Abstract
Lipoxins (LXs) are endogenously produced eicosanoids that inhibit neutrophil trafficking and stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. In this study we assessed the effect of LXs on cell ultrastructure and actin reorganization in human leukocytes and investigated the signaling events that subserve LX bioactivity in this context. LXA4 (10−9 mol/L), the stable synthetic analogues 15-(R/S)-methyl-LXA4 and 16-phenoxy-LXA4 (10−11 mol/L), but not the LX precursor 15-(S)-HETE, induced marked changes in ultrastructure and rearrangement of actin in monocytes and macrophages. In contrast, LXA4 did not modify actin distribution in neutrophils under basal conditions and after stimulation with leukotriene B4. Blockade of Rho kinases by the inhibitor Y-27632 prevented LXA4-triggered actin reorganization in macrophages. To investigate the role of the specific small GTPases in LX-induced actin rearrangement we used THP-1 cells differentiated to a macrophage-like phenotype. THP-1 cells stimulated with LXs, but not with 15-(S)-HETE, showed an increase in membrane-associated RhoA and Rac by immunoblotting. Additionally, a twofold increase in Rho activity was seen in response to LXA4. LX-induced actin rearrangement and RhoA activation were inhibited by the cell permeable cAMP analogue 8-Br-cAMP, whereas Rp-cAMP, an inhibitor of protein kinase A, mimicked the effect of LXA4. These data demonstrate that LXs stimulate RhoA- and Rac-dependent cytoskeleton reorganization, contributing to the potential role of LXs in the resolution of inflammation.
Lipoxins (lipoxygenase interaction products, LXs) are trihydroxytetraene-containing eicosanoids generated via biosynthetic pathways catalyzed initially by the sequential actions of two lipoxygenases (LO), either 5-LO and 15-LO or 5-LO and 12- LO, on arachidonic acid. 1,2 In a cytokine-primed milieu, aspirin acetylation of cyclooxygenase type 2 switches the catalytic activity of the enzyme from a prostaglandin endoperoxide synthase to an R-LO that initiates the biosynthesis of the 15-epimer LXs (aspirin-triggered LXs, ATL) that share many of the bioactions of the nonepimeric forms. 3 The rapid metabolic degradation of the native LXs has prompted the development of stable analogues that retain the biological activity of the native compounds. 4
Because of their effects on modulation of leukocyte trafficking, LXs have been proposed to act as braking signals in inflammation. 5 With respect to polymorphonuclear neutrophils (PMNs), LXs do not influence cell motility or adhesiveness under basal conditions, but are potent inhibitors of chemotaxis, adhesion to endothelium, and transmigration across endothelial cell and epithelial cell monolayers induced by leukotriene B4 (LTB4) and other chemoattractants in vitro. 6-9
In contrast to their inhibitory effects on PMN function, LXs are potent activators of monocytes, stimulating their chemotaxis and adherence without causing degranulation or release of reactive species. 10,11 We recently reported that LXs also rapidly stimulate the nonphlogistic phagocytosis of apoptotic PMNs by monocyte-derived macrophages (Mφ), both in vitro and in vivo (Mitchell et al, manuscript submitted). 12 Such phagocytic clearance without the provocation of an inflammation response plays an essential role in the resolution of inflammation. 13 In addition to the clearance of apoptotic cells and their potential noxious contents, such phagocytosis is associated with the production of quiescing intermediates such as transforming growth factor-β that actively suppress cytokine release. 14 Thus LXs are not only braking signals for PMN-mediated inflammation 5 but also actively promote the resolution of inflammatory processes on monocytes and Mφ.
Multiple cell surface molecules have been implicated in the recognition of apoptotic cells by phagocytic Mφ. 13,15,16 We have demonstrated that LX-stimulated phagocytosis may modulate several of these including CD36 and CD51/61 integrins. 12 Remodeling of the actin cytoskeleton is a prerequisite for all phagocytic processes. 17 Such cytoskeletal rearrangement may reflect a response to the phagocytic target.
In this article we have examined the effects of LXs on actin cytoskeleton rearrangement in monocyte/Mφ and PMNs. We report that the native LXA4 and the stable analogues 15-(R/S)-methyl-LXA4 and 16-phenoxy-LXA4 induce changes in the actin cytoskeleton in monocytes and in Mφ, but not in PMNs and that these changes correlate to differential activation of the small GTPases RhoA and Rac.
Materials and Methods
Materials
5S,6R,15S-trihydroxy-7,9,13-trans-11-cis-eicosatetraenoic acid (LXA4), LTB4, and (5Z,8Z,11Z,13E,15(S))-15-hydroxyeicosatetraenoic acid (15-(S)-HETE) were purchased from Cascade Biochem Ltd. (Berkshire, UK). Synthetic analogues 15(R/S)-methyl-LXA4 (5S,6R,15R/S-trihydroxy-15-methyl-7,9,13-trans-11-cis-eicosatetraenoic acid methyl ester) and 16-phenoxy-LXA4 (15S−16-phenoxy-17,18,19,20-tetranor-LXA4 methyl ester) were prepared by total organic synthesis as previously described. 4 The anti-RhoA, anti-Rac2, and anti-Cdc42 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The compounds Rp-cAMP and LY294002 were purchased from Calbiochem, San Diego, CA; 8-bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP) was obtained from Sigma (St. Louis, MO). The cDNA for the GTPase-binding domain (RBD) from Rhotekin was a gift from Dr. Joan Heller Brown (Dept. of Pharmacology, University of California, San Diego, CA). Reagents were dissolved in dimethyl sulfoxide or ethanol and further diluted in medium (final concentration, 0.1%) or phosphate-buffered saline (PBS) as appropriate. Equivalent concentrations of dimethyl sulfoxide or ethanol, used as vehicle in controls, had no effects in our experiments.
Isolation of Peripheral Blood Monocytes and Cell Culture
Human PMNs and monocytes were isolated from peripheral venous blood drawn from healthy volunteers, after informed written consent in accordance with institutional ethical guidelines (Mater Misericordiae Hospital, Dublin, Ireland). PMNs and mononuclear cells were separated by centrifugation on Ficoll-Paque (Pharmacia, Uppsala, Sweden). PMNs, after dextran sedimentation (Dextran T500, Pharmacia) and hypotonic lysis of red cells, were suspended in PBS (Bio-Whittaker, Walkersville, MD) at 2 × 106 cells/ml. Monocytes were isolated from the mononuclear cell fraction. To eliminate platelet contamination, mononuclear cells were washed twice with PBS containing 5 mmol/L of ethylenediaminetetraacetic acid. Cells were then resuspended at 2 × 106 cells/ml in RPMI 1640 supplemented with 10% autologous serum, 2 mmol/L glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin (Life Technologies, Inc., Grand Island, NY). Cells were placed on 18-mm glass coverslips for fluorescence studies or in Lab-Tek chamber slides (Nunc, Naperville, IL) for electron microscopy studies. Monocytes were allowed to attach for 2 hours at 37°C in a 5% CO2 humidified atmosphere and nonadherent cells were removed by two washes with RPMI 1640. Mφ were obtained by culturing monocytes for 7 days in RPMI 1640 supplemented with 10% autologous serum, 2 mmol/L glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin.
The human myelomonocytic cell line THP-1 (European Collection of Cell Cultures, Salisbury, UK) was maintained in suspension in RPMI 1640 supplemented with 2 mmol/L glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum (Life Technologies, Inc.). THP-1 cells at 2 × 106/ml were differentiated to a Mφ-like phenotype by treatment with phorbol 12-myristate, 13-acetate (PMA) (16 nmol/L, Sigma) for 48 hours in 12- or 6-well plates (Costar, Cambridge, MA).
