Abstract
CD40 is a protein on microglia that is up-regulated with interferon (IFN)-γ and is engaged by CD40L, found on CD4+ T cells, B cells, and monocytes. These interactions may be important in central nervous system inflammatory diseases. Microglia have been shown to be a source of chemokines, whose expression plays a key role in central nervous system pathologies. We examined the expression of CD40 on microglia in human immunodeficiency virus (HIV) encephalitic brain, and the effects of CD40-CD40L interactions on the expression of chemokines by cultured microglia. We found significantly increased numbers of CD40-positive microglia in HIV-infected brain tissue. Treatment of cultured microglia with IFN-γ and CD40L increased expression of several chemokines. IFN-γ- and CD40L-induced MCP-1 protein was mediated by activation of the ERK1/2 MAPK pathway, and Western blot analysis demonstrated phosphorylation of ERK1/2 upon stimulation of microglia. In contrast, IFN-γ- and CD40L-induced IP-10 protein production was mediated by the p38 MAPK pathway. Our data suggest a mechanism whereby CD40L+ cells can induce microglia to secrete chemokines, amplifying inflammatory processes seen in HIV encephalitis and multiple sclerosis, and implicate CD40-CD40L interactions as a target for interventional strategies.
CD40 is a phosphorylated 48-kd glycoprotein expressed on the surface of various cells including monocytes 1,2 and microglia. 3 CD40 is a member of the tumor necrosis factor (TNF) receptor superfamily that also includes TNFR1, TNFR2, and FAS (CD95). The receptor for CD40, CD40 ligand (CD40L), is expressed on several cell types, including activated CD4+ T cells 4,5 and monocytes/macrophages. 6 CD40-CD40L interactions were originally believed to be necessary specifically for B-cell isotype switching, 7 but are now known to play a more general role in immune regulation and inflammatory processes. 8
A role for CD40-CD40L interactions has been suggested for a variety of central nervous system (CNS) inflammatory models. CD40L knockout animals cannot be induced to develop experimental autoimmune encephalomyelitis (EAE), a T-cell-dependent autoimmune disease of the CNS used as an animal model for multiple sclerosis (MS). 9 Antibody to CD40L blocks the development of clinical disease progression and CNS inflammation in EAE. 9,10 CD40L+ cells have been detected in MS tissue by immunohistochemistry, and these co-localized with CD40+ cells of the monocytic/microglial lineage. 9
Activated T cells may enter the CNS under a variety of pathological conditions, including MS, 11-13 simian immunodeficiency virus, 14,15 and early HIV encephalitis. 16 These T cells secrete interferon (IFN)-γ, which is a mediator of a number of proinflammatory effects. It has been demonstrated that IFN-γ can up-regulate CD40 on a number of cell types, including mouse 17 and human 18 microglia in culture.
Chemokine production plays a major role in CNS inflammation. Chemokines are low-molecular weight cytokines that function in leukocyte recruitment as well as in cell activation. 19 The chemokines can be divided into different families based on the position of their N-terminal cysteine residues. The C-X-C family contains IFN-inducible protein (IP)-10 (CXCL10) among others, which is chemotactic for monocytes and activated T cells. 20 Members of the CC family include monocyte chemoattractant protein (MCP)-1 (CCL2), macrophage inflammatory protein (MIP)-1α (CCL3), MIP-1β (CCL4), and regulated upon activation, normal T-cell expressed and secreted (RANTES; CCL5), which also attract monocytes and activated T cells.
Microglia, the resident macrophages of the brain, are believed to function as the primary antigen-presenting cell of the CNS, 21 and have been shown to express chemokines. 22 Chemokines play an important role in CNS pathologies. Antibodies against MIP-1α inhibited adoptively transferred EAE and reduced inflammation in the CNS, whereas antibodies against MCP-1 inhibited relapses. 23 An increase in RANTES and IP-10 protein levels has been detected in the cerebrospinal fluid of MS patients. 24 Expression of several CC chemokines has been demonstrated within MS lesions, including MCP-1, MCP-2, MCP-3, 25 RANTES, 24 MIP-1α, and MIP-1β. 26 A role for chemokines in HIV encephalitis and HIV dementia has also been established. MCP-1, MIP-1α, and MIP-1β expressions have been detected in the CNS of individuals with HIV. 27,28
The importance of chemokines in the development of CNS pathologies led us to determine whether ligation of CD40 on microglia can induce these cells to secrete various chemotactic factors. In this study, we analyzed the expression of CD40 in HIV encephalitic brain tissue and the response of cultured microglia to CD40 ligation. We demonstrated up-regulation of CD40 expression in HIV-infected brains co-localized with CD68, a microglial marker. CD40 expression on cultured microglia was also up-regulated after treatment with IFN-γ. Treatment of cultured microglia with IFN-γ- and CD40L-induced expression of the chemokines MCP-1, IP-10, MIP-1α, MIP-1β, and RANTES. IFN-γ and CD40L induction of MCP-1 protein was mediated by the extracellular regulated kinase (ERK)1/2 mitogen-activated protein kinase (MAPK) pathway, whereas IP-10 protein induction was mediated via the p38 MAPK pathway. These results suggest a mechanism for the increase in chemokine production seen in the CNS in certain inflammatory diseases, such as MS and HIV encephalitis, and may indicate novel pathways for therapeutic intervention.
Materials and Methods
Cell Culture and Reagents
Human fetal CNS tissue (16 to 24 weeks) was obtained at the time of elective abortuses from healthy females. The tissue was used as part of an ongoing research protocol approved by the Albert Einstein College of Medicine. Microglia were established according to a modified protocol. 29 Briefly, the meninges were removed; the tissue was minced and shaken for 45 minutes at 37°C in 1× Hepes-buffered salt solution (Life Technologies, Inc., Baltimore, MD), 1× trypsin-ethylenediaminetetraacetic acid (Boehringer-Mannheim, Indianapolis, IN) and DNaseI (Life Technologies, Inc.). The slurry was passed through a 250-μm nylon mesh filter followed by a 150-μm filter, washed once with Hepes-buffered salt solution, and then with complete Dulbecco’s modified Eagle’s medium (DMEM plus 25 mmol/L Hepes, 10% fetal calf serum, 1% penicillin-streptomycin, 1% nonessential amino acids). Cells were resuspended in complete DMEM, seeded at 9 × 10 7 per 150-cm 2 flask, and maintained at 5% CO2, 37°C for 12 days. After this time, the media (containing microglia) was removed and centrifuged for 5 minutes at 220 × g. The microglia were resuspended in complete DMEM and seeded at 5 × 10 5 per well of a 24-well plate. The media was changed after 6 to 8 hours. Twenty-four hours after plating, microglia were treated with 200 μl of DMEM without fetal calf serum containing either 100 U/ml IFN-γ (R&D Systems, Minneapolis, MN), 5 μg/ml soluble trimeric human CD40 ligand (a generous gift from Immunex Corporation, Seattle, WA), both IFN-γ and CD40L, or left untreated for 24 and 48 hours. For inhibitor studies, microglia were pretreated with 10 or 30 μmol/L of the ERK1/2 MAPK inhibitor, PD98059 30 (Sigma-Aldrich, St. Louis, MO) or 5, 10, or 20 μmol/L of the p38 MAPK inhibitor, SB203580 31 (Sigma-Aldrich) for 1 hour, followed by treatment with IFN-γ and CD40L for 24 or 48 hours. Supernatants were then collected.
