Abstract
The conformational change in amyloid β (Aβ) peptide from its monomeric form to aggregates is crucial in the pathogenesis of Alzheimer's disease (AD). In the healthy brain, some unidentified chaperones appear to prevent the aggregation of Aβ. Here we reported that lipocalin-type prostaglandin D synthase (L-PGDS)/β-trace, the most abundant cerebrospinal fluid (CSF) protein produced in the brain, was localized in amyloid plaques in both AD patients and AD-model Tg2576 mice. Surface plasmon resonance analysis revealed that L-PGDS/β-trace tightly bound to Aβ monomers and fibrils with high affinity (KD = 18–50 nM) and that L-PGDS/β-trace recognized residues 25–28 in Aβ, which is the key region for its conformational change to a β-sheet structure. The results of a thioflavin T fluorescence assay to monitor Aβ aggregation disclosed that L-PGDS/β-trace inhibited the spontaneous aggregation of Aβ (1–40) and Aβ (1–42) within its physiological range (1–5 μM) in CSF. L-PGDS/β-trace also prevented the seed-dependent aggregation of 50 μM Aβ with Ki of 0.75 μM. Moreover, the inhibitory activity toward Aβ (1–40) aggregation in human CSF was decreased by 60% when L-PGDS/β-trace was removed from the CSF by immunoaffinity chromatography. The deposition of Aβ after intraventricular infusion of Aβ (1–42) was 3.5-fold higher in L-PGDS-deficient mice and reduced to 23% in L-PGDS-overexpressing mice as compared with their wild-type levels. These data indicate that L-PGDS/β-trace is a major endogenous Aβ-chaperone in the brain and suggest that the disturbance of this function may be involved in the onset and progression of AD. Our findings may provide a diagnostic and therapeutic approach for AD.
Keywords: aggregation, Alzheimer's disease, mouse, surface plasmon resonance, thioflavin T
The conformational change in amyloid β (Aβ) peptides, Aβ (1–40) and Aβ (1–42), from their soluble monomeric forms to insoluble aggregates is central to the pathogenesis of Alzheimer's disease (AD). Aβ (1–42) peptide is probably the pathogenic one in AD (1–3). Mutations in either amyloid precursor protein or presenilin 1 and 2 genes result in Aβ overproduction (1–3) and have been detected in early onset familial cases of AD, which account for ≈3% of AD patients (4, 5). In contrast, Aβ production in late-onset sporadic cases of AD, which represent the majority of AD cases, remains unchanged, yet the aggregation of Aβ is enhanced. Functional abnormalities in the deterrents against Aβ aggregation would seem, therefore, to be involved in the pathogenesis of sporadic AD. Aβ is secreted into human cerebrospinal fluid (CSF) under normal conditions (6), and in the healthy brain, it appears to be efficiently controlled by some unidentified extracellular chaperones so as not to aggregate. In the present study, we focused on the possibility of lipocalin-type prostaglandin D synthase (L-PGDS) (7, 8), a major human CSF protein known as β-trace (9), being such a chaperone that functions to prevent Aβ misfolding and aggregation.
Results
Immunostaining of L-PGDS/β-Trace in Amyloid Plaques.
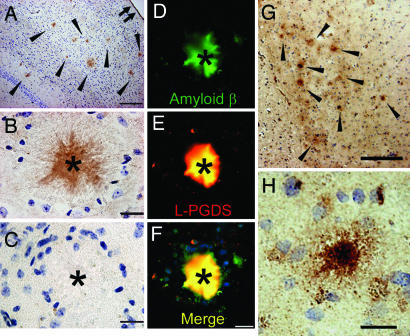
When we immunostained for L-PGDS/β-trace in the brain of 2-year-old male AD model Tg2576 mice, L-PGDS/β-trace was detected in many amyloid plaques (Fig. 1A, arrowheads, and B, asterisk), as well as in the leptomeninges (Fig. 1A, small double arrow) as described previously (10, 11). The L-PGDS/β-trace immunoreactivity was not observed in amyloid plaques when preabsorbed antibody was used (Fig. 1C). Double immunofluorescence staining with anti-Aβ antibody (Fig. 1D) and anti-L-PGDS/β-trace antibody (Fig. 1E) also revealed that L-PGDS/β-trace was localized in Aβ-positive amyloid plaques (Fig. 1F, asterisks). In the brain of a late-onset sporadic AD patient, we also confirmed that L-PGDS/β-trace was immunohistochemically detectable in senile plaques in the frontal cortex (Fig. 1G, arrowheads, and H, asterisk). These data suggest that L-PGDS/β-trace may bind to Aβ fibrils.
Fig. 1.