Electron Microscopy
Monocytes or Mφ were treated with vehicle or LXA4 (10−9 mol/L) for 15 minutes at 37°C and washed twice with PBS. PMNs were treated with vehicle or LXA4 (10−9 mol/L) for 15 minutes at 37°C followed by further treatment with vehicle or LTB4 (10−7 mol/L) for 30 seconds at 37°C. All samples were fixed in 2.5% glutaraldehyde in PBS, pH 7.4, postfixed in 1% osmium tetroxide and embedded in Epon using standard methods. The plastic of the Lab-Tek chamber was removed from the polymerized Epon to expose the basal region of the cells and sections to a depth of 5 μ were discarded. Thus podosomal regions of cells were excluded before 50-nm ultrathin sections were taken for analysis. Sections were stained with uranyl acetate and lead citrate and examined in a JEOL 2000EX microscope (Tokyo, Japan) using an acceleration potential of 80 kV and objective aperture of 20 μ.
Actin Staining
Adherent monocytes or Mφ on glass coverslips were incubated with the appropriate stimuli (LXA4, 10−9 mol/L; 15-(R/S)-methyl-LXA4, 10−11 mol/L; 16-phenoxy-LXA4, 10−11 mol/L; 15-(S)-HETE, 10−9 mol/L) for 15 minutes at 37°C. 8-Br-cAMP (2 mmol/L), Rp-cAMP (100 μmol/L), and LY294002 (10 μmol/L) were co-incubated with vehicle or LXA4 (10−9 mol/L) for 15 minutes at 37°C. Mφ were pretreated with Y-27632 (10 μmol/L) for 30 minutes at 37°C before exposure to LXA4 (15 minutes at 37°C). At the end of the incubations, cells were rinsed with PBS and fixed in 3.8% paraformaldehyde-PBS for 20 minutes at room temperature.
PMNs were incubated with LXA4 (10−9 mol/L) or vehicle for 15 minutes at 37°C before the addition of LTB4 (10−7 mol/L) or the appropriate vehicle for 10, 30, 60, or 300 seconds at 37°C. Cells were fixed in 3.8% paraformaldehyde-PBS for 20 minutes at room temperature and spread on poly-l-lysine-coated glass slides.
Cells were permeabilized with 0.1% Triton X-100 in PBS for 5 minutes and stained with Oregon Green-phalloidin (0.33 μmol/L; Molecular Probes, Eugene, OR) for 30 minutes at room temperature. Coverslips were mounted on microscope glass slides with Probing Antifade medium (Molecular Probes). Cells were viewed on a Leica fluorescent microscope and photographed with Ektachrome 400 film. Images of the cells were recorded at focal planes that excluded cell/substrate interfaces.
Western Blotting
Differentiated THP-1 cells were incubated with the different LXs or with 15-(S)-HETE for 15 minutes at 37°C. The reaction was stopped by washing twice with ice-cold PBS and cells were scraped with ice-cold lysis buffer (20 mmol/L HEPES, 2 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L MgCl2) containing 50 μl/ml of a protease inhibitor cocktail [2 mmol/L 4-(2-aminoethyl) benzenesulfonyl fluoride, 1 mmol/L ethylenediaminetetraacetic acid, 130 μmol/L bestatin, 1.4 μmol/L trans-epoxysuccinyl-l-leucyl-amido(4-guanidino)butane (E64), 1 μmol/L leupeptin, 0.3 μmol/L aprotinin; Sigma]. Cells were incubated for 20 minutes at 4°C, homogenized and centrifuged at 22,000 × g for 60 minutes at 4°C. The supernatants were retained as cytosolic fractions and the membrane pellets were resuspended in lysis buffer and Laemmli sample buffer. Samples from the same number of cells (1 × 106 cells) were resolved by electrophoresis on a 12% sodium dodecyl sulfate polyacrylamide gel and transferred to Immobilon-P polyvinylidene difluoride membranes (Millipore, Bedford, MA). The membranes were incubated with blocking buffer [Tris-buffered saline (20 mmol/L Tris, 137 mmol/L NaCl) containing 0.1% Tween-20 (TBS-T) and 5% milk] before probing with either 1 μg/ml of monoclonal anti-RhoA antibody, rabbit anti-Rac2, or anti-Cdc42 antibodies in TBS-T. After incubation with a horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibody as appropriate (1/2000 in TBS-T; New England Biolabs, Beverly, MA), bound antibody was visualized with an enhanced chemiluminescence detection system (Santa Cruz Biotechnology) and blots were quantified by densitometry.
PMNs stimulated with the chemoattractant fMLP (1 μmol/L for 30 seconds) were used as positive controls for Cdc42 Western blotting.
RhoA Activity Assay
For expression and purification of GST-RBD (Rho binding domain of Rhotekin), 18 bacteria expressing GST-RBD were cultured to the log phase and protein expression induced on incubation with 0.5 mmol/L of isopropyl-β-d-thiogalactopyranoside for 2 hours. Proteins were purified on glutathione-coupled Sepharose 4B beads (Pharmacia). After six washes in washing buffer (50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 5 mmol/L MgCl2, 0.5% Triton X-100, 1 mmol/L dithiothreitol, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 0.1 mmol/L phenylmethyl sulfonyl fluoride), the beads were resuspended in washing buffer supplemented with 10% glycerol, aliquoted, and stored at −80°C. The purity and the concentration of the preparation were determined by Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gels by comparison to bovine serum albumin standards.
Differentiated THP-1 cells (4 × 106 cells), after exposure to LXA4 (10−9 mol/L) or vehicle for 15 minutes at 37°C, were washed with ice-cold PBS and lysed in cold lysis buffer (50 mmol/L Tris, pH 7.2, 0.5% Triton X-100, 150 mmol/L NaCl, 5 mmol/L MgCl2) containing 1 mmol/L of phenylmethyl sulfonyl fluoride and 10 μg/ml each of leupeptin and aprotinin. Cell lysates were centrifuged at 13,000 × g for 10 minutes at 4°C. Twenty μg of bacterially expressed GST-RBD fusion protein bound to glutathione-coupled Sepharose 4B beads were incubated with equal volumes of lysates for 45 minutes at 4°C. The beads and protein bound to the fusion protein were washed four times with ice-cold lysis buffer containing 0.1 mmol/L of phenylmethyl sulfonyl fluoride and 10 μg/ml each of leupeptin and aprotinin. The bead pellets were resuspended in Laemmli sample buffer. Bound and total Rho proteins were detected by Western blotting with an antibody against RhoA as described above.
Results
LXs Induce Ultrastructural Changes and Actin Reorganization in Human Monocytes and Mφ, But Not in PMNs
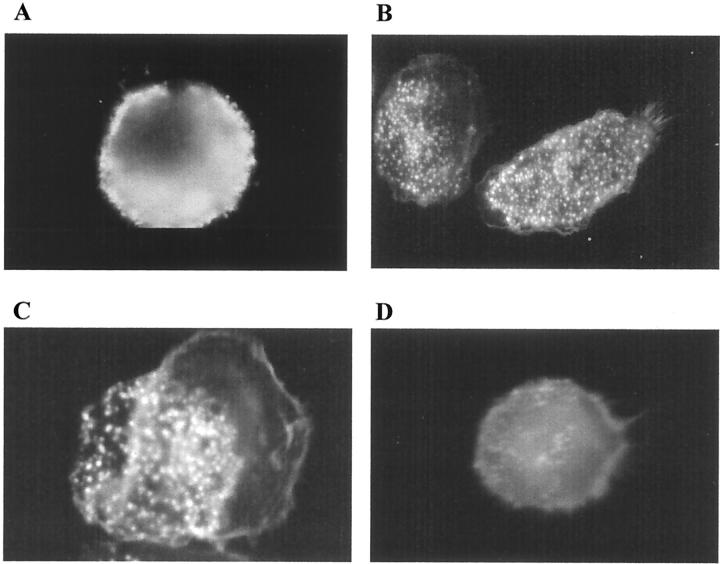
To evaluate the effect of LXs on monocytes and Mφ, cells were exposed to LXA4 (10−9 mol/L) or vehicle for 15 minutes at 37°C and F-actin staining and cellular ultrastructure were investigated. In control monocytes, the staining of F-actin was homogenous with an even distribution of actin at the level of the surface of the cell (Figure 1A) ▶ . Stimulation with LXA4 induced a shape change with the formation of lamellipodia and focal intensities across the cytoplasm (Figure 1B) ▶ . These morphological changes were confirmed by electron microscopy. Control monocytes had smooth cellular surfaces with few short pseudopodia (Figure 1C) ▶ . LXA4-stimulated monocytes showed a profusion of elongated pseudopodia and cell organelles peripherally distributed (Figure 1D) ▶ .
