HER-2/neu. None of these subgroupings correlated significantly to MT-3 staining or increased the association of MT-3 staining with outcome. The small numbers of malignancies examined in special histological types precluded reliable statistical analysis by histological type, however, when two-way correlation tables examined MT-3 staining either eliminating the histological subtypes with a more favorable prognosis (mucinous, medullary) or when the analysis was limited to infiltrating ductal carcinoma, tumors that were MT 3-positive were more frequent in patients with bad outcomes, although this did not meet, on the numbers in this study, a level of statistical significance (Pearson’s χ 2 = 2.334, probability = 0.127). The presence of residual invasive carcinoma within the surgically therapeutic mastectomy or lumpectomy specimen did correlate with poor outcome (α = 0.001), even when the presence of a single microscopic focus was present in the mastectomy specimen (α = 0.006).
Figure 3.
Semiquantitative analysis of MT-3 antibody staining in DCIS and invasive breast carcinoma grouped by outcome. The MT-3 staining intensity of DCIS lesions was stronger in patients with bad outcomes than patients with good outcomes.
In contrast, when the tumor subgrouping was further defined on the basis of DCIS, there was a particularly marked difference in the MT-3 staining between patients with good and bad outcomes (Figure 3) ▶ . In DCIS, the MT-3 staining was significantly increased in patients with bad outcomes compared to those with good outcomes. The Bonferroni adjusted probability for this comparison was 0.039 with a power of 0.532 (N = 27). Because patients in the good prognostic group had a higher incidence of tumors that were MT-3-negative, the analysis was repeated using only the MT-3-positive tumors (N = 18). These results were even more conclusive, with an Bonferroni adjusted probability of 0.009 with a power of 0.632. Two-way correlation tables with MT-3 staining in DCIS demonstrated a Pearson’s χ 2 = 3.481 (α = 0.062), which improved in significance when MT-3 staining was subdivided into negative (quickscore = 0,1), weak (QS = 2,3) and positive (QS >3), Pearson’s χ 2 = 8.341, α = 0.015 (Table 1) ▶ . Two-way correlation tables were constructed to determine whether other features of DCIS would correlate with good or poor prognosis or the degree of MT-3 immunostaining. Using Pearson’s χ 2 testing, no significant correlations with the degree of MT-3 immunostaining were disclosed. Parameters examined included whether the DCIS involvement was extensive or focal, the primary histological pattern of the breast carcinoma (invasive ductal, invasive lobular, comedocarcinoma, adenosquamous, medullary, or mucinous), whether the histological pattern was uniform, or, if it was mixed with a secondary histological pattern, the histological type of the secondary pattern (infiltrating ductal or infiltrating lobular), the location of DCIS relative to invasive carcinoma (within, adjacent, or present in otherwise benign breast tissue), the nuclear grade of both the DCIS and invasive carcinoma, the presence of LCIS, nodal status at initial diagnosis, the hormone receptor status (ER, PR), and, when available, the ploidy analysis and HER-2/neu receptor status. The only positive correlation noted was that the presence of both DCIS and LCIS was more frequent in carcinomas that were MT-3-negative (Pearson’s χ2, α = 0.099). When corrected for ordered variables with the Goodman-Kruskal and Yule Q statistics, the significance rose to α = 0.018; however, due to the small sample size, the power analysis was only 0.460. The immunostaining of MT-3 in LCIS lesions demonstrated a similar trend as DCIS, with more intensity of staining in patients with bad outcomes, however, the occurrence of LCIS in this study sample was much lower than DCIS, with only 15 patients having LCIS, 12 of those with concomitant DCIS, thus precluding statistical analysis due to the small sample size. However, the DCIS findings and identical trend of LCIS immunostaining with MT-3 demonstrates that overexpression of MT-3 in CIS lesions of the breast is associated with tumors having a poor prognosis.
Table 1.
Patients with MT3 Immunostaining in DCIS
| Positive | Weak | Negative | |
|---|---|---|---|
| Good outcome | 3 | 6 | 6 |
| Bad outcome | 9 | 2 | 1 |
| Pearson χ2 | 8.341 | ||
| Probability | 0.015 |
A theoretical basis does exist to explain why MT-3 overexpression would be associated with breast cancers having a bad outcome. Central to this explanation is the hypothesis that the overexpression of MT-3 would produce an excess of metal-free MT (apoMT) and in so doing generate a zinc-deficient state. Although not present in normal cells, apoMT has been shown to have a widespread presence in animal tumors. 14 It has also been shown that the microinjection of apoMT into living cells is capable of removing zinc from the zinc finger DNA binding proteins, Sp-1, and transcription factor IIIA, 15-18 allowing one to hypothesize that the overexpression of MT-3 could cause the dysregulation of zinc finger transcription factors, as well as other zinc-requiring proteins, and in so doing affect cell growth, differentiation, and programmed cell death. Furthermore, apoMT, and the resulting zinc-depleted state of the cell, can be implicated in the generation of the genetic instability necessary for breast tumor progression by induction of a p53-null state. The inactivation of the transcriptional regulatory activity of p53 most frequently occurs due to alterations in the central region of the protein, which contains the sequence-specific DNA-binding activity, and this core portion of p53 folds into a compact structural domain with the assistance of a zinc atom. It has been shown that apoMT has the potential to remove and/or sequester zinc from p53 and inactivate it similarly to other zinc chelators. 19-21 The recent demonstration that MT-3 has the ability, in contrast to the other MT family members, to remain undegraded under zinc-deficient conditions would enhance the possibility that MT-3 overexpression could result in a cellular environment in which p53 and other zinc-requiring factors could be deprived of essential zinc. 22 Thus, the early overexpression of MT-3 in tumors may cause a zinc-limiting cellular environment that favors tumor progression.