L-PGDS/β-trace immunostaining of amyloid plaques in Tg2576 mice and AD patients. (A–F) Amyloid plaques in the cerebral cortex of Tg2576 mice (A, arrowheads; B and C, asterisk) were immunopositive with anti-mouse L-PGDS/β-trace antibody (A and B), but not with preabsorbed antibody (C). Double immunofluorescence staining of Aβ (D) and L-PGDS/β-trace (E) showed that they were colocalized (F, merged image). (G and H) In the frontal cortex of a 70-year-old AD patient, amyloid plaques (G, arrowheads; H, asterisk) were immunostained by anti-human L-PGDS/β-trace polyclonal antibody. (Scale bars: A and G, 200 μm; B–F and H, 20 μm.)
Binding of L-PGDS/β-Trace to Aβ Peptides.
To investigate the binding of L-PGDS/β-trace to Aβ peptides, we monitored the changes in molecular mass of Aβ (1–40) immobilized on a sensor chip after infusion of various concentrations of L-PGDS/β-trace solution on the chip by surface plasmon resonance (SPR) analysis. L-PGDS/β-trace purified from human CSF rapidly bound to the immobilized Aβ (1–40) in a dose-dependent manner and thereafter very slowly dissociated from it after washing with 50 mM sodium phosphate (pH 7.5) and 100 mM NaCl. According to the association and dissociation kinetics, the dissociation constant (KD) value for the binding affinity was calculated to be 50 nM (Fig. 2A). Conversely, Aβ (1–40) monomer also bound to the immobilized L-PGDS/β-trace with a similar high affinity at the KD value of 60 nM (Fig. 2B). L-PGDS/β-trace also bound to immobilized Aβ (1–42), Aβ (1–40) fibrils, and Aβ (1–42) fibrils with high affinities (KD = 40–43 nM; Table 1).
Fig. 2.
SPR analysis of binding between L-PGDS/β-trace and Aβ. (A) Binding of L-PGDS/β-trace to 2 ng of immobilized Aβ (1–40). (B) Binding of Aβ (1–40) to 8 ng of immobilized Aβ L-PGDS/β-trace. Filled and open arrows show the starting points for sample injection and washing with buffer, respectively. Human serum albumin did not bind to Aβ (1–40) in the same concentration range.
Table 1.
KD values of L-PGDS/β-trace and recombinant L-PGDS to various Aβ peptides by SPR analysis
| Peptides |
KD, nM |
|
|---|---|---|
| L-PGDS/β-trace | Recombinant human L-PGDS | |
| Aβ (1–40) | 50 | 20 |
| Aβ (1–42) | 43 | 27 |
| Aβ (1–40) fibril | 42 | 31 |
| Aβ (1–42) fibril | 40 | 26 |
| Aβ (25–35) | 20 | 18 |
| Aβ (1–28) | 40 | 19 |
| Aβ (1–16) | Not detected | Not detected |
L-PGDS/β-trace purified from human CSF or recombinant human L-PGDS was applied to the various immobilized Aβ peptides. According to the association and dissociation curves, KD values for the binding affinity were calculated.
To address which regions of Aβ were involved in the binding to L-PGDS/β-trace, we used several segments of Aβ peptides for SPR analysis. L-PGDS/β-trace tightly bound to Aβ (25–35) and Aβ (1–28) peptides with KD values of 20 and 40 nM, respectively, but not to Aβ (1–16) (Table 1), thus indicating that L-PGDS/β-trace recognized residues 25–28 of Aβ. Recombinant human L-PGDS expressed in Eschericha coli also tightly bound to Aβ peptides with KD values of 18–31 nM.
Inhibition of Aβ Aggregation by L-PGDS/β-Trace in Vitro.
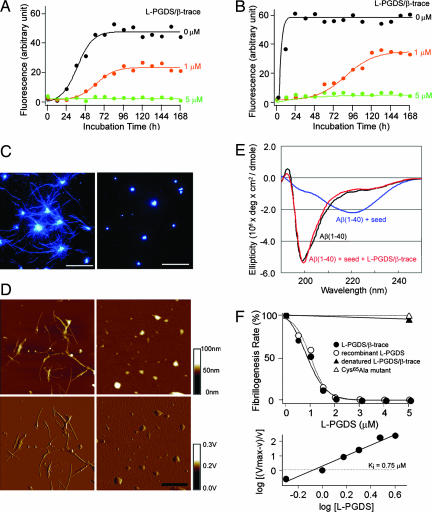
Next, we investigated the effect of L-PGDS/β-trace on spontaneous Aβ aggregation in vitro (Fig. 3). The results of a thioflavin T (ThT) fluorescence assay to monitor Aβ aggregation revealed that the fibrillogenesis phase of 50 μM Aβ (1–40) aggregation commenced 24 h subsequent to initiation of the nucleation phase and reached a plateau 48 h thereafter. L-PGDS/β-trace at 1 μM extended the nucleation phase and decreased the final amount of Aβ aggregates to 49% of that in its absence. L-PGDS/β-trace at 5 μM inhibited all spontaneous Aβ aggregation for at least 168 h (Fig. 3A). The fibrillogenesis of Aβ (1–42) commenced even earlier, 6 h after the nucleation phase had begun, and Aβ aggregation reached a plateau within 24 h. L-PGDS/β-trace at 1 μM extended the nucleation phase and decreased the final amount of Aβ aggregates to 62% of that in its absence. The aggregation of Aβ (1–42) was also completely inhibited for at least 168 h in the presence of 5 μM L-PGDS/β-trace (Fig. 3B). The aggregation of both Aβ (1–40) and Aβ (1–42) was prevented in a dose-dependent manner by the addition of either 1 or 5 μM L-PGDS/β-trace, and concentrations are in the physiological range in human CSF (12).