Figure 1.
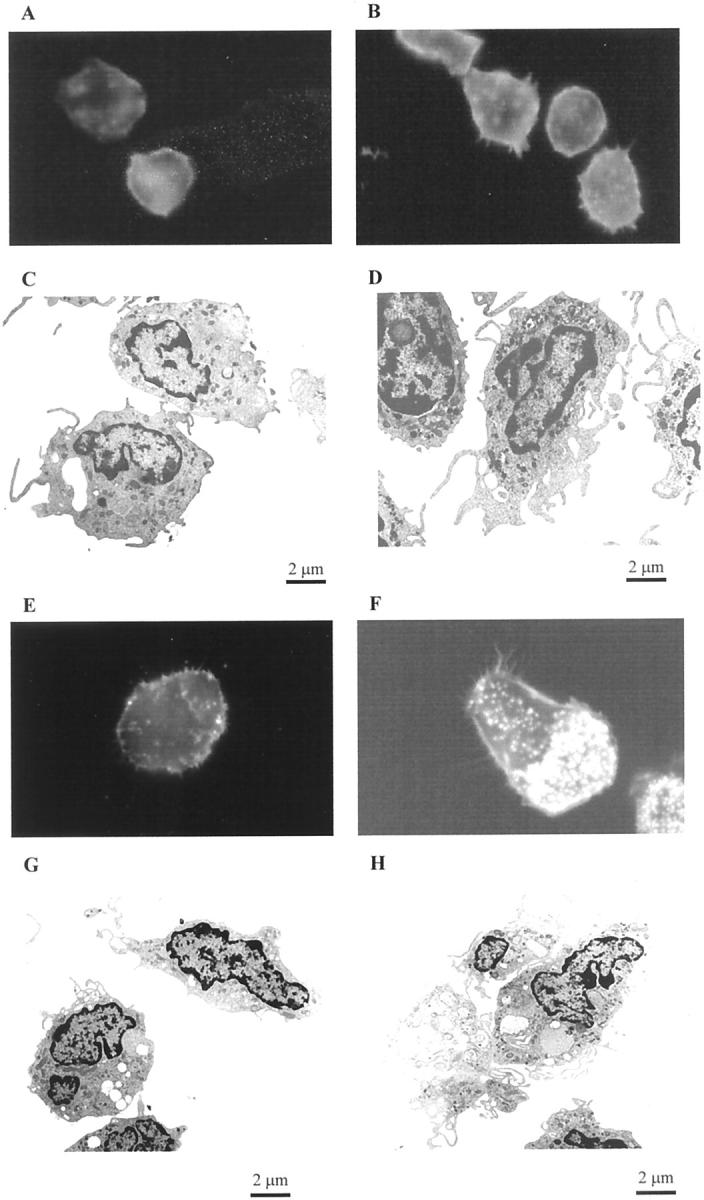
Effects of LXA4 on ultrastructure and actin reorganization in human monocytes and monocyte-derived macrophages. Adherent monocytes (A–D) or monocyte-derived macrophages (E–H) were treated with vehicle (A, C, E, G) or with LXA4 (10−9 mol/L) (B, D, F, H) for 15 minutes at 37°C. Cells were fixed with glutaraldehyde and osmium tetroxide for electron microscopy and with paraformaldehyde for fluorescence microscopy. Localization of actin was determined using Oregon Green phalloidin and visualized by fluorescence microscopy using a ×100 oil objective. The electron microscopy images were all taken at the same magnification. These images are representative of several fields derived from two independent experiments for electron microscopy and of five for fluorescence microscopy.
Adherent monocytes were cultured in medium for 7 days to obtain mature Mφ. The latter were more heterogeneous in shape compared to monocytes, however, ∼70% of the control Mφ showed a spherical shape with a diffuse distribution of actin and discrete extensions (Figure 1E) ▶ . After LXA4 treatment, the cells appeared more polarized and presented extensive filopodia and lamellipodia, with punctate F-actin staining on the apical surface, representative of filopodia (Figure 1F) ▶ . Electron microscopy of control Mφ showed smooth-surfaced cells almost bereft of pseudopodia (Figure 1G) ▶ , whereas LXA4-stimulated Mφ exhibited innumerable filamentous membrane protrusions of filopodia and a broad membrane-structure characteristic of lamellipodia (Figure 1H) ▶ .
LXs are rapidly inactivated by monocytes, the major route of degradation being the formation of the inactive metabolites 15-oxo-LXA4, 13,14-dihydro-15-oxo-LXA4, and 13,14-dihydro-LXA4. 19 15-(R/S)-methyl-LXA4 and 16-phenoxy-LXA4 are synthetic stable LXA4 analogues that resist to the metabolic inactivation and retain many of the anti-inflammatory activities of the native compound with respect to PMNs and Mφ including stimulation of Mφ phagocytosis of apoptotic PMNs. 4,12 In the present study, both analogues (10−11 mol/L, 15 minutes at 37°C) stimulated actin reorganization in monocytes (data not shown) and Mφ (Figure 2, B and C) ▶ . In contrast, when Mφ were exposed to the LXA4 precursor, 15-(S)-HETE (Figure 2D) ▶ the actin distribution was similar to that seen in unstimulated control cells (Figure 2A) ▶ .
Figure 2.
Effects of stable analogues of LXA4 and 15-(S)-HETE on actin reorganization in human monocyte-derived macrophages. Mφ were exposed to vehicle (A), 15-(R/S)-methyl-LXA4 (10−11 mol/L) (B), 16-phenoxy-LXA4 (10−11 mol/L) (C), or 15-(S)-HETE (10−9 mol/L) (D) for 15 minutes at 37°C. Cells were fixed with paraformaldehyde and localization of actin was determined using Oregon Green phalloidin and visualized by fluorescence microscopy using a ×100 oil objective. These images are representative of several fields derived from one experiment of five independent experiments.
Given the evidence that LXs stimulate the functions of monocytes and Mφ, but inhibit many of the bioactivities of PMNs, it was of interest to investigate the effects of LXs on PMN ultrastructure and actin rearrangement. PMNs were exposed to LXA4 (10−9 mol/L) or appropriate vehicle (0.1% ethanol) for 15 minutes before the addition of LTB4 (10−7 mol/L) for different times (10, 30, 60, 300 seconds). Control PMNs were round with actin filaments homogeneously distributed through the cytoplasm and a minor enrichment at the cell periphery (Figure 3A) ▶ and electron microscopy revealed cells with few pseudopodia (Figure 3B) ▶ . After 30 seconds of exposure to LTB4, PMNs showed accumulation of F-actin on the margin of the cells (Figure 3C) ▶ and a large increase in the number of pseudopodia (Figure 3D) ▶ . LXA4 did not affect actin rearrangement in PMNs (Figure 3E) ▶ , an observation confirmed by electron microscopy that showed PMNs greatly resembling control cells (Figure 3F) ▶ . In addition, LXA4 did not inhibit the actin reorganization (Figure 3G) ▶ and the ultrastructural changes (Figure 3H) ▶ induced by LTB4. Similar results were observed for all of the incubation times (data not shown).
Figure 3.