Immunohistochemistry
Immunohistochemical studies were performed on brain tissue taken at autopsy. Six patients with HIV, HIV encephalitis, or HIV dementia were studied. Three control brains with non-CNS pathologies were included (Table 1) ▶ . The post mortem interval for all cases was between 12 and 24 hours, with the exception of case 2, which was 37 hours. Paraffin-embedded tissue was dehydrated and deparaffinized. After rehydration, the sections were placed in 10 mmol/L of sodium citrate at 95°C for 20 minutes. Sections were cooled and washed in Tris-buffered saline, quenched in 0.8% H2O2 in methanol, incubated in 2% normal horse serum/Tris-buffered saline (Vector Laboratories, Burlingame, CA) for 1 hour at 37°C, and incubated overnight at 4°C in primary antibody (mouse anti-human CD68, KP1, 0.82 μg/ml; DAKO, Carpinteria, CA) or an isotype-matched antibody (mouse IgG1; Cappel, Los Angeles, CA). The sections were washed, incubated with a biotinylated secondary antibody (1:750, Vector Laboratories), followed by incubation in avidin-biotin complex (Vector Laboratories). Slides were developed with 3′3′-diaminobenzidine (Sigma-Aldrich) to give a brown reaction product. Slides were then requenched and reblocked in 2% normal goat serum/1% bovine serum albumin/Tris-buffered saline. Sections were incubated overnight at 4°C with primary antibody (rabbit anti-human CD40, 1 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA), or with rabbit IgG (Santa Cruz Biotechnology). Sections were washed and incubated with peroxidase-coupled goat anti-rabbit secondary antibody (1:500; Southern Biotechnology Associates, Pittsburgh, PA). Slides were developed with Vector-VIP (Vector Laboratories), which results in a purple reaction product, dehydrated, and mounted with Cytoseal (VWR Scientific, Willard, OH).
Table 1.
Pathological Analysis of Brain Tissue Taken at Autopsy Used for Immunohistochemical Study
| Case | HIV status | Age/gender | Brain pathology | Cause of death |
|---|---|---|---|---|
| 1 | − | 52 yr/F | Normal brain | Cardiac arrest |
| 2 | − | 22 yr/F | Normal brain | Sickle cell disease |
| 3 | − | 13 yr/F | Normal brain | Osteogenic sarcoma |
| 4 | + | 23 mo/F | HIVE,* CST† degeneration, microglial nodules | AIDS-associated pneumonia |
| 5 | + | 20 mo/F | HIV, CST degeneration, large cell lymphoma | AIDS-associated pneumonia |
| 6 | + | 9 yr/F | AIDS, septic shock | Endstage AIDS |
| 7 | + | 22 yr/F | HIVE, sepsis | Endstage AIDS |
| 8 | + | 40 wk/M | AIDS, HIV leukoencephalopathy | Endstage AIDS |
| 9 | + | 6 yr/M | HIVE, microglial nodules | AIDS-associated pneumonia |
*HIV encephalitis.
†Corticospinal tract.
Fluorescence-Activated Cell Sorting Analysis
Microglia were plated at 1 × 10 6 cells/100-mm dish for 36 hours, and were either left untreated or treated with IFN-γ (100 U/ml). Microglia were washed once in Hepes-buffered salt solution, once in 0.5 μmol/L of ethylenediaminetetraacetic acid/phosphate-buffered saline (PBS), and were detached with 0.5 μmol/L of ethylenediaminetetraacetic acid/PBS. Microglia were collected and centrifuged for 5 minutes at 220 × g at 4°C. Microglia were then incubated for 30 minutes on ice with primary antibody (mouse anti-human CD40, 0.25 μg/10 6 cells; Santa Cruz Biotechnology) or an isotype-matched negative control antibody (mouse IgG1, 0.25 μg/10 6 cells; Cappel). Cells were washed with block buffer (2% horse serum/1% bovine serum albumin/0.1% NaN3/PBS) and incubated with a biotinylated secondary antibody (1:750, Vector Laboratories) for 30 minutes on ice, washed, and incubated with StreptAvidin-conjugated CyChrome (1:50, PharMingen, San Diego, CA) for an additional 30 minutes, covered, on ice. After a final wash, the cells were transferred to fluorescence-activated cell sorting tubes and analyzed with a FACScan Flow Cytometer (Becton Dickinson, San Jose, CA) using WinMDI 2.8 software (Scripps Research Institute, La Jolla, CA).
Chemokine Enzyme-Linked Immunosorbent Assay
Supernatants were analyzed for chemokine proteins using a sandwich enzyme-linked immunosorbent assay according to the manufacturer’s protocol. MCP-1, MIP-1α, MIP-1β, and RANTES enzyme-linked immunosorbent assay antibody pairs were purchased from R&D Systems. The antibody pairs for IP-10 were from Pharmingen. The sensitivities for these assays are 4 pg/ml, 8 pg/ml, 1 pg/ml, 3 pg/ml, and 5 pg/ml, respectively.
RNA Extraction and Analysis
Microglia were plated at 1 × 10 6 cells/100-mm dish for 12 hours. Total RNA was extracted using Tri-Reagent (Molecular Research Center, Cincinnati, OH). Chemokine mRNA expression was analyzed using the human chemokine ribonuclease protection assay kit hCK5 from Pharmingen. Densitometry was performed using Ambis QuantProbe software, with values normalized to glyceraldehyde-3-phosphate dehydrogenase.
Western Blot Analysis
Microglia were plated at 5 × 10 5 cells/well of a 24-well plate. Cells were pretreated with or without inhibitor for 1 hour, after which some cells were left untreated, and others were treated with IFN-γ and CD40L for 10 minutes. Microglia were washed and lysed (62.5 mmol/L Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate, 10% glycerol, 50 mmol/L dithiothreitol, 0.1% bromophenol blue, PA, 10 mmol/L, protease inhibitor cocktail 50 μl/ml, okadaic acid, 1:5000, 10 μl/ml; Sigma-Aldrich). The slurry was passed through an 18-gauge needle five times and heated at 95°C for 5 minutes. Equal amounts of whole-cell lysates (15 μl) were loaded onto each lane of a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel. Proteins were transferred electrophoretically to Protran nitrocellulose (Schleicher & Schuell, Keene, NH). Membranes were blocked with 5% nonfat dry milk in 0.1% Tween-20/Tris-buffered saline and incubated with primary antibodies (p44/42 MAP kinase Ab, phospho-p44/42 MAP kinase Ab, p38 MAP kinase Ab, phospho-p38 MAP kinase Ab; Cell Signaling, Beverly, MA) at a concentration of 1:1000 overnight at 4°C. After washing, membranes were incubated with anti-rabbit horseradish peroxidase secondary antibody (1:2000, Cell Signaling) for 1 hour at room temperature. Proteins were visualized using an enhanced chemiluminescence detection kit (ECL; Amersham-Pharmacia, Piscataway, NJ). Densitometry was performed using Ambis QuantProbe software, with values normalized to total protein levels.