Fig. 3.
Inhibition of Aβ aggregation by L-PGDS/β-trace. (A and B) Spontaneous aggregation of 50 μM Aβ (1–40) (A) and Aβ (1–42) (B) in the absence (black) or presence of L-PGDS/β-trace (orange, 1 μM; green, 5 μM). (C and D) Observations of Aβ (1–40) seed-dependent aggregation by fluorescence abscence (black) or microscopy (C) and AFM (D) in the absence (Left) or presence (Right) of L-PGDS/β-trace. (Scale bars: C, 10 μm; D, 1 μm.) (E) CD spectra of 50 μM Aβ (1–40) before (black) and after incubation for 2 h with Aβ seed (10 μg/ml) in the absence (blue) or presence of 5 μM L-PGDS/β-trace (red). (F Upper) Inhibition of seed-dependent fibrillogenesis of 50 μM Aβ (1–40) by incubation for 1 h with L-PGDS/β-trace purified from human CSF (closed circles), recombinant human L-PGDS (open circles), heat-denatured L-PGDS/β-trace (closed triangles), or recombinant inactive Cys65Ala mutant of human L-PGDS (open triangles). (F Lower) Hill plot of the data obtained for L-PGDS/β-trace purified from human CSF.
When Aβ fibrils were added as a “seed,” the fibrillogenesis was remarkably accelerated. This seed-dependent aggregation of Aβ was also inhibited by L-PGDS/β-trace [Fig. 3 C and D and supporting information (SI) Fig. 7A]. Albumin is the most abundant human CSF protein with the physiological range ≈2–8 μM in human CSF (13). Albumin partially inhibited Aβ aggregation, yet did not completely prevent the seed-dependent aggregation of 50 μM Aβ (1–40) within its physiological range (SI Fig. 7B). Total internal reflection fluorescence microscopy revealed that large Aβ fibrils were produced after incubation of 50 μM Aβ (1–40) monomer with the seed (Fig. 3C Left); however, such fibrils were not observed in the presence of 5 μM L-PGDS/β-trace (Fig. 3C Right). The inhibitory effect of L-PGDS/β-trace on Aβ fibrillogenesis was confirmed by inspection by atomic force microscopy (AFM) (Fig. 3D).
As revealed by circular dichroism (CD) spectrum analysis, the Aβ monomer possessed a predominantly unfolded conformation (Fig. 3E, black). When the Aβ monomer was incubated with a seed, a CD spectrum with a minimum at ≈220 nm appeared, indicating the formation of amyloid fibrils with the β-sheet structure (14) (Fig. 3E, blue). However, in the presence of L-PGDS/β-trace, Aβ did not assume the β-sheet-rich structure (Fig. 3E, red), indicating that L-PGDS/β-trace prevented the conformational change to the β-sheet structure. These results are consistent with the results of the ThT fluorescence assay described above.
The rate of fibrillogenesis of the seed-dependent Aβ (1–40) aggregation was decreased in a concentration-dependent manner by either L-PGDS/β-trace purified from human CSF or the recombinant L-PGDS (Fig. 3F Upper). The kinetic inhibitory constant (Ki) of L-PGDS/β-trace with respect to Aβ (1–40) aggregation was calculated by Hill plot analysis to be 0.75 μM (Fig. 3F Lower). The inhibitory activity against the Aβ (1–40) aggregation was observed with neither heat-denatured L-PGDS/β-trace nor a recombinant inactive Cys65 Ala mutant of human L-PGDS (the catalytically active Cys65 residue was replaced; Fig. 3F Upper). These results indicate that L-PGDS/β-trace has a specific conformation required for its interaction with Aβ, and that the Cys65 residue is crucial for the chaperone activity of L-PGDS/β-trace to prevent Aβ aggregation.
Preventive Effect of L-PGDS/β-Trace in Human CSF on Aβ Aggregation.
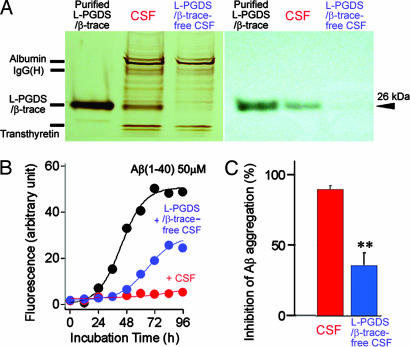
L-PGDS/β-trace could be almost completely removed from human CSF by passage through a mouse monoclonal anti-L-PGDS/β-trace antibody-conjugated column (Fig. 4A). When we compared the inhibitory effect of human CSF and L-PGDS/β-trace-free CSF on Aβ aggregation, 50% human CSF prevented spontaneous aggregation of Aβ (1–40) for 96 h and 50% L-PGDS/β-trace-free CSF for 48 h (Fig. 4B). After incubation for 96 h, human CSF and L-PGDS/β-trace-free CSF prevented 90% and 36%, respectively, of the spontaneous aggregation of Aβ (1–40), indicating that L-PGDS/β-trace was the major CSF component responsible for the inhibition of Aβ aggregation (Fig. 4C).