Footnotes
Address reprint requests to Mary Ann Sens, M.D., Ph.D., Department of Pathology, West Virginia University, P.O. Box 9203, Morgantown, WV 26506-9203. E-mail: msens@hsc.wvu.edu.
Supported by National Institute of Environmental Health Sciences, NIH grant ES10039.
References
- 1.Hamer DH: Metallothionein. Annu Rev Biochem 1986, 55:913-951 [DOI] [PubMed] [Google Scholar]
- 2.Andrews GK: Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol 2000, 59:95-104 [DOI] [PubMed] [Google Scholar]
- 3.Kägi JHR, Hunziker P: Mammalian metallothionein. Biol Trace Element Res 1989, 21:111-118 [DOI] [PubMed] [Google Scholar]
- 4.Palmiter RD, Findley SD, Whitmore TE, Durnam DM: MT-III, a brain-specific member of the metallothionein gene family. Proc Natl Acad Sci USA 1992, 89:6333-6337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchida Y, Takio K, Titani K, Ihara Y, Tomonaga M: The growth inhibitory factor that is deficient in Alzheimer’s disease is a 68 amino acid metallothionein-like protein. Neuron 1991, 7:337-347 [DOI] [PubMed] [Google Scholar]
- 6.Amoureux C, Wurch T, Pauwels PJ: Modulation of metallothionein-III mRNA content and growth rate of rat C6-glial cells by transfection with human 5-HT1D receptor genes. Biochem Biophys Res Commun 1995, 214:639-645 [DOI] [PubMed] [Google Scholar]
- 7.Garrett SH, Sens MA, Todd JH, Somji S, Sens DA: Expression of MT-3 protein in the human kidney. Toxicol Lett 1999, 105:207-214 [DOI] [PubMed] [Google Scholar]
- 8.Sens MA, Somji S, Lamm DL, Garrett SH, Slovinsky F, Todd JH, Sens DA: Metallothionein isoform 3 as a potential biomarker for human bladder cancer. Environ Health Perspect 2000, 108:413-418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrett SH, Sens MA, Shukla D, Nestor S, Somji S, Todd JH, Sens DA: Metallothionein isoform 3 expression in the human prostate and cancer-derived cell lines. Prostate 1999, 41:196-202 [DOI] [PubMed] [Google Scholar]
- 10.Detre S, Jotti GS, Dowsett M: A quickscore method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol 1995, 48:876-878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrett SH, Sens MA, Shukla D, Flores L, Somji S, Todd JH, Sens DA: Metallothionein isoform 1 and 2 gene expression in the human prostate: down-regulation of MT-1X in advanced prostate cancer. Prostate 2000, 43:125-135 [DOI] [PubMed] [Google Scholar]
- 12.Hoey JG, Garrett SH, Sens MA, Todd JH, Sens DA: Expression of MT-3 mRNA in human kidney, proximal tubule cell cultures, and renal cell carcinoma. Toxicol Lett 1999, 92:149-160 [DOI] [PubMed] [Google Scholar]
- 13.Jasani B, Schmid KW: Significance of metallothionein overexpression in human tumours. Histopathology 1997, 31:211-214 [DOI] [PubMed] [Google Scholar]
- 14.Pattanaik A, Shaw CF, III, Petering DH, Garvey J, Kraker AJ: Basal metallothionein in tumors: widespread presence of apoprotein. J Inorg Biochem 1994, 54:91-105 [DOI] [PubMed] [Google Scholar]
- 15.Zeng J, Vallee BL, Kagi JHR: Zinc transfer from transcription factor IIIA fingers to thionein clusters. Proc Natl Acad Sci USA 1991, 88:9984-9988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng J, Heuchel R, Schaffner W, Kagi JHR: Thionein (apothionein) can modulate DNA binding and transcription activation by zinc finger containing factor SP1. FEBS Lett 1991, 279:310-312 [DOI] [PubMed] [Google Scholar]
- 17.Predki PF, Sarkar B: Metal replacement in zinc finger and its effect on DNA binding. Environ Health Perspec 1994, 102(S3):195-198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob C, Maret W, Vallee BL: Control of zinc transfer between thionein, metallothionein, and zinc proteins. Proc Natl Acad Sci USA 1998, 93:3489-3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hainaut R, Milner J: A structural role for metal ions in the wild type conformation of the tumor suppressor protein p53. Cancer Res 1993, 53:1739-1742 [PubMed] [Google Scholar]
- 20.Oren M, Prives C: p53: upstream, downstream and off stream-a review of the 8th p53 workshop (Dundee, July 5–9). Biochim Biophys Acta 1996, 1288:R13-R19 [DOI] [PubMed] [Google Scholar]
- 21.Méplan C, Richard M-J, Hainaut P: Metalloregulation of the tumor suppressor protein p53; zinc mediates renaturation of p53 after exposure to metal chelators in vitro and in intact cells. Oncogene 2000, 19:5227-5236 [DOI] [PubMed] [Google Scholar]
- 22.Palmiter RD: Constitutive expression of metallothionein-III (MT-III), but not MT-1, inhibits growth when cells become zinc deficient. Toxicol Appl Pharmacol 1995, 135:139-146 [DOI] [PubMed] [Google Scholar]