Statistical Analysis
The paired Student’s t-test (one-tailed) was used to determine statistical significance. A value of P < 0.05 was considered to be significant.
Results
CD40/CD68 Double-Positive Cells Are Increased in Sections of HIV-Infected Brain Tissue
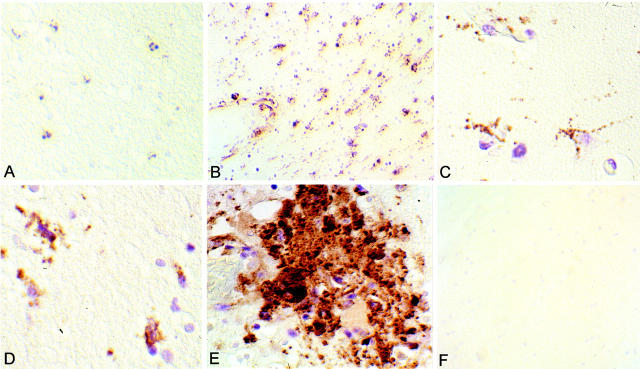
Sections of normal brain and HIV-infected brain were analyzed for reactivity with antibodies to CD40 as well as CD68, a marker of cells of the monocyte/macrophage lineage that includes microglia, using immunohistochemistry (Table 1) ▶ . There was some reactivity with both reagents in the three normal brains tested (Figure 1A ▶ , case 2). It is not possible to distinguish microglia from monocytes/macrophages on the basis of surface markers. However, activated microglia have been identified by their enlarged cell bodies and stout processes, 32 as well as by their location within the cortex. 33-35 HIV-infected CNS tissue is characterized by a large number of activated microglia. 36 This activation was apparent in all six of the HIV-infected brains analyzed. All HIV-infected tissues had significant double staining with CD40 and CD68. An example of this reactivity is illustrated (Figure 1B ▶ , case 9). The CD68-positive cells with the phenotype of activated microglia (brown) were reactive and were also double-stained for CD40 (purple). CD40+/CD68+ microglia are illustrated in two other HIV-infected brain sections (Figure 1C ▶ , case 6; Figure 1D ▶ , case 4). As shown, the CD40 staining was localized to the surface of the cell body, whereas the CD68 staining was punctate and localized to the processes. One of the hallmarks of HIV infection is the microglial nodule. Staining of the nodule demonstrated that there were many double-labeled microglia within it (Figure 1E ▶ , case 9). There was little background or nonspecific reactivity with isotype-matched negative control antibodies in the HIV-infected brain (Figure 1F ▶ , case 9).
Figure 1.
HIV infection increases the number of CD40/CD68 double-positive cells. CD68 is visualized with 3′3′-diaminobenzidine (brown) and CD40 is visualized with Vector VIP (purple). A: Normal brain with little CD68 or CD40 staining (original magnification, ×82.5; case 2). B: Reactive microglia in an HIV-infected brain (original magnification, ×82.5; case 9). There are numerous double-labeled cells. C and D: Reactive microglia staining for CD68 on their processes and for CD40 on their cell bodies (original magnifications, ×165; C is case 6 and D is case 4). E: Prominent staining seen in a microglia nodule, which is a hallmark of HIV (original magnification, ×165; case 9). There is abundant CD68 reactivity and many of these cells are also staining intensely for CD40. B to E demonstrate increased expression of CD40 on microglia after HIV infection when compared to A, which is a normal brain. F: Reactivity of isotype-matched control antibodies for CD40 and CD68 (original magnification, ×82.5; case 9). There is little background or nonspecific staining with either antibody.
IFN-γ Up-Regulates CD40 Expression on Cultured Human Fetal Microglia
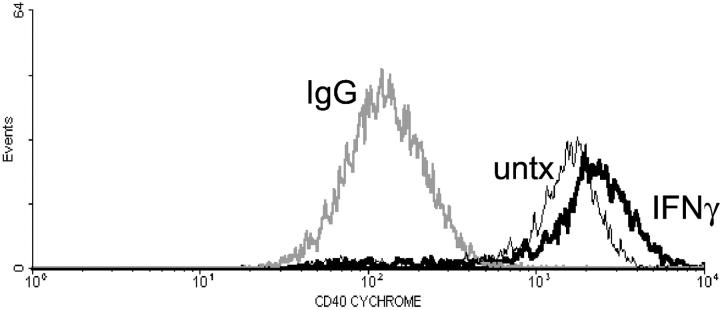
Others have demonstrated that IFN-γ can up-regulate CD40 on both mouse 17,37-39 and human 18 microglia in culture, therefore, we determined whether IFN-γ would also up-regulate CD40 expression on microglia isolated using our methods and tissue source. Microglia were either left untreated or treated with IFN-γ for 36 hours after which CD40 expression was detected by flow cytometry. There was constitutive expression of CD40 on untreated microglia (Figure 2 ▶ , thin black line), compared to IgG-stained microglia (Figure 2 ▶ , light line). After treatment with IFN-γ, CD40 expression was up-regulated on cultured microglia (Figure 2 ▶ , thick black line).
Figure 2.
IFN-γ up-regulates CD40 expression of human fetal microglia in culture. CD40 expression was analyzed on untreated microglia, and microglia treated with 100 U/ml IFN-γ for 36 hours. Shown is a representative histogram with cell number (y axis) versus log fluorescence intensity (x axis). Positive (CD40-expressing) cells are displayed. Fluorescence-activated cell sorting analysis was repeated three times with similar results. Light line, IgG stained; thin black line, untreated microglia stained with CD40; thick black line, IFN-γ-treated microglia stained with CD40.
IFN-γ and CD40L Induce Chemokine Protein Secretion by Microglia
We analyzed purified human microglial cells for their expression of chemokines in response to CD40L. IFN-γ and CD40L stimulation of microglial cultures induced protein secretion of MCP-1, IP-10, MIP-1α, MIP-1β, and RANTES (Figure 3, a to e) ▶ . The IFN-γ- and CD40L-induced increase in chemokine secretion is significant at both the 24-hour treatment compared to untreated, IFN-γ treatment alone, and CD40L treatment alone (P < 0.04) and the 48-hour treatment compared to untreated, IFN-γ treatment alone, and CD40L treatment alone (P < 0.04).
Figure 3.