Fig. 4.
Inhibitory effect of L-PGDS/β-trace in human CSF on Aβ aggregation. (A) SDS/PAGE and Western blot analysis revealed that L-PGDS/β-trace is a major protein in human CSF with a molecular mass of 26 kDa and is almost completely removed from the CSF by passage through a mouse monoclonal anti-L-PGDS antibody-conjugated column. (B) Representative time course of the spontaneous Aβ aggregation in the absence (black) or presence of 50% human CSF (red) or 50% L-PGDS/β-trace-free CSF (blue). (C) Inhibition of Aβ aggregation in the presence of 50% CSF or the L-PGDS/β-trace-free CSF. The percentage inhibition of Aβ aggregation was calculated by using the formula [1− (Fa/Fb)] × 100, where Fb and Fa are the ThT fluorescence intensities of 50 μM Aβ (1–40) incubated for 96 h or in the presence of 50% CSF or L-PGDS/β-trace-free CSF, respectively. Data are expressed as the mean ± SEM of three independent experiments. ∗∗, P < 0.01 vs. CSF (Student's t test).
Inhibition of Aβ Deposition by L-PGDS/β-Trace in Vivo.
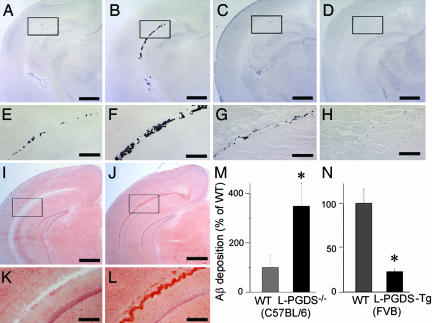
To test whether L-PGDS/β-trace could prevent Aβ aggregation in vivo, we injected biotin-labeled human Aβ (1–42) into the lateral ventricle of the brain of WT, L-PGDS-knockout (L-PGDS−/−; C57BL/6 strain), and human L-PGDS-transgenic (L-PGDS-Tg; FVB strain) mice. As examined by avidin–biotin–peroxidase staining, deposits of biotin-labeled Aβ (1–42) were detected along the lateral ventricle at 3 h postinjection in all sections examined (Fig. 5). As compared with WT mice (Fig. 5 A, C, E, and G), Aβ (1–42) deposition was histologically accelerated in L-PGDS−/− mice (Fig. 5 B and F) and reduced in L-PGDS-Tg mice (Fig. 5 D and H). Moreover, Aβ deposits were positively stained by Congo-red staining (Fig. 5 I–L), indicating that the deposits were composed of Aβ fibrils rather than Aβ monomers. Next deposition of Aβ (1–42) was quantified by binding of [125I]-streptavidin to brain sections prepared from L-PGDS−/− (7BL/6 strain; Fig. 5M) and L-PGDS-Tg (Fig. 5N) mice and their WT counterparts, and sections had been immunostained with biotin-labeled Aβ (1–42). The deposition of Aβ (1–42) was 3.5-fold higher in L-PGDS−/− mice than in WT mice (Fig. 5M) and 77% lower in L-PGDS-Tg mice than in WT mice (Fig. 5N). These results clearly indicate that L-PGDS/β-trace strongly inhibited Aβ aggregation and deposition in vivo.
Fig. 5.
Inhibition of Aβ deposition by L-PGDS/β-trace in vivo. (A–H) Aβ deposition in the brain of WT mice (C57BL/6; A and E), L-PGDS−/− mice (C57BL/6; B and F), WT mice (FVB; C and G), and L-PGDS-Tg mice (FVB; D and H). (Scale bars: A–D, 1 mm; E–H, 200 μm.) (I–L) Congo-red staining of Aβ deposition in WT (C57BL/6; I and K) and L-PGDS−/− mice (C57BL/6; J and L) brain as described above. (Scale bar: I and J, 1 mm; K and L, 200 μm.) (M and N) The Aβ deposition was quantified by binding of [125I]-streptavidin to tissue sections prepared from the brain of WT and L-PGDS−/− mice (C57BL/6; M) and of WT and L-PGDS-Tg mice (FVB; N), which sections had been reacted with biotin-labeled Aβ (1–42). Data are expressed as the mean ± SEM (n = 3–4). Significant difference was based on Student's t test; ∗, P < 0.05.