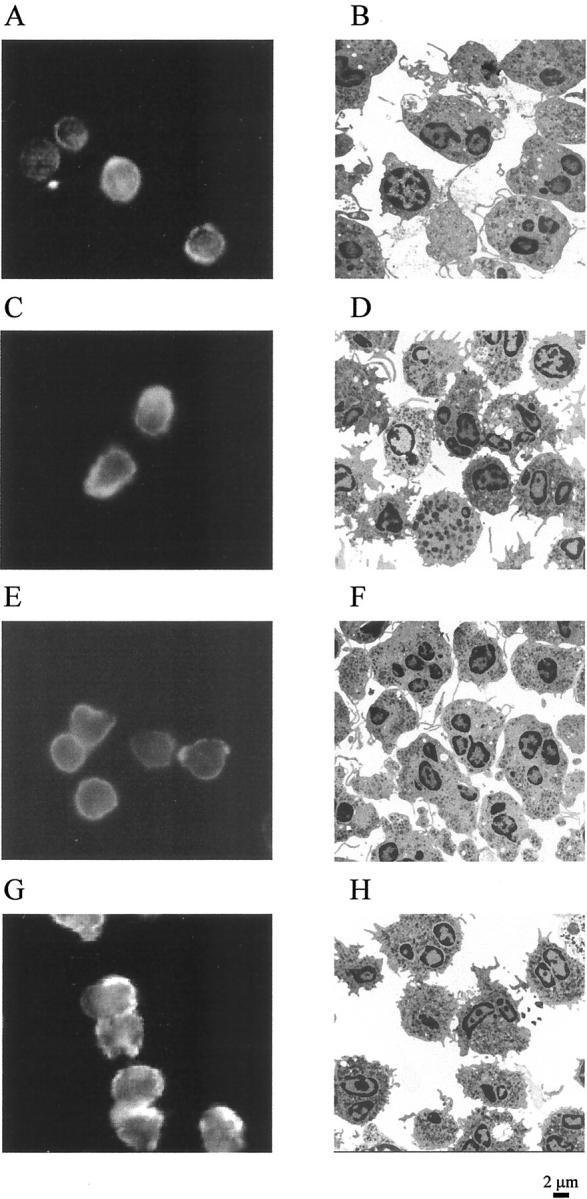
Effects of LXA4 on actin reorganization in human PMNs. Human PMNs (2 × 106 cells/ml) were treated with vehicle (A and B) or LXA4 (10−9 mol/L) (E and F) for 15 minutes at 37°C before the addition of LTB4 (10−7 mol/L) for 30 seconds at 37°C [vehicle + LTB4 (C and D); LXA4 + LTB4 (G and H)]. At the end of the incubation time, cells were fixed with 3.8% paraformaldehyde-PBS for 20 minutes at room temperature and spread on poly-l-lysine-coated glass slides for fluorescence studies or with glutaraldehyde for electron microscopy. Localization of actin was determined using Oregon Green phalloidin and visualized by fluorescence microscopy using a ×100 oil objective. The electron microscopy images were all taken at the same magnification. These images are representative of several fields derived from two independent experiments for electron microscopy and of three for fluorescence microscopy.
Cell Signaling Pathways Involved in LXA4-Induced Actin Reorganization
We have previously demonstrated that LX-stimulated phagocytosis was modulated by cAMP. 12 In the present study, the involvement of cAMP in LXA4-triggered actin reorganization in Mφ was studied using both a stable analogue of cAMP, 8-Br-cAMP to mimic elevation of intracellular levels of cAMP and Rp-cAMP, a cAMP-dependent protein kinase (PKA) inhibitor. Incubation of Mφ for 15 minutes with 8-Br-cAMP (2 mmol/L) did not result in significant changes in F-actin distribution (Figure 4A) ▶ . Co-incubation of 8-Br-cAMP and LXA4 resulted in an inhibition of LXA4-induced actin reorganization, leading to round cells with incomplete development of cytoskeletal extensions (Figure 4B) ▶ . In contrast, Rp-cAMP (100 μmol/L, 15 minutes) mimicked the effect of LXA4 on actin cytoskeleton, increasing the accumulation of F-actin throughout the cytoplasm (Figure 4C) ▶ . Co-incubation of Mφ with Rp-cAMP and LXA4 induced similar effects on F-actin distribution as LXA4 alone (Figure 4D ▶ compared to Figure 1F ▶ ). These data parallel our observations that LX-stimulated phagocytosis is inhibited by 8-Br-cAMP and mimicked by Rp-cAMP. 12
Figure 4.
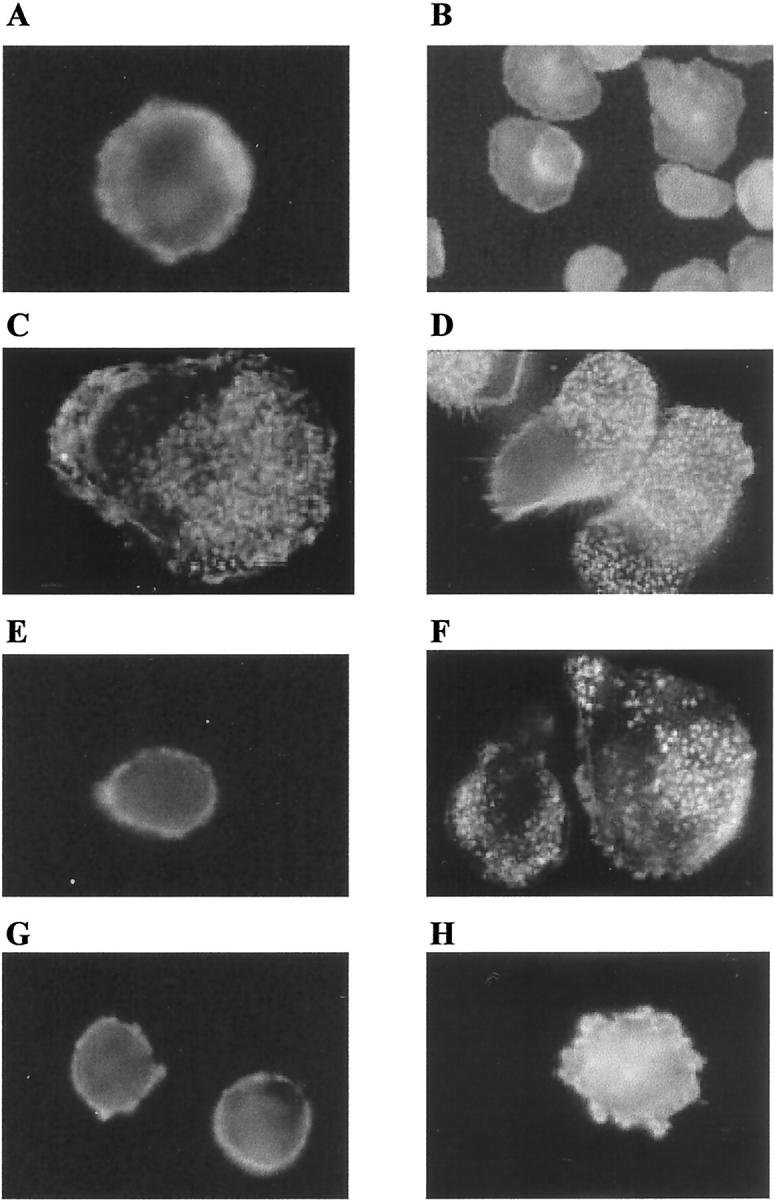
Roles of cAMP, PI 3-kinase, and Rho kinase in LXA4-induced rearrangement of the actin cytoskeleton in human monocyte-derived macrophages. Mφ were treated with 8-Br-cAMP (2 mmol/L), Rp-cAMP (100 μmol/L), or LY294002 (10 μmol/L) simultaneously with vehicle or LXA4 (10−9 mol/L) for 15 minutes at 37°C. Mφ were incubated with the inhibitor Y-27632 (10 μmol/L) for 30 minutes before exposure to LXA4 (10−9 mol/L) for 15 minutes. Cells were fixed with paraformaldehyde and localization of actin was determined using Oregon Green phalloidin and visualized by fluorescence microscopy using a ×100 oil objective. These images are representative of several fields derived from three independent experiments. A: vehicle + 8-Br-cAMP; B: LXA4 + 8-Br-cAMP; C: vehicle + Rp-cAMP; D: LXA4 + Rp-cAMP; E: vehicle + LY294002; F: LXA4 + LY294002; G: Y-27632 + vehicle; H: Y-27632 + LXA4.