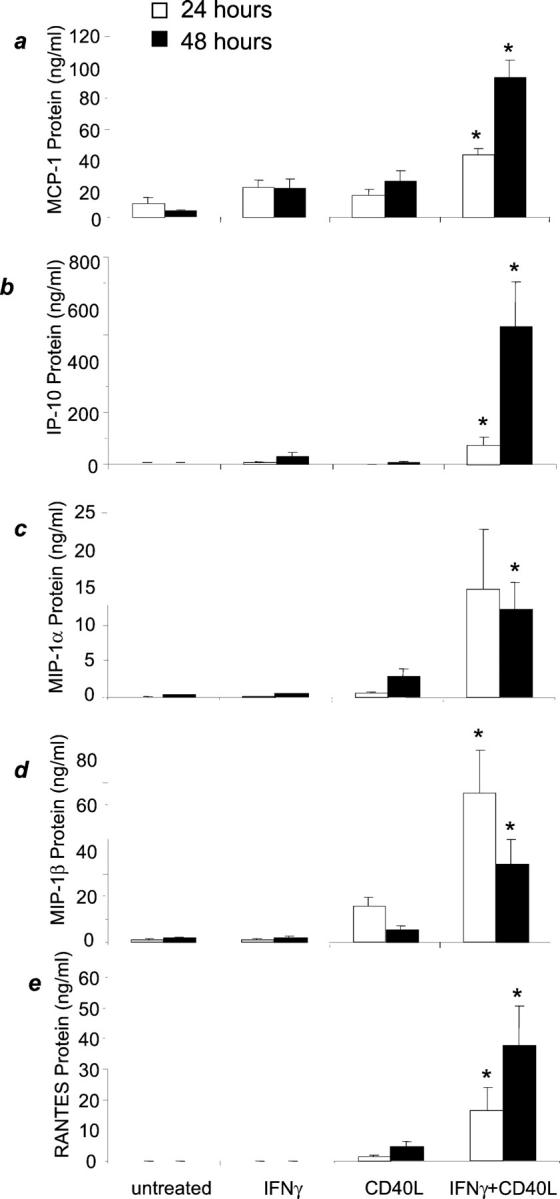
IFN-γ plus CD40L induces chemokine protein secretion by microglia. Microglia were incubated with media alone, IFN-γ, CD40L, or both IFN-γ and CD40L. Graphs represent the means of four to six experiments ± SEM. a: MCP-1 protein was induced by IFN-γ and CD40L after 24 hours and 48 hours. b: IP-10 protein was induced by IFN-γ and CD40L after 24 hours and 48 hours. c: MIP-1α protein was significantly induced by IFN-γ and CD40L only after 48 hours. d: MIP-1β protein was induced by IFN-γ and CD40L after 24 hours and 48 hours. e: RANTES protein was induced by IFN-γ and CD40L after 24 hours and 48 hours. *, P < 0.04 compared to untreated, IFN-γ treatment alone or CD40L treatments alone.
IFN-γ and CD40L Treatment Induce Chemokine mRNA
We analyzed microglial chemokine mRNA expression by ribonuclease protection assay after a 12-hour treatment with IFN-γ, CD40L, or IFN-γ and CD40L treatment (Figure 4a) ▶ . The densitometric analysis of these data shows that IFN-γ and CD40L treatment increases MCP-1, IP-10, MIP-1α, MIP-1β, and RANTES mRNA expression compared to untreated, IFN-γ treatment, or CD40L treatment alone (Figure 4b) ▶ .
Figure 4.
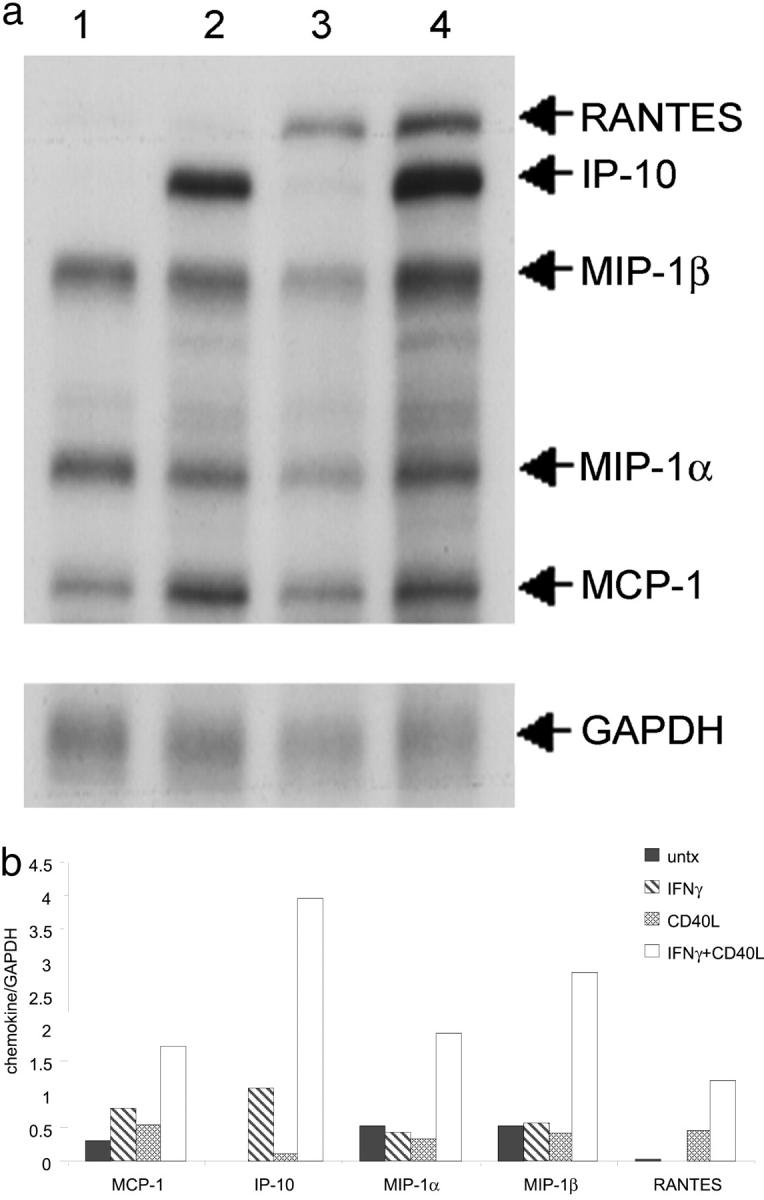
IFN-γ plus CD40L induces chemokine mRNA expression from microglia. a: Microglia were incubated with media alone (lane 1), IFN-γ (lane 2), CD40L (lane 3), or both IFN-γ and CD40L (lane 4). b: Densitometric analysis confirms that IFN-γ and CD40L treatment increases RNA expression for every chemokine tested. Shown is a representative ribonuclease protection assay of two experiments. Filled bars, untreated; hatched bars, IFN-γ treated; stippled bars, CD40L treated; open bars, IFN-γ and CD40L treated.