Discussion
In this study, we demonstrated that L-PGDS/β-trace plays a critical role as an endogenous Aβ-chaperone, thereby inhibiting both Aβ (1–40) and Aβ (1–42) aggregation in vitro and in vivo. SPR analysis showed that L-PGDS/β-trace tightly bound to Aβ (1–28) and Aβ (25–35), but not to Aβ (1–16), indicating that the hydrophobic region from residues 25–28 of Aβ was crucial for the binding to L-PGDS/β-trace. Interestingly, these same residues in Aβ are the key region for the conformational change in Aβ peptides from the random-coiled to the β-sheet structure (15). During Aβ aggregation, soluble Aβ peptides are known to change their conformation to the β-sheet structure and aggregate to form insoluble fibrils enriched in β-sheet structure (15, 16). The interaction of L-PGDS/β-trace with residues 25–28 in Aβ may prevent this conformational change to the β-sheet structure (Fig. 6), as was suggested by far-UV CD spectrum analysis (Fig. 3E). L-PGDS/β-trace completely prevented Aβ aggregation even at a molar ratio of 1:10. These results suggest that L-PGDS/β-trace catalyzes this conformational change in Aβ from the β-sheet to the random-coiled structure.
Fig. 6.
Proposed schema for inhibition of Aβ aggregation by L-PGDS/β-trace. Soluble Aβ monomers change their conformation from a random-coil dominant structure to the β-sheet-rich structure and then aggregate to insoluble fibrils. L-PGDS/β-trace can prevent Aβ aggregation by inhibiting the transformation of Aβ monomers to β-sheet-rich structure and the sequential seed-dependent Aβ fibrillogenesis.
The aggregation of Aβ was earlier shown to be inhibited by apolipoprotein (Apo) E (17, 18) and transthyretin (19). Because of the disturbance of Aβ metabolism (17, 18), ApoE is a risk factor for late-onset AD. The ε4 allele of ApoE shows an incidence of 17.5% among all AD cases (4, 5). However, ApoE is not a CNS-specific protein (13, 20), whereas Aβ is primarily produced in the brain (6, 7). On the other hand, L-PGDS/β-trace is the most abundant CSF protein produced in the brain and is dominantly expressed in the CNS rather than in the peripheral organs. L-PGDS/β-trace exists more abundantly in the brain than ApoE. Furthermore, L-PGDS/β-trace binds to Aβ with an affinity (KD = 18–50 nM) comparable with that of ApoE (KD = 20 nM) (21). Therefore, L-PGDS/β-trace could be considered more essential than ApoE for the prevention of Aβ aggregation in the brain.
Based on these findings, the incidence of Aβ aggregation would be expected to increase when the L-PGDS/β-trace concentration is decreased or the Aβ-chaperone activity of L-PGDS/β-trace is inactivated in the brain. Two-dimensional gel electrophoresis analysis (22) revealed that L-PGDS/β-trace is decreased in CSF of AD patients as compared with control healthy individuals. In postmenopausal depression, a known risk factor for AD (23), the decrease in the estradiol level is known to elicit a decrease in L-PGDS/β-trace expression (24). In addition, a polymorphism in L-PGDS/β-trace with a C-terminal exon 6-truncation was found in AD patients and claimed to be another risk factor for AD (38). Because C-terminal deletion results in the unfolding and inactivation of L-PGDS/β-trace (25), such mutation is predicted to decrease L-PGDS/β-trace chaperone activity. Moreover, the CSF levels of small hydrophobic molecules such as bilirubin and biliverdin, which bind to L-PGDS/β-trace (26), have been reported to be increased in AD patients (27) and inactivate the Aβ-chaperone activity of L-PGDS/β-trace (T.K., unpublished results). It is reasonable, therefore, to posit that functional disturbances of L-PGDS/β-trace may lead to the sporadic late-onset cases of AD.
Quantitative and qualitative changes in the Aβ-chaperone activity of L-PGDS/β-trace, therefore, may serve as biomarkers for predicting the onset of AD. Further, as the high-resolution x-ray crystallographic structure of L-PGDS has already been determined, it may be possible to design recombinant L-PGDS with a higher affinity for Aβ or to develop novel drugs to increase the affinity of L-PGDS/β-trace for Aβ for use in the therapy of AD. Interestingly, environmental enrichment, as compared with standard animal housing conditions, increased L-PGDS/β-trace gene expression and reduced Aβ deposition in the brain of amyloid precursor protein transgenic mice (28), indicating that the up-regulation of L-PGDS/β-trace may suppress Aβ deposition. That report is in good agreement with our conclusion that L-PGDS/β-trace is the major endogenous Aβ-chaperone to prevent Aβ aggregation in the brain. Our findings thus provide a new insight into the molecular mechanism of AD pathogenesis and a potential therapeutic strategy for AD.
Materials and Methods
Animals.
Tg2576 mice (29) were purchased from The Jackson Laboratory (Bar Harbor, ME). L-PGDS−/− mice (30, 31) and human L-PGDS-Tg mice (B20) (32) were generated at the Osaka Bioscience Institute (Osaka, Japan). The experimental protocols employing mice were approved by the Animal Care Committee of Osaka Bioscience Institute, and every effort was made to minimize the number of animals used as well as any pain and discomfort.