PI 3-kinases have been implicated in many cellular responses such as macrophage chemotaxis 20 and phagocytosis. 21,22 To evaluate the effects PI 3-kinases have on actin cytoskeleton, Mφ were co-incubated for 15 minutes with the inhibitor LY294002 (10 μmol/L) alone or in combination with LXA4 (10−9 mol/L). Inhibition of PI-3 kinase did not affect actin reorganization in control and LXA4-stimulated Mφ (Figure 4, E and F) ▶ .
LXA4-Induced F-Actin Reorganization Is Blocked by Y-23632 and Involves RhoA and Rac Activation
Rho GTPases seem to be important modulators of actin cytoskeleton function during many phagocytic responses. 17,22-26 Rho kinases are effectors of Rho and they can be specifically inhibited by the compound Y-27632. 27,28 In the present study we evaluated the effect of Y-27632 on LXA4-induction of actin rearrangement. Whereas Y-27632 itself (10 μmol/L) did not influence F-actin distribution (Figure 4G) ▶ , previous exposure of Mφ to this inhibitor almost completely inhibited the LXA4-induced changes in the actin reorganization (Figure 4H) ▶ , implicating the involvement of small GTPases in the LXA4-elicited effect on actin cytoskeleton.
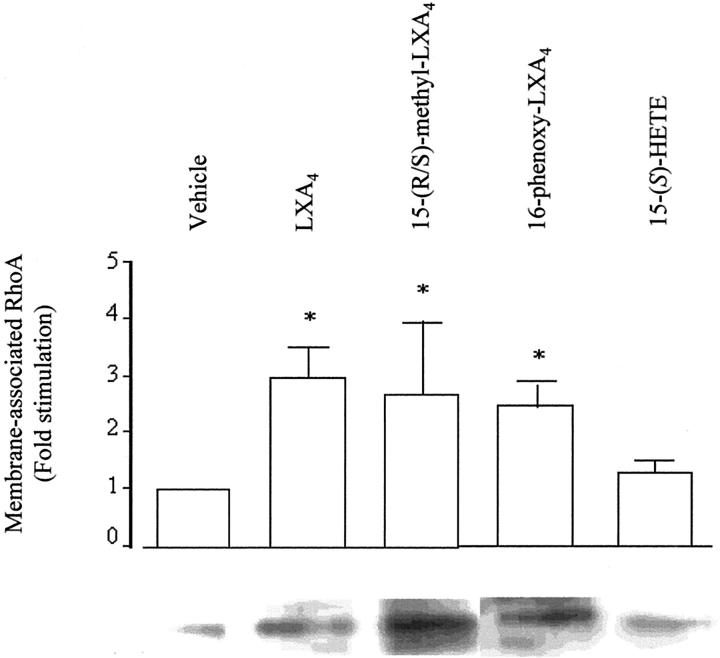
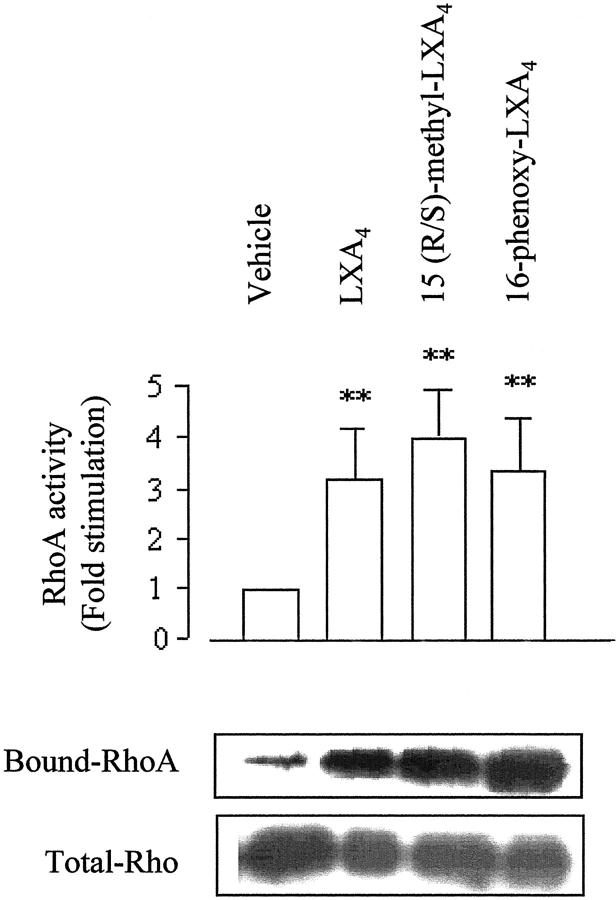
To examine the ability of LXA4 to activate small GTPases of the Rho family, we measured changes in membrane-associated RhoA, Rac, and Cdc42 in THP-1 cells differentiated to a Mφ-like phenotype by treatment with PMA. In THP-1 cells, LXA4 induces ultrastructural changes and actin redistribution comparable to those observed in Mφ (data not shown). When cells were stimulated with LXA4 (10−9 mol/L) or the two analogues (10−11 mol/L) for 15 minutes, a threefold increase in membrane-associated RhoA, an index of Rho activation, 27 was observed, whereas 15-(S)-HETE was without effect (Figure 5) ▶ . Consistent with the observation of others on Rho activation, a decrease in cytosolic RhoA could not be detected in our experimental conditions (data not shown) because of the fact that a large fraction of the total Rho protein still remained cytosolic. 29 Using a pull-down assay with Rho-binding domain of Rhotekin we found that LXA4 and the stable analogues caused a significant increase in Rho activity in THP-1 lysates (Figure 6) ▶ . There is not any statistically significant difference in the effect of native LXA4 and the stable analogues on in vitro Rho activation assay. However, the difference in the active concentrations between LXA4 and the analogues can be related to the rapid inactivation of LXA4 19 and the enhanced stability of the analogues, bypassing the initial rapid inactivation, can lead to more sustained actions in in vivo models of inflammation.
Figure 5.
LX-induced translocation of RhoA. THP-1 cells were differentiated into a Mφ-like phenotype by treatment with PMA (16 nmol/L) for 48 hours. Cells were exposed to vehicle, LXA4 (10−9 mol/L), 15-(R/S)-methyl-LXA4 (10−11 mol/L), 16-phenoxy-LXA4 (10−11 mol/L), or 15-(S)-HETE for 15 minutes at 37°C. Cells were lysed and a crude preparation of membranes was isolated. Samples from the same number of cells (1 × 106 cells) were resolved by electrophoresis on a 12% sodium dodecyl sulfate-polyacrylamide gel and Western blot analysis was performed with an antibody against RhoA. Blots were quantified by densitometry. Values are expressed as fold stimulation relative to vehicle (unity) and are the means ± SEM of five independent experiments. A representative blot is shown. *, P < 0.05 versus vehicle.
Figure 6.
LXs activate RhoA in THP-1 cells. THP-1 cells were differentiated into a Mφ-like phenotype by treatment with PMA (16 nmol/L) for 48 hours. Cells were exposed to vehicle, LXA4 (10−9 mol/L), 15-(R/S)-methyl-LXA4 (10−11 mol/L), 16-phenoxy-LXA4 (10−11 mol/L), or 15-(S)-HETE for 15 minutes at 37°C. Activity of the GTP-bound Rho in THP-1 lysates was assessed by a pull-down assay using glutathione-Sepharose 4B beads coupled with bacterially expressed GST-RBD fusion protein. Bound and total Rho proteins were detected by Western blotting with an antibody against RhoA. RhoA activity is indicated by the amount of RBD-bound Rho normalized to the amount of Rho in whole-cell lysates. Values represent Rho activity relative to vehicle. Results are the means ± SEM of three independent experiments and a representative blot is shown. **, P < 0.01 versus vehicle.