MCP-1 Induction by IFN-γ and CD40L Is Regulated through the ERK1/2 MAPK Pathway
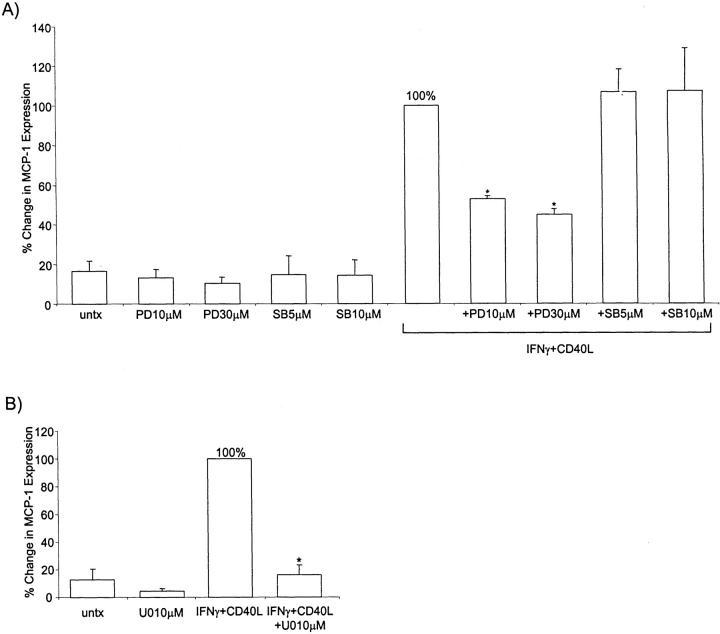
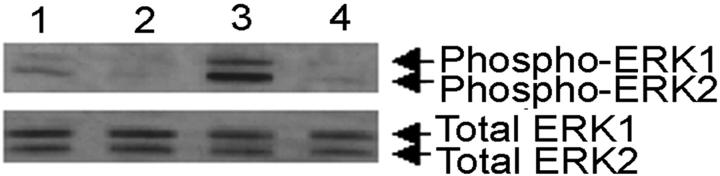
MCP-1 protein expression induced by IFN-γ and CD40L (24 hours) was significantly decreased (P < 0.0001) by the ERK1/2 MAPK pathway inhibitor, PD98059 at both 10 μmol/L and 30 μmol/L (Figure 5A) ▶ . The inhibitor alone had no effect on microglial MCP-1 expression compared to untreated cells. To confirm that MCP-1 protein production was occurring through the ERK1/2 MAPK pathway, microglia were pretreated with the highly specific ERK1/2 MAPK inhibitor, U0126. U0126 treatment at 10 μmol/L significantly reduced (P < 0.0001) IFN-γ- and CD40L-induced MCP-1 protein production, bringing the levels of MCP-1 to baseline (Figure 5B) ▶ . Pretreatment with the p38 MAPK inhibitor, SB203580, did not significantly change MCP-1 protein expression as compared to IFN-γ and CD40L treatment. We analyzed phosphorylated ERK1/2 levels by Western blot (Figure 6) ▶ . Densitometric analysis demonstrated that IFN-γ and CD40L treatment induced phosphorylation of ERK1/2 after 10 minutes, and pretreatment with PD98059 at 30 μmol/L abrogated this induction (data not shown).
Figure 5.
MCP-1 protein expression is ERK 1/2 MAPK-dependent. MCP-1 protein expression was determined as percent change compared to IFN-γ and CD40L (set to 100%). A: MCP-1 protein expression after treatment with either the ERK1/2 MAPK inhibitor or the p38 MAPK inhibitor for 24 hours. Shown are the means of five separate experiments ± SEM. B: MCP-1 protein expression after treatment with the ERK1/2 MAPK inhibitor U0126 for 24 hours. Shown are the means of four separate experiments. *, P < 0.0001 compared to IFN-γ and CD40L. PD10, PD30 = PD98059 at 10 μm and 30 μmol/L, respectively; SB5, SB10 = SB203580 at 5 μmol/L and 10 μmol/L, respectively; U010 = U0126 at 10 μmol/L.
Figure 6.
IFN-γ and CD40L induces phosphorylation of ERK1/2, and PD98059 pretreatment abrogates phosphorylation. Western blot analysis demonstrates the phosphorylation of ERK1/2 (top) and total ERK1/2 expression (bottom). Shown is a representative blot of three experiments. Lane 1,untreated; lane 2, untreated plus PD30; lane 3, IFN-γ and CD40L; lane 4, IFN-γ and CD40L plus PD30. PD30 = PD98059 at 30 μmol/L.
IP-10 Induction by IFN-γ and CD40L Is Regulated through the p38 MAPK Pathway
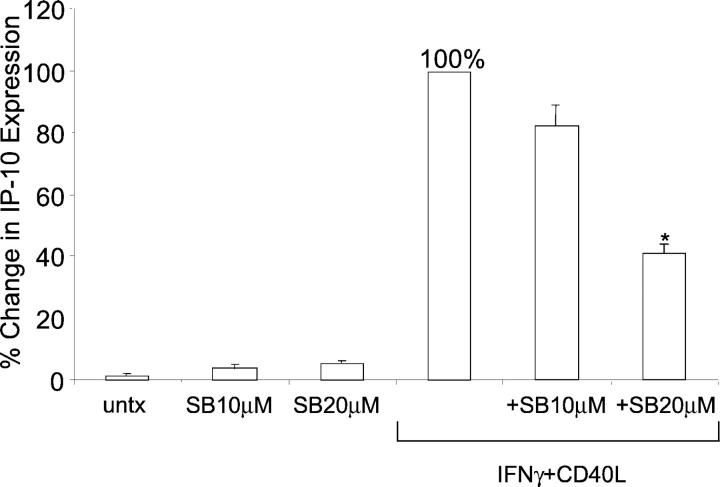
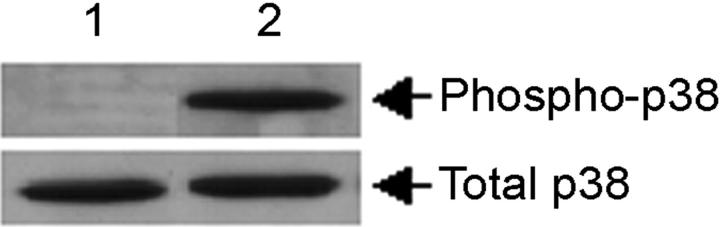
IP-10 protein expression was analyzed at 24 hours after pretreatment with both PD98059 and the p38 MAPK inhibitor, SB203580. No difference in IP-10 protein expression by microglia was detected when pretreated with PD98059 at either 10 μmol/L or 30 μmol/L, but there was a small decrease in IP-10 protein expression when pretreated with SB203580 (data not shown). Therefore, these experiments were repeated for an expanded time frame of 48 hours. Pretreatment of microglia at 10 μmol/L modestly inhibited IP-10 protein production compared to IFN-γ and CD40L, and pretreatment with 20 μmol/L of the inhibitor significantly decreased (P < 0.001) IP-10 protein expression compared to IFN-γ and CD40L alone (Figure 7) ▶ . The inhibitor alone had no effect on microglial IP-10 expression compared to untreated cells. Phosphorylation of p38 after treatment with IFN-γ and CD40L was analyzed by Western blot (Figure 8) ▶ . Densitometric analysis demonstrated that IFN-γ and CD40L treatment induced phosphorylation of p38 after 10 minutes compared to untreated cultures (data not shown).