Autopsy Brain Tissues.
Brain tissues from pathologically diagnosed AD patients were obtained from the Brain Bank of Tokyo Metropolitan Geriatric Hospital and Tokyo Metropolitan Institute of Gerontology (Tokyo, Japan). This study was approved by the institutional review boards of Osaka Bioscience Institute, Tokyo Metropolitan Geriatric Hospital, Tokyo Metropolitan Institute of Gerontology, and Osaka University Graduate School of Medicine.
Antibodies.
Rabbit polyclonal anti-human L-PGDS (1:1,000 dilution) and anti-mouse L-PGDS (1:4,000 dilution) antisera were raised and purified at the Osaka Bioscience Institute. Monoclonal anti-human Aβ (11–28) antibody (1:100; IBL, Gunma, Japan) was also used.
Immunohistochemistry.
Deparaffinized sections were preincubated with 0.3% H2O2 in methanol followed by 50 mM sodium phosphate (pH 7.5) and 100 mM NaCl containing 0.2% Triton X-100. After pretreatment with formic acid for 5 min and trypsin for 15 min, they were sequentially incubated with primary antibody, biotinylated secondary antibody (Vector Laboratories, Burlingame, CA), and avidin–biotin complex (2 μg/ml; Vector Laboratories) by using the ABC elite kit according to the manufacturer's protocol. Immunoreactivity was visualized with diaminobenzidine (DAB; Dotite, Kumamoto, Japan) solution. For double immunostaining, deparaffinized sections were incubated at 4°C overnight with anti-Aβ and anti-L-PGDS anti-body, followed by FITC-conjugated anti-mouse IgG (Vector Laboratories) and Texas Red-conjugated anti-rabbit IgG (ICN Biomedicals, Costa Mesa, CA) for 2 h at room temperature.
Preparation of Aβ Solutions.
Aβ (1–16), Aβ (25–35), Aβ (1–40), Aβ (1–42) (Peptide Institute, Osaka, Japan), and Aβ (1–28) (AnaSpec, San Jose, CA) were dissolved in 0.02% ammonia solution at 200 μM. The Aβ fibril seed solution was prepared by incubation of 50 μM Aβ peptides in 50 mM sodium phosphate (pH 7.5) and 100 mM NaCl for 7 days at 37°C.
Purification of Human L-PGDS/β-Trace in CSF.
Human CSF samples were obtained from the Department of Neurosurgery, Nagoya City University Hospital. Informed consent was obtained from all patients for use of their CSF, sampled by lumbar puncture as part of a diagnostic workup. Human L-PGDS/β-trace was purified from the CSF by immunoaffinity chromatography with a monoclonal anti-human L-PGDS/β-trace antibody 6F5-conjugated column as reported previously (33).
SPR Analysis.
The SPR experiments were performed with a BIAcore 2000 (BIAcore AB, Uppsala, Sweden). Aβ monomers, fibrils or L-PGDS/β-trace were immobilized by amine coupling onto a CM5 chip (BIAcore AB) that had been preactivated with a mixture of N-ethyl-N′-(3-dymethylaminopropyl)carbodimide hydrochloride and N-hydroxysuccinimide. After blocking of the remaining activated carboxyl groups on the sensor chip with ethanolamine, the analyte was applied at a flow rate of 20 μl/min in 50 mM sodium phosphate (pH 7.5) and 100 mM NaCl at 25°C. Following measurement, the chip surface was regenerated with 50 mM NaOH. The reference cell was prepared by amine coupling without the ligand.
Fluorescence Spectroscopy.
Aβ peptides (50 μM) were incubated at 37°C in the absence or presence of human L-PGDS/β-trace purified from human CSF (34), recombinant human L-PGDS expressed in E. coli (35), or human CSF in 50 mM sodium phosphate (pH 7.5) and 100 mM NaCl. The seed-dependent and spontaneous Aβ aggregations were monitored with or without addition of Aβ seed (final concentration 10 μg/ml) in a Hitachi F-4500 fluorescence spectrophotometer (Hitachi Software Engineering, Yokohama, Japan) at excitation and emission wavelengths of 446 and 490 nm, respectively, after the components had been mixed with a 200-fold volume of 50 mM glycine-NaOH (pH 8.5) containing 5 μM ThT (Wako Pure Chemicals, Osaka, Japan) as described earlier (36).
Fluorescence Microscopy and AFM.
Aβ (1–40; 50 μM) was incubated for 2 h with the Aβ seed (10 μg/ml) in 50 mM sodium phosphate (pH 7.5) and 100 mM NaCl in the absence or presence of 5 μM L-PGDS/β-trace. The solution was then diluted 5-fold and incubated with ThT of a final concentration of 5 μM. The fibril formation of Aβ on glass slides was examined by fluorescence microscopy (37). In the case of AFM, 10 μl of Aβ solution was spotted on freshly cleaved mica surfaces and left undisturbed for 2 min, after which the excess solution was blown off with compressed air. AFM images were obtained by using a dynamic force microscope (Nanoscope-RIIIa; Digital Instruments, Santa Barbara, CA).