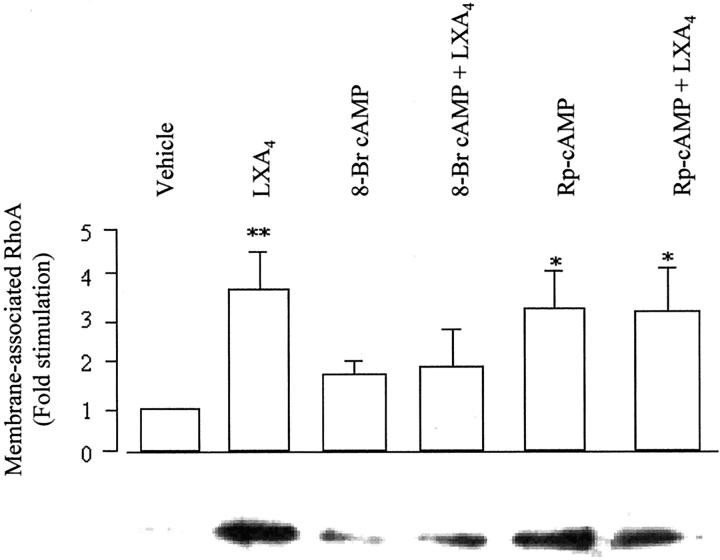
As discussed above, cAMP/PKA pathways are involved in LXA4-stimulated rearrangement of the actin cytoskeleton. Inhibition of RhoA translocation to the membrane was observed in differentiated THP-1 cells treated with 8-Br-cAMP and LXA4 (Figure 7) ▶ . When THP-1 cells were exposed to Rp-cAMP alone or in combination with LXA4, a threefold increase in membrane-associated RhoA was detected, an increase comparable to that observed with LXA4 alone (Figure 7) ▶ .
Figure 7.
Role of cAMP in LXA4-induced Rho translocation. THP-1 cells were differentiated into a Mφ-like phenotype by treatment with PMA (16 nmol/L) for 48 hours. Cells were treated with 8-Br-cAMP (2 mmol/L) or Rp-cAMP (100 μmol/L), simultaneously with vehicle or LXA4 (10−9 mol/L) for 15 minutes at 37°C. Cells were lysed and a crude preparation of membranes was isolated. Samples from the same number of cells (1 × 106 cells) were resolved by electrophoresis on a 12% sodium dodecyl sulfate-polyacrylamide gel and Western blot analysis was performed with an antibody against RhoA. Blots were quantified by densitometry. Values are expressed as fold stimulation relative to vehicle (unity) and are the means ± SEM of three independent experiments. A representative blot is shown. *, P < 0.05; **, P < 0.01 versus vehicle.
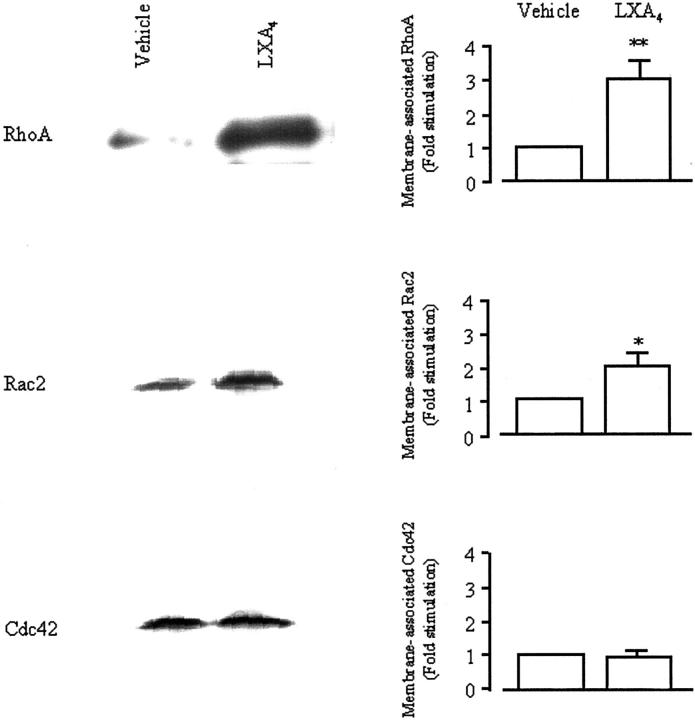
THP-1 cells exposed to LXA4 (10−9 mol/L) showed a twofold increase in the membrane-associated Rac, whereas the levels of membrane-associated Cdc42 were unaffected by LXA4 (Figure 8) ▶ .
Figure 8.
LX-induced translocation of Rho small GTPases. THP-1 cells were differentiated into a Mφ-like phenotype by treatment with PMA (16 nmol/L) for 48 hours and then exposed to vehicle or LXA4 (10−9 mol/L) for 15 minutes at 37°C. Cells were lysed and a crude preparation of membranes was isolated. Samples from the same number of cells (1 × 106 cells) were resolved by electrophoresis on a 12% sodium dodecyl sulfate-polyacrylamide gel and Western blot analysis was performed with antibodies against RhoA, Rac2, or Cdc42. Blots were quantified by densitometry. Values are expressed as fold stimulation relative to vehicle (unity) and are the means ± SEM of three independent experiments and in each case a representative blot is shown. *, P < 0.05; **, P < 0.01 versus vehicle.
Discussion
In this study we present evidence that LXs induce remarkable changes in the ultrastructure and F-actin reorganization in human monocytes and Mφ, but not in PMNs. LXs are endogenous mediators that play an important role in the resolution of inflammation through distinct effects on specific cell types. 1,2 The anti-inflammatory activities of LXs on PMN trafficking have been demonstrated in vitro and in vivo. 1-9,30-32 LXs inhibit PMN chemotaxis, 8 adhesion, and transmigration across endothelia and epithelia in response to pro-inflammatory stimuli such as LTB4 and fMLP. 7,9 The data presented here, indicating that LXs are without effect on LTB4-induced changes in the actin cytoskeleton on PMNs, suggest that the locus of LX inhibition of LTB4-stimulated PMN activation is not at the level of actin cytoskeletal rearrangement and migration and provides further circumstantial evidence for LX modulating PMN trafficking predominantly through inhibition of LTB4-stimulated β2 integrin-mediated adhesion. 9,33,34
In contrast to the inhibitory effects of LXs on PMN function, LXs are potent stimuli for monocytes. LXA4 is rapidly metabolized by monocytes to the inactive metabolites 15-oxo-LXA4, 13–14-dihydro-15-oxo-LXA4, and 13,14-dihydro-LXA4. 11,19 LXA4 activates Rainbow trout macrophages, increasing the intracellular calcium concentrations. 35 The effect of LXA4 on the calcium levels correlates with the chemotactic mechanisms, but not with phagocytosis of yeast particles in this system. 35 LXA4 and LXB4 stimulate human monocyte chemotaxis and adhesion, without degranulation or release of reactive oxygen species, 10,11 suggesting that the actions of LXs may be related to the recruitment of monocytes to sites of injury. LXs were shown to accelerate the resolution of allergic pleural edema 36 and to stimulate nonphlogistic phagocytosis of apoptotic PMN by Mφ in vitro 12 and in vivo in a mouse thioglycollate-elicited peritonitis model (Mitchell et al, manuscript submitted). These data provide compelling evidence for a role for LXs in promoting the resolution of inflammation. The locus of the effect of LXs on promoting phagocytosis of apoptotic PMNs is unresolved. Multiple cell surface molecules on the phagocyte have been implicated in the recognition and binding of apoptotic cells 13,15,16 and functional inhibition of several of these is associated with modulation of LX-stimulated phagocytosis. 12 The work presented here has investigated whether LXA4 and its stable analogues might modulate rearrangement of the actin cytoskeleton in monocytes and Mφ. Our data demonstrate that LXs induce significant changes in the reorganization of actin in human monocytes and Mφ resulting in the promotion of cytoplasmic extensions and in the formation of pseudopodia. Given that the process of phagocytosis is highly dependent on the localized polymerization of actin filaments that facilitate the formation of filopodia that surround the cells or the microorganisms to be engulfed, 17 our data are of a particular significance. It is reasonable to propose that enhanced phagocytosis of apoptotic PMNs is an inevitable consequence of interaction with such primed Mφ.