Figure 7.
IP-10 protein expression is p38 MAPK-dependent. IP-10 protein expression was determined as percent change compared to IFN-γ and CD40L (set to 100%). IP-10 protein expression was examined after pretreatment with the p38 MAPK inhibitor for 1 hour, and then treatment with IFN-γ and CD40L for 48 hours. Shown are the means of two separate experiments ± SEM. *, P < 0.001 compared to IFN-γ and CD40L. SB10, SB20 = SB203580 at 10 μmol/L and 20 μmol/L, respectively.
Figure 8.
IFN-γ and CD40L induces phosphorylation of p38. Western blot analysis demonstrates the phosphorylation of p38 (top) and total p38 expression (bottom) at 10 minutes. Shown is a representative blot of two experiments. Lane 1, untreated; lane 2, IFN-γ and CD40L. PD30 = PD98059 at 30 μmol/L.
Discussion
We studied the expression of CD40 in HIV encephalitic brains as well as the effect of CD40 ligation in cultured primary human fetal microglia using recombinant human CD40L. We showed by immunohistochemistry that there is an abundance of CD40/CD68 double-positive cells. These cells stain brightly in HIV-infected brains as compared to normal brains that show minimal reactivity. Using cultured microglia we demonstrated an increase in CD40 expression after treatment with IFN-γ, a product of activated T cells. We also demonstrated that when microglia were co-treated with IFN-γ and CD40L, chemokine expression was increased at both 24 hours and 48 hours. Our immunohistochemical and chemokine data demonstrate that as microglia become activated, they increase their expression of CD40. This would facilitate their interaction with infiltrating CD40L-positive leukocytes, promoting the secretion of chemokines by microglia and further infiltration of leukocytes, thereby amplifying the inflammatory processes characteristic of HIV encephalitis and MS.
In several CNS pathologies there is an abundance of inflammation resulting from the transmigration of monocytes/macrophages and T cells across the blood-brain barrier. 40 In MS, SIV encephalitis, as well as early HIV encephalitis, one of the cells that enters the CNS is the activated T cell that is able to secrete IFN-γ. In fact, in individuals infected with HIV, there is an increased number of CD8+CD28−T cells, and these cells have been demonstrated to secrete IFN-γ. 41 Others have demonstrated that IFN-γ can up-regulate CD40 on various cell types, and infiltrating T cells are the major source of IFN-γ in the CNS. CD40L is expressed on T cells and monocytes, and CD40 is expressed on microglial cells and astrocytes. 42 HIV-infected patients on highly active antiretrovial therapy (HAART) have higher counts of CD40L-expressing CD4+ T cells 43 and many exhibit an abundance of macrophages within the CNS, suggesting the possibility that these cells could interact with microglia via CD40. Our data are the first to our knowledge to demonstrate expression of CD40 in HIV-infected CNS tissue.
The role of CD40-CD40L interactions in the development of EAE has been studied extensively. Treatment of mice with anti-CD40L antibodies blocked or attenuated the development of EAE. CD40L knockout mice expressing a myelin basic protein-specific transgene could not be induced to develop EAE, whereas the wild-type transgenic animals did develop disease. 44 CD40 was shown to be expressed in the CNS of individuals with MS. CD40L+ cells were found in active MS lesions that co-localized with CD40+ cells, and these CD40+ cells were of the monocytic lineage (macrophages and microglia). 9
Chemokines play an important role in CNS inflammatory disease. MCP-1 expression has been demonstrated in individuals infected with HIV. The HIV transactivator protein, Tat, has been shown to induce MCP-1 expression in astrocytes and microglial cells in tissue culture 28 and MCP-1 expression has been found in both brain tissue 28,45 and cerebrospinal fluid 27 of patients with HIV encephalitis or dementia. MCP-1 has also been shown to be important in EAE and MS. There is expression of MCP-1 at the beginning of acute EAE, 46,47 as well as MCP-1 and IP-10 expression during spontaneous relapse of the disease. 47,48 Anti-MCP-1 antibodies were able to reduce significantly relapses of EAE, 23 and mice deficient for CCR2, the receptor for MCP-1, were resistant to EAE induction. 49 MCP-1 has also been found in active MS lesions from autopsied brains. 25 It has been shown that there is an increased amount of IP-10 in the cerebrospinal fluid of MS patients during active attacks and IP-10 has also been shown to be present in active MS lesions. 50 Thus, both MCP-1 and IP-10 are important chemokines in CNS pathologies.
Chemokines may play multiple roles in the pathogenesis of HIV encephalitis. They can recruit inflammatory cells into the CNS, thereby facilitating the entry of HIV-infected cells, as well as amplifying the inflammatory response. Yet they may also act on resident cells within the CNS to inhibit further infection. For example, certain strains of HIV use CCR5 as a co-receptor for entry into cells. 51-55 The ligands for this receptor, MIP-1α, MIP-1β, and RANTES, have been shown to suppress HIV infection. 56 MIP-1α and MIP-1β mRNA 57 and proteins 28,45,58 were found to be expressed in glial cells in the CNS of individuals with HIV dementia or encephalitis and not in normal brains. MIP-1α and MIP-1β elevation was also demonstrated in the macaque model of SIV encephalitis, along with increased RANTES and IP-10. 59,60 Our data that CD40L induces MIP-1α, MIP-1β, and RANTES from microglia suggest that CD40-CD40L interactions within the CNS could also serve a protective, as well as a proinflammatory, role during HIV infection of the brain.
Ligation of mouse microglial CD40 results in activation of ERK1/2 MAPK. 37 We demonstrated that IFN-γ- and CD40L-induced MCP-1 protein secretion by human microglia is dependent on ERK1/2 activation. Inhibition of the upstream activator of ERK1/2 MAPK with PD98059 and U0126 decreased MCP-1 secretion, and PD98059 pretreatment inhibited ERK1/2 phosphorylation. Interestingly, IP-10 signaling differed from that for MCP-1 in that it is dependent on p38 MAPK. The specific inhibitor for the p38 MAPK pathway, SB203580, partially blocked IFN-γ- and CD40L-induced IP-10 secretion, whereas the ERK1/2 inhibitor had no effect. A role for p38 signaling after CD40 ligation of dendritic cells and B cells has also been shown. 61 Thus, CD40 seems to induce a variety of signaling pathways.