CD Spectroscopy.
Aβ (1–40) monomer (50 μM) was dissolved in 50 mM sodium phosphate (pH 7.5) and 100 mM NaCl and incubated in the absence or presence of 5 μM L-PGDS/β-trace at 37°C for 2 h. These samples were diluted 2-fold in the buffer described above, and far-UV CD spectra were recorded at 37°C by using a Jasco-600 spectropolarimeter equipped with a thermostat-controlled cell holder (Jasco, Tokyo, Japan). A quartz cuvette with a 0.1-cm path length was used. The data were presented as the mean residue mass ellipticity for the Aβ peptide.
Analysis of Aβ Deposition in Vivo.
Under pentobarbital (50 mg/kg, i.p.) anesthesia, biotin-labeled Aβ (1–42) (100 μM; AnaSpec, San Jose, CA) was infused at a rate of 0.66 μl/min for 60 min into the lateral ventricle (coordinates relative to bregma: anterior–posterior 0.0-mm, lateral 2.0-mm, and 2.3-mm depth) of 4-month-old male L-PGDS−/−mice (30, 31) and human L-PGDS-Tg mice (32). At 3 h after administration, the mice were killed. Cryosections (30 μm) of mouse brain were prepared, fixed in ethanol, and incubated with avidin–biotin–peroxidase complex (2 μg/ml; Vector Laboratories) according to the manufacturer's protocol. Cryosections were also stained with 1% Congo-red solution. For quantification of Aβ deposition, cryosections of mouse brain (30 μm) were fixed in ethanol and incubated with [125I]-streptavidin (9.25 kBq/ml, 1,850 kBq/μg; Amersham Biosciences, Little Chalfont, U.K.) in 50 mM sodium phosphate (pH 7.5) and 100 mM NaCl for 2 h. After wash by 50 mM sodium phosphate (pH 7.5) and 100 mM NaCl containing 0.2% Triton X-100, the amount of biotin-labeled Aβ (1–42) within the brain was quantified with a Micro Imager radioisotope detector (Biospace Measures, Paris, France).
SI.
Additional results about the inhibition of seed-dependent Aβ aggregation by L-PGDS/β-trace or human serum albumin can be found in SI Fig. 7.
Supplementary Material
Acknowledgments
We thank Dr. O. Hayaishi for his critical advice in this study, Dr. K. Yamaguchi for help with AFM measurements, and Dr. M. Mase for providing the human CSF samples.
Abbreviations
- Aβ
amyloid β
- AD
Alzheimer's disease
- AFM
atomic force microscopy
- CSF
cerebrospinal fluid
- L-PGDS
lipocalin-type prostaglandin D synthase
- SPR
surface plasmon resonance
- ThT
thioflavin T.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701585104/DC1.
References
- 1.Sisodia SS. J Clin Invest. 1999;104:1169–1170. doi: 10.1172/JCI8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.Tanzi RE, Kovacs DM, Kim TW, Moir RD, Guenette SY, Wasco W. Neurobiol Dis. 1996;3:159–168. doi: 10.1006/nbdi.1996.0016. [DOI] [PubMed] [Google Scholar]
- 5.Tanzi RE. J Clin Invest. 1999;104:1175–1179. doi: 10.1172/JCI8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, et al. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 7.Urade Y, Hayaishi O. Biochim Biophys Acta. 2000;1482:259–271. doi: 10.1016/s0167-4838(00)00161-8. [DOI] [PubMed] [Google Scholar]
- 8.Urade Y, Eguch N, Hayaishi O. In: Lipocalins. Akerstrom B, Borregaard N, Flower D, Salier JP, editors. Georgetown, TX: Eurekah.com; 2006. pp. 99–109. [Google Scholar]
- 9.Clausen J. Proc Soc Exp Biol Med. 1961;107:170–172. doi: 10.3181/00379727-107-26569. [DOI] [PubMed] [Google Scholar]
- 10.Urade Y, Kitahama K, Ohishi H, Kaneko T, Mizuno N, Hayaishi O. Proc Natl Acad Sci USA. 1993;90:9070–9074. doi: 10.1073/pnas.90.19.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beuckmann CT, Lazarus M, Gerashchenko D, Mizoguchi A, Nomura S, Mohri I, Uesugi A, Kaneko T, Mizuno N, Hayaishi O, Urade Y. J Comp Neurol. 2000;428:62–78. doi: 10.1002/1096-9861(20001204)428:1<62::aid-cne6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Hiraoka A, Arato T, Tominaga I, Anjyo A. J Pharm Biomed Anal. 1997;15:1257–1263. doi: 10.1016/s0731-7085(96)01986-3. [DOI] [PubMed] [Google Scholar]