The small Rho GTPases (Rho, Rac, and Cdc42) regulate the assembly and reorganization of the actin cytoskeleton in response to external stimuli, providing the driving force for cells to migrate, to adhere, and to phagocytose particles. 37 Rho GTPases cycle between an inactive GDP-bound form and an active GTP-bound form and once activated, they interact with downstream effectors, 38,39 regulating different signal transduction pathways linking various membrane receptors to the assembly of actin. 40 In fibroblasts Rac is involved in the formation of lamellipodia causing actin polymerization at the plasma membrane, Cdc42 mediates the formation of filopodia, and Rho mediates the formation of stress fibers and focal adhesions. 41,42 However the function of the Rho family in shape control, actin reorganization, and integrin activity might be different in cells that do not possess stress fibers such as leukocytes. 37,43 Differential involvement of specific Rho family members has been demonstrated in phagocytosis in response to stimulation of immunoglobulin (Fcγ) or complement receptors. Rac and Cdc42 have been shown to regulate actin reorganization during Fcγ receptor-mediated phagocytosis by promoting pseudopod extension and phagosome closure, 22-26 whereas complement-mediated phagocytosis, a process not associated with the release of pro-inflammatory molecules, requires the activation of RhoA. 24 Phagocytosis of apoptotic cells shares some of the characteristics of both mechanisms. Similar to the Fcγ receptor-mediated phagocytosis, internalization of the apoptotic cells is because of extension of the phagocyte membrane around the cell to be engulfed 44 and require Rac and Cdc42, whereas inhibition of Rho enhanced phagocytosis. 22 However uptake of apoptotic cells does not evoke release of inflammatory mediators, 14 a scenario comparable to complement-mediated phagocytosis. We have investigated whether LX-stimulated actin reorganization is associated with Rho small GTPases. The effect of LXA4 on actin cytoskeleton was impaired by Y-27632, an inhibitor of Rho kinase, a downstream kinase of Rho, suggesting a role for these signaling molecules in LX-triggered cytoskeletal reorganization. In addition, we have measured the activation of the small GTPases in THP-1 cells treated with LXA4 by evaluating the membrane-associated levels of RhoA, Rac, and Cdc42. To measure Rho activation, we have also used a more sensitive assay by measuring the ability of RhoA to associate with the Rho-binding domain of Rhotekin exploiting the fact that Rho effectors intersect only with GTP-bound Rho. 18 Our data demonstrate that LXA4 activates RhoA and Rac, but not Cdc42 in THP-1 cells, a Mφ-like phenotype. It has been proposed that inhibition of Rho enhances phagocytosis, but reduces monocyte transmigration and Mφ chemotaxis. 22,37 The effect of LXA4 on Rho activation suggests that RhoA might be differentially involved in the stimulatory activity of LXs on monocyte/Mφ such as chemotaxis and phagocytosis. The increase of the levels of active RhoA is of interest in the context of the nonphlogistic phagocytosis induced by LXA4, a characteristic shared with the complement-receptor mediated phagocytosis. On the other hand, the increase in Rac levels may be responsible for the protrusion of the plasma membrane around the apoptotic cell. 17,22
cAMP-dependent mechanisms have been found to produce changes in the rearrangement of the actin cytoskeleton in different cell types through RhoA. 45-47 Elevated intracellular cAMP levels cause activation of PKA, which phosphorylates many target proteins including RhoA. It has been suggested that such phosphorylation down-regulates the binding of Rho to Rho kinases. 45,46 We have previously demonstrated that the effect of LXs on phagocytosis involves cAMP/PKA pathways. 12 A role for cAMP/PKA pathways on the effects of LXA4 on actin cytoskeleton are suggested by the observation that LXA4-induced actin reorganization is inhibited by the cell permeable cAMP analogue, 8-Br-cAMP, and is mimicked by an inhibitor of PKA.
Interestingly, we find that LX-stimulated actin cytoskeletal rearrangement is insensitive to the PI 3-kinase inhibitor LY294002, whereas LX-stimulated phagocytosis is PI 3-kinase-dependent (Michell et al, manuscript submitted). This may reflect the independence of PI 3-kinase in the regulation of the initial actin polymerization, but its critical role is in more distal events in the phagocytic process involving membrane fusion. 22
In conclusion, we demonstrate that LXs and LX-stable analogues induce reorganization of the actin cytoskeleton in monocytes and Mφ but not in PMNs and that this effect is associated with differential activation of small Rho GTPases. This observation is of particular interest in view of the fact that LXs stimulate nonphlogistic phagocytosis of apoptotic PMN and that a role for LX in wound healing has been suggested on the basis of activation of monocyte function and chemotaxis. 11 Given the role of Rho small GTPases in cell motility it may be proposed that LX stimulation of RhoA and Rac activity modulates chemotactic and phagocytic responses. In particular, data presented here suggest that LX pretreatment of Mφ might prime them for chemotaxis and phagocytosis by promoting RhoA- and Rac-dependent cytoskeleton reorganization and pseudopodia formation, contributing to the potential role of LXs in the resolution of inflammation.
Acknowledgments
We thank Dr. J. H. Brown and David Goldstein (Department of Pharmacology, University of California, San Diego, CA) for providing the Rho-RBD-containing bacteria and advice on the Rho pull-down assay, and Dr. Cormac Taylor (Department of Medicine and Therapeutics, University College Dublin) for his critical comments.
Footnotes
Address reprint requests to Dr. Catherine Godson, Department of Medicine and Therapeutics, University College Dublin, 41 Eccles St., Dublin 7, Ireland. E-mail: cgodson@mater.ie.
Supported by the Irish Heart Foundation (to P. M.), the Health Research Board Ireland (to C. G.), the Wellcome Trust (to H. R. B. and C. G.), the European Union (to H. R. B. and C. G.), the Irish National Program for Research in Third Level Institution, and the National Institutes of Health (PO1DE13499 to N. A. P.).