Our data suggest a mechanism by which infiltration of inflammatory cells into the CNS can induce microglia to secrete chemoattractants. These chemokines may serve to recruit additional cells into the CNS as well as to activate resident cells, thereby contributing to CNS pathology. These findings suggest that CD40-CD40L interactions may serve as targets for therapeutic intervention.
Acknowledgments
We thank Dr. Tina Calderon, Dr. James Martiney, and Mr. Harry Ma for their critical reading of this manuscript; the Fetal Tissue Repository at the Albert Einstein College of Medicine; Dr. Brad Poulos for providing tissue; and Immunex Corporation for their generous gift of the human CD40L.
Footnotes
Address reprint requests to Joan W. Berman, Albert Einstein College of Medicine, Department of Pathology, Forchheimer 727, 1300 Morris Park Ave., Bronx, NY 10461. E-mail: berman@aecom.yu.edu.
Supported by the National Institutes of Mental Health grant MH52974 (to J. W. B.), National Institutes of Health grant NS11920 (to J. W. B.), and the National Institutes of Health Experimental Neuropathology Training grant T32NS07098 (to T. G. D.).
References
- 1.Alderson MR, Armitage RJ, Tough TW, Strockbine L, Fanslow WC, Spriggs MK: CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med 1993, 178:669-674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stout RD, Suttles J, Xu J, Grewal IS, Flavell RA: Impaired T cell-mediated macrophage activation in CD40 ligand-deficient mice. J Immunol 1996, 156:8-11 [PubMed] [Google Scholar]
- 3.Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, Anderson DM, Gimpel SD, Davis-Smith T, Maliszewski CR, Clark EA, Smith CA, Grabstein KH, Cosman D, Spriggs MK: Molecular and biological characterization of a murine ligand for CD40. Nature 1992, 357:80-82 [DOI] [PubMed] [Google Scholar]
- 4.Armitage RJ, Sato TA, Macduff BM, Clifford KN, Alpert AR, Smith CA, Fanslow WC: Identification of a source of biologically active CD40 ligand. Eur J Immunol 1992, 22:2071-2076 [DOI] [PubMed] [Google Scholar]
- 5.Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A: A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci USA 1992, 89:6550-6554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mach F, Schonbeck U, Sukhova GK, Bourcier T, Bonnefoy JY, Pober JS, Libby P: Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci USA 1997, 94:1931-1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipsky PE, Attrep JF, Grammer AC, McIlraith MJ, Nishioka Y: Analysis of CD40-CD40 ligand interactions in the regulation of human B cell function. Ann N Y Acad Sci 1997, 815:372-383 [DOI] [PubMed] [Google Scholar]
- 8.van Kooten C, Banchereau J: Functional role of CD40 and its ligand. Int Arch Allergy Immunol 1997, 113:393-399 [DOI] [PubMed] [Google Scholar]
- 9.Gerritse K, Laman JD, Noelle RJ, Aruffo A, Ledbetter JA, Boersma WJ, Claassen E: CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA 1996, 93:2499-2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howard LM, Miga AJ, Vanderlugt CL, Dal Canto MC, Laman JD, Noelle RJ, Miller SD: Mechanisms of immunotherapeutic intervention by anti-CD40L (CD154) antibody in an animal model of multiple sclerosis. J Clin Invest 1999, 103:281-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paterson PY, Day ED: Current perspectives of neuroimmunologic disease: multiple sclerosis and experimental allergic encephalomyelitis (1,2). Clin Immunol Rev 1981, 1:581-697 [PubMed] [Google Scholar]
- 12.Traugott U, Raine CS: Further lymphocyte characterization in the central nervous system in multiple sclerosis. Ann N Y Acad Sci 1984, 436:163-180 [DOI] [PubMed] [Google Scholar]
- 13.Traugott U: Characterization and distribution of lymphocyte subpopulations in multiple sclerosis plaques versus autoimmune demyelinating lesions. Springer Semin Immunopathol 1985, 8:71-95 [DOI] [PubMed] [Google Scholar]
- 14.Lackner AA, Smith MO, Munn RJ, Martfeld DJ, Gardner MB, Marx PA, Dandekar S: Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am J Pathol 1991, 139:609-621 [PMC free article] [PubMed] [Google Scholar]
- 15.Lackner AA, Vogel P, Ramos RA, Kluge JD, Marthas M: Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIVmac1A11) molecular clones of simian immunodeficiency virus. Am J Pathol 1994, 145:428-439 [PMC free article] [PubMed] [Google Scholar]
- 16.Weidenheim KM, Epshteyn I, Lyman WD: Immunocytochemical identification of T-cells in HIV-1 encephalitis: implications for pathogenesis of CNS disease. Mod Pathol 1993, 6:167-174 [PubMed] [Google Scholar]
- 17.Nguyen VT, Walker WS, Benveniste EN: Post-transcriptional inhibition of CD40 gene expression in microglia by transforming growth factor-beta. Eur J Immunol 1998, 28:2537-2548 [DOI] [PubMed] [Google Scholar]
- 18.Becher B, Blain M, Antel JP: CD40 engagement stimulates IL-12 p70 production by human microglial cells: basis for Th1 polarization in the CNS. J Neuroimmunol 2000, 102:44-50 [DOI] [PubMed] [Google Scholar]
- 19.Rollins BJ: Chemokines. Blood 1997, 90:909-928 [PubMed] [Google Scholar]
- 20.Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ: Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med 1993, 177:1809-1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreutzberg GW: Microglia: a sensor for pathological events in the CNS. Trends Neurosci 1996, 19:312-318 [DOI] [PubMed] [Google Scholar]
- 22.McManus CM, Brosnan CF, Berman JW: Cytokine induction of MIP-1 alpha and MIP-1 beta in human fetal microglia. J Immunol 1998, 160:1449-1455 [PubMed] [Google Scholar]
- 23.Karpus WJ, Kennedy KJ: MIP-1alpha and MCP-1 differentially regulate acute and relapsing autoimmune encephalomyelitis as well as Th1/Th2 lymphocyte differentiation. J Leukoc Biol 1997, 62:681-687 [PubMed] [Google Scholar]
- 24.Sorensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, Qin S, Rottman J, Sellebjerg F, Strieter RM, Frederiksen JL, Ransohoff RM: Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest 1999, 103:807-815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McManus C, Berman JW, Brett FM, Staunton H, Farrell M, Brosnan CF: MCP-1, MCP-2 and MCP-3 expression in multiple sclerosis lesions: an immunohistochemical and in situ hybridization study. J Neuroimmunol 1998, 86:20-29 [DOI] [PubMed] [Google Scholar]
- 26.Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN: Expression of monocyte chemoattractant protein-1 and other beta-chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J Neuroimmunol 1998, 84:238-249 [DOI] [PubMed] [Google Scholar]