- 13.Einstein ER. Proteins of the Brain and CSF in Health and Disease. Springfield, IL: Thomas; 1982. [Google Scholar]
- 14.Raman B, Ban T, Sakai M, Pasta SY, Ramakrishna T, Naiki H, Goto Y, Rao Ch M. Biochem J. 2005;392:573–581. doi: 10.1042/BJ20050339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petkova AT, Ishii Y, Balbach JJ, Antzutkin ON, Leapman RD, Delaglio F, Tycko R. Proc Natl Acad Sci USA. 2002;99:16742–16747. doi: 10.1073/pnas.262663499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harper JD, Lansbury PT., Jr Annu Rev Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- 17.Wisniewski T, Frangione B. Neurosci Lett. 1992;135:235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- 18.Naslund J, Thyberg J, Tjernberg LO, Wernstedt C, Karlstrom AR, Bogdanovic N, Gandy SE, Lannfelt L, Terenius L, Nordstedt C, et al. Neuron. 1995;15:219–228. doi: 10.1016/0896-6273(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 19.Schwarzman AL, Gregori L, Vitek MP, Lyubski S, Strittmatter WJ, Enghilde JJ, Bhasin R, Silverman J, Weisgraber KH, Coyle PK, et al. Proc Natl Acad Sci USA. 1994;91:8368–8372. doi: 10.1073/pnas.91.18.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitas RE, Boyles JK, Lee SH, Hui D, Weisgraber KH. J Biol Chem. 1987;262:14352–14360. [PubMed] [Google Scholar]
- 21.Golabek AA, Soto C, Vogel T, Wisniewski T. J Biol Chem. 1996;271:10602–10606. doi: 10.1074/jbc.271.18.10602. [DOI] [PubMed] [Google Scholar]
- 22.Puchades M, Hansson SF, Nilsson CL, Andreasen N, Blennow K, Davidsson P. Brain Res Mol Brain Res. 2003;118:140–146. doi: 10.1016/j.molbrainres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, III, Assaf AR, Jackson RD, et al. J Am Med Assoc. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 24.Mong JA, Devidze N, Frail DE, O'Connor LT, Samuel M, Choleris E, Ogawa S, Pfaff DW. Proc Natl Acad Sci USA. 2003;100:318–323. doi: 10.1073/pnas.262663799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urade Y, Tanaka T, Eguchi N, Kikuchi M, Kimura H, Toh H, Hayaishi O. J Biol Chem. 1995;270:1422–1428. doi: 10.1074/jbc.270.3.1422. [DOI] [PubMed] [Google Scholar]
- 26.Beuckmann CT, Aoyagi M, Okazaki I, Hiroike T, Toh H, Hayaishi O, Urade Y. Biochemistry. 1999;38:8006–8013. doi: 10.1021/bi990261p. [DOI] [PubMed] [Google Scholar]
- 27.Kimpara T, Takeda A, Yamaguchi T, Arai H, Okita N, Takase S, Sasaki H, Itoyama Y. Neurobiol Aging. 2000;21:551–554. doi: 10.1016/s0197-4580(00)00128-7. [DOI] [PubMed] [Google Scholar]
- 28.Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 30.Eguchi N, Minami T, Shirafuji N, Kanaoka Y, Tanaka T, Nagata A, Yoshida N, Urade Y, Ito S, Hayaishi O. Proc Natl Acad Sci USA. 1999;96:726–730. doi: 10.1073/pnas.96.2.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qu WM, Huang ZL, Xu XH, Aritake K, Eguchi N, Nambu F, Narumiya S, Urade Y, Hayaishi O. Proc Natl Acad Sci USA. 2006;103:17949–17954. doi: 10.1073/pnas.0608581103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinzar E, Kanaoka Y, Inui T, Eguchi N, Urade Y, Hayaishi O. Proc Natl Acad Sci USA. 2000;97:4903–4907. doi: 10.1073/pnas.090093997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oda H, Shiina Y, Seiki K, Sato N, Eguchi N, Urade Y. Clin Chem. 2002;48:1445–1453. [PubMed] [Google Scholar]
- 34.Oda H, Eguchi N, Urade Y, Hayaishi O. Proc Jpn Acad. 1996;72:108–111. [Google Scholar]
- 35.Inui T, Ohkubo T, Emi M, Irikura D, Hayaishi O, Urade Y. J Biol Chem. 2003;278:2845–2852. doi: 10.1074/jbc.M209934200. [DOI] [PubMed] [Google Scholar]
- 36.Naiki H, Higuchi K, Hosokawa M, Takeda T. Anal Biochem. 1989;177:244–249. doi: 10.1016/0003-2697(89)90046-8. [DOI] [PubMed] [Google Scholar]
- 37.Ban T, Goto Y. Methods Enzymol. 2006;413:91–102. doi: 10.1016/S0076-6879(06)13005-0. [DOI] [PubMed] [Google Scholar]
- 38.Fabien S, Annelies R, Laurent D. FR2848573A1. 2004 Unexamined Patent.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.