H. R. B. and C. G. are both of equal seniority.
References
- 1.Serhan CN: Lipoxins and novel aspirin-triggered 15-epi-lipoxins (ATL): a jungle of cell-cell interactions or a therapeutic opportunity? Prostaglandins 1997, 53:107-137 [DOI] [PubMed] [Google Scholar]
- 2.McMahon B, Mitchell S, Brady HR, Godson C: Lipoxins: revelations on resolution. Trends Pharmacol Sci 2001, 22:391-395 [DOI] [PubMed] [Google Scholar]
- 3.Claria J, Serhan CN: Aspirin triggers novel bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci USA 1995, 92:9475-9479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan CN, Maddox JF, Petasis NA, Akritopoulou-Zanze I, Papayianni A, Brady HR, Colgan SP, Madara JL: Design of lipoxin A4 analogs that block transmigration and adhesion of human neutrophils. Biochemistry 1995, 34:14609-14615 [DOI] [PubMed] [Google Scholar]
- 5.Brady HR, Serhan CN: Potential vascular roles for lipoxins in the “stop programs” of host defense and inflammation. Trends Cardiovasc Med 1995, 5:186-192 [DOI] [PubMed] [Google Scholar]
- 6.Hachicha M, Pouliot M, Petasis NA, Serhan CN: Lipoxin (LX)A4 and aspirin-triggered 15-epi-LXA4 inhibit tumor necrosis factor α-induced neutrophil responses and trafficking: regulators of a cytokine-chemokine axis. J Exp Med 1999, 189:1923-1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL: Lipoxin A4 modulates transmigration of human neutrophils across intestinal epithelial cell monolayers. J Clin Invest 1993, 92:75-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee TH, Horton CE, Kyan-Aungm V, Haskard D, Crea AEG, Spur W: Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by LTB4 and N-formyl-L-methionyl-L-leucyl-L-phenylalanine Clin Sci (London) 1989, 77:195-203 [DOI] [PubMed] [Google Scholar]
- 9.Papayianni A, Serhan CN, Brady HR: Lipoxins inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells. J Immunol 1996, 156:2264-2272 [PubMed] [Google Scholar]
- 10.Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN: Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J Biol Chem 1997, 272:6972-6978 [DOI] [PubMed] [Google Scholar]
- 11.Maddox JF, Serhan CN: Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med 1996, 183:137-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR: Cutting edge: lipoxins rapidly stimulate non-phlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol 2000, 164:1663-1667 [DOI] [PubMed] [Google Scholar]
- 13.Savill J, Fadok V: Corpse clearance defines the meaning of cell death Nature 2000, 407:784-788 [DOI] [PubMed] [Google Scholar]
- 14.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM: Macrophages that have ingested apoptotic cells in vitro inhibit pro-inflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2 and PAF. J Clin Invest 1998, 101:890-898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren Y, Savill J: Apoptosis: the importance of being eaten. Cell Death Differ 1998, 5:563-568 [DOI] [PubMed] [Google Scholar]
- 16.Henson PM, Bratton DL, Fadok VA: Apoptotic cell removal. Curr Biol 2001, 11:R795-R805 [DOI] [PubMed] [Google Scholar]
- 17.May RC, Machesky LM: Phagocytosis and the actin cytoskeleton. J Cell Sci 2000, 114:1061-1077 [DOI] [PubMed] [Google Scholar]
- 18.Ren XD, Kiosses WB, Schwartz MA: Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J 1999, 18:578-585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serhan CN, Fiore S, Brezinski DA, Lynch S: Lipoxin A4 metabolism by differentiated HL-60 cells and human monocytes: conversion to novel 15-oxo and dihydro products. Biochemistry 1993, 32:6313-6319 [DOI] [PubMed] [Google Scholar]
- 20.Vanhaesebroeck B, Jones GE, Allen WE, Zicha D, Hooshmand-Rad R, Sawyer C, Wells C, Waterfield MD, Ridley AJ: Distinct PI(3)Ks mediated mitogenic signaling and cell migration in macrophages. Nat Cell Biol 1999, 1:69-71 [DOI] [PubMed] [Google Scholar]
- 21.Cox D, Tseng CC, Bjekic G, Greenberg S: A requirement for phosphatidyl inositol 3-kinase in pseudopod extension. J Biol Chem 1999, 274:1240-1247 [DOI] [PubMed] [Google Scholar]
- 22.Leverrier Y, Ridley AJ: Requirement for Rho GTPases and PI 3-kinases during apoptotic cell phagocytosis by macrophages. Curr Biol 2001, 11:195-199 [DOI] [PubMed] [Google Scholar]
- 23.Cox D, Chang P, Zhang Q, Reddy PG, Bokoch GM, Greenberg S: Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J Exp Med 1997, 186:1487-1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caron E, Hall A: Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 1998, 282:1717-1721 [DOI] [PubMed] [Google Scholar]
- 25.Castellano F, Montcourrier P, Chavrier P: Membrane recruitment of rac1 triggers phagocytosis. J Cell Sci 2000, 113:2955-2961 [DOI] [PubMed] [Google Scholar]
- 26.May RC, Caron E, Hall A, Machesky LM: Involvement of the Arp2/3 complex in phagocytosis mediated by FcγR or CR3. Nat Cell Biol 2000, 2:246-248 [DOI] [PubMed] [Google Scholar]
- 27.Glomset JA, Farnsworth CC: The role of protein modification reaction in programming interaction between Ras-related GTPases and cell membranes. Annu Rev Cell Biol 1994, 10:181-205 [DOI] [PubMed] [Google Scholar]
- 28.Narumiya S, Ishizaki T, Uehata M: Use and properties of ROCK-specific inhibitor Y-27632. Methods Enzymol 2000, 325:273-284 [DOI] [PubMed] [Google Scholar]
- 29.Lang P, Gesbert F, Thiberge J, Troalen F, Dutartre H, Chavrier P, Bertoglio J: Characterization of a monoclonal antibody specific for the ras-related GTP-binding protein RhoA. Biochem Biophys Res Commun 1993, 196:1522-1528 [DOI] [PubMed] [Google Scholar]
- 30.Chiang N, Gronert K, Clish CB, O’Brien JA, Freeman MW, Serhan CN: Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J Clin Invest 1999, 104:309-316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serhan CN: Cutting edge: nociceptin stimulates neutrophil chemotaxis and recruitment: inhibition by aspirin-triggered-15-epi-lipoxin A4. J Immunol 2001, 166:3650-3654 [DOI] [PubMed] [Google Scholar]
- 32.Clish CB, O’Brien JA, Gronert K, Stahl GL, Petasis NA, Serhan CN: Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc Natl Acad Sci USA 1999, 96:8247-8252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiore S, Serhan CN: Lipoxin A4 receptor activation is distinct from that of the formyl peptide receptor in myeloid cells: inhibition of CD11/18 expression by lipoxin A4-lipoxin A4 receptor interaction. Biochemistry 1995, 34:16669-16678 [DOI] [PubMed] [Google Scholar]
- 34.Filep JG, Zouki C, Petasis NA, Hachicha M, Serhan CN: Anti-inflammatory actions of lipoxin A4 stable analogues are demonstrable in human whole blood: modulation of leukocyte adhesion molecules and inhibition of neutrophil-endothelial interactions. Blood 1999, 94:4132-4142 [PubMed] [Google Scholar]
- 35.Knight J, Lloyd-Evans P, Rowley AF, Barrow SE: Effect of lipoxins and other eicosanoids on phagocytosis and intracellular calcium mobilisation in rainbow trout (Oncorhynchus mykiss) leukocytes. J Leukoc Biol 1993, 54:518-522 [DOI] [PubMed] [Google Scholar]
- 36.Bandeira-Melo C, Serra MF, Dias BL, Cordeiro RSB, Silva PMR, Lenzi HL, Bakhle YS, Serhan CN, Martins MA: Cyclooxygenase-2-derived prostaglandin E2 and lipoxin A4 accelerate resolution of allergic edema in Angiostrongylus costaricensis-infected rats: relationship with concurrent eosinophilia. J Immunol 2000, 164:1029-1036 [DOI] [PubMed] [Google Scholar]
- 37.Ridley A: Rho proteins, PI 3-kinases and monocyte/macrophage motility. FEBS Lett 2001, 498:168-171 [DOI] [PubMed] [Google Scholar]
- 38.Kaibuchi K, Kuroda S, Amano M: Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem 1999, 68:459-486 [DOI] [PubMed] [Google Scholar]
- 39.Bishop AL, Hall A: Rho GTPases and their effector proteins Biochem J 2000, 348:241-255 [PMC free article] [PubMed] [Google Scholar]
- 40.Hall A: RhoGTPases and the actin cytoskeleton. Science 1998, 279:509-514 [DOI] [PubMed] [Google Scholar]
- 41.Nobes CD, Hall A: Rho, Rac and Cdc42 regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell 1995, 81:53-62 [DOI] [PubMed] [Google Scholar]
- 42.Ridley AJ, Hall A: The small GTP-binding protein Rho regulates the assembly of focal adhesions and stress fibers in response to growth factors. Cell 1992, 70:389-399 [DOI] [PubMed] [Google Scholar]
- 43.Allen WE, Jones GE, Pollard JW, Ridley AJ: 1Rho, Rac and Cdc42 regulate actin organisation and cell adhesion in macrophages. J Cell Sci 1997, 110:707-720 [DOI] [PubMed] [Google Scholar]
- 44.Parnaik R, Raff MC, Scholes J: Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr Biol 2000, 10:857-860 [DOI] [PubMed] [Google Scholar]
- 45.Laudanna C, Campbell JJ, Butcher EC: Elevation of intracellular cAMP inhibits RhoA activation and integrin-dependent leukocyte adhesion induced by chemoattractants. J Biol Chem 1997, 272:24141-24144 [DOI] [PubMed] [Google Scholar]
- 46.Dong JM, Leung T, Manser E, Lim L: cAMP-induced morphological changes are counteracted by the activated RhoA small GTPase and the Rho kinase ROKα. J Biol Chem 1998, 273:22554-22562 [DOI] [PubMed] [Google Scholar]
- 47.Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J: Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J 1996, 15:510-519 [PMC free article] [PubMed] [Google Scholar]