- 27.Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO: Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA 1998, 95:3117-3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McManus CM, Weidenheim K, Woodman SE, Nunez J, Hesselgesser J, Nath A, Berman JW: Chemokine and chemokine-receptor expression in human glial elements: induction by the HIV protein, Tat, and chemokine autoregulation. Am J Pathol 2000, 156:1441-1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarthy KD, de Vellis J: Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol 1980, 85:890-902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR: PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem 1995, 270:27489-27494 [DOI] [PubMed] [Google Scholar]
- 31.Tong L, Pav S, White DM, Rogers S, Crane KM, Cywin CL, Brown ML, Pargellis CA: A highly specific inhibitor of human p38 MAP kinase binds in the ATP pocket. Nat Struct Biol 1997, 4:311-316 [DOI] [PubMed] [Google Scholar]
- 32.Kato H, Walz W: The initiation of the microglial response. Brain Pathol 2000, 10:137-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hickey WF, Vass K, Lassmann H: Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J Neuropathol Exp Neurol 1992, 51:246-256 [DOI] [PubMed] [Google Scholar]
- 34.Hickey WF, Kimura H: Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science 1988, 239:290-292 [DOI] [PubMed] [Google Scholar]
- 35.Lassmann H, Schmied M, Vass K, Hickey WF: Bone marrow derived elements and resident microglia in brain inflammation. Glia 1993, 7:19-24 [DOI] [PubMed] [Google Scholar]
- 36.Adle-Biassette H, Chretien F, Wingertsmann L, Hery C, Ereau T, Scaravilli F, Tardieu M, Gray F: Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol 1999, 25:123-133 [DOI] [PubMed] [Google Scholar]
- 37.Tan J, Town T, Paris D, Placzek A, Parker T, Crawford F, Yu H, Humphrey J, Mullan M: Activation of microglial cells by the CD40 pathway: relevance to multiple sclerosis. J Neuroimmunol 1999, 97:77-85 [DOI] [PubMed] [Google Scholar]
- 38.Becher B, Blain M, Antel JP: CD40 engagement stimulates IL-12 p70 production by human microglial cells: basis for Th1 polarization in the CNS. J Neuroimmunol 2000, 102:44-50 [DOI] [PubMed] [Google Scholar]
- 39.Nguyen VT, Walker WS, Benveniste EN: Post-transcriptional inhibition of CD40 gene expression in microglia by transforming growth factor-beta. Eur J Immunol 1998, 28:2537-2548 [DOI] [PubMed] [Google Scholar]
- 40.Dickson DW, Lee SC, Mattiace LA, Yen SH, Brosnan C: Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer’s disease. Glia 1993, 7:75-83 [DOI] [PubMed] [Google Scholar]
- 41.Eylar EH, Lefranc C, Baez I, Colon-Martinez SL, Yamamura Y, Rodriguez N, Yano N, Breithaupt TB: Enhanced interferon-gamma by CD8+ CD28− lymphocytes from HIV+ patients. J Clin Immunol 2001, 21:135-144 [DOI] [PubMed] [Google Scholar]
- 42.Abdel-Haq N, Hao HN, Lyman WD: Cytokine regulation of CD40 expression in fetal human astrocyte cultures. J Neuroimmunol 1999, 101:7-14 [DOI] [PubMed] [Google Scholar]
- 43.Sousa AE, Chaves AF, Doroana M, Antunes F, Victorino RM: Early reduction of the over-expression of CD40L, OX40 and Fas on T cells in HIV-1 infection during triple anti-retroviral therapy: possible implications for lymphocyte traffic and functional recovery. Clin Exp Immunol 1999, 116:307-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grewal IS, Foellmer HG, Grewal KD, Xu J, Hardardottir F, Baron JL, Janeway CA, Flavell RA: Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science 1996, 273:1864-1867 [DOI] [PubMed] [Google Scholar]
- 45.Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL: Chemokines and receptors in HIV encephalitis. Aids 1998, 12:1021-1026 [PubMed] [Google Scholar]
- 46.Berman JW, Guida MP, Warren J, Amat J, Brosnan CF: Localization of monocyte chemoattractant peptide-1 expression in the central nervous system in experimental autoimmune encephalomyelitis and trauma in the rat. J Immunol 1996, 156:3017-3023 [PubMed] [Google Scholar]
- 47.Ransohoff RM, Hamilton TA, Tani M, Stoler MH, Shick HE, Major JA, Estes ML, Thomas DM, Tuohy VK: Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J 1993, 7:592-600 [DOI] [PubMed] [Google Scholar]
- 48.Glabinski AR, Tani M, Strieter RM, Tuohy VK, Ransohoff RM: Synchronous synthesis of alpha- and beta-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of chronic experimental autoimmune encephalomyelitis. Am J Pathol 1997, 150:617-630 [PMC free article] [PubMed] [Google Scholar]
- 49.Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ: CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med 2000, 192:899-906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balashov KE, Rottman JB, Weiner HL, Hancock WW: CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA 1999, 96:6873-6878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA: CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 1996, 272:1955-1958 [DOI] [PubMed] [Google Scholar]
- 52.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J: The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 1996, 85:1135-1148 [DOI] [PubMed] [Google Scholar]
- 53.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR: Identification of a major co-receptor for primary isolates of HIV-1. Nature 1996, 381:661-666 [DOI] [PubMed] [Google Scholar]
- 54.Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC, Parmentier M, Collman RG, Doms RW: A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 1996, 85:1149-1158 [DOI] [PubMed] [Google Scholar]
- 55.Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA: HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 1996, 381:667-673 [DOI] [PubMed] [Google Scholar]
- 56.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P: Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995, 270:1811-1815 [DOI] [PubMed] [Google Scholar]
- 57.Schmidtmayerova H, Nottet HS, Nuovo G, Raabe T, Flanagan CR, Dubrovsky L, Gendelman HE, Cerami A, Bukrinsky M, Sherry B: Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci USA 1996, 93:700-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonwetsch R, Croul S, Richardson MW, Lorenzana C, Valle LD, Sverstiuk AE, Amini S, Morgello S, Khalili K, Rappaport J: Role of HIV-1 Tat and CC chemokine MIP-1alpha in the pathogenesis of HIV associated central nervous system disorders. J Neurovirol 1999, 5:685-694 [DOI] [PubMed] [Google Scholar]
- 59.Westmoreland SV, Rottman JB, Williams KC, Lackner AA, Sasseville VG: Chemokine receptor expression on resident and inflammatory cells in the brain of macaques with simian immunodeficiency virus encephalitis. Am J Pathol 1998, 152:659-665 [PMC free article] [PubMed] [Google Scholar]
- 60.Sasseville VG, Smith MM, Mackay CR, Pauley DR, Mansfield KG, Ringler DJ, Lackner AA: Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am J Pathol 1996, 149:1459-1467 [PMC free article] [PubMed] [Google Scholar]
- 61.Aicher A, Shu GL, Magaletti D, Mulvania T, Pezzutto A, Craxton A, Clark EA: Differential role for p38 mitogen-activated protein kinase in regulating CD40-induced gene expression in dendritic cells and B cells. J Immunol 1999, 163:5786-5795 [PubMed] [Google Scholar]