Abstract
Social concepts such as “tactless” or “honorable” enable us to describe our own as well as others' social behaviors. The prevailing view is that this abstract social semantic knowledge is mainly subserved by the same medial prefrontal regions that are considered essential for mental state attribution and self-reflection. Nevertheless, neurodegeneration of the anterior temporal cortex typically leads to impairments of social behavior as well as general conceptual knowledge. By using functional MRI, we demonstrate that the anterior temporal lobe represents abstract social semantic knowledge in agreement with this patient evidence. The bilateral superior anterior temporal lobes (Brodmann's area 38) are selectively activated when participants judge the meaning relatedness of social concepts (e.g., honor–brave) as compared with concepts describing general animal functions (e.g., nutritious–useful). Remarkably, only activity in the superior anterior temporal cortex, but not the medial prefrontal cortex, correlates with the richness of detail with which social concepts describe social behavior. Furthermore, this anterior temporal lobe activation is independent of emotional valence, whereas medial prefrontal regions show enhanced activation for positive social concepts. Our results demonstrate that the superior anterior temporal cortex plays a key role in social cognition by providing abstract conceptual knowledge of social behaviors. We further speculate that these abstract conceptual representations can be associated with different contexts of social actions and emotions through integration with frontolimbic circuits to enable flexible evaluations of social behavior.
Keywords: functional MRI, semantics, social cognition, temporal lobe, frontal lobe
“What is honor?” asks Shakespeare's Falstaff (The First Part of King Henry the Fourth5.1.133). Although the meaning of a social or moral concept, such as honor, changes with cultural context, we are nonetheless able to understand its core meaning in a 16th-century play. Here, we explore the neuroanatomical basis of this remarkably stable social domain of conceptual knowledge. One hypothesis is that such abstract social semantic knowledge necessary to describe psychological characteristics is mainly subserved by the same medial prefrontal regions (1–3) that are essential for attributing mental states (theory of mind) and self-reflection (4–7). This study provides evidence for an alternative view, which predicts separable abstract representations of social concepts (e.g., “ambitious,” “polite,” “tactless,” and “stingy”) in the anterior temporal lobe.
Most of what we know about the neural organization of conceptual knowledge is based on studies of names for living and nonliving objects. These studies indicate that different conceptual domains [animals, fruits/vegetables, tools (8)] and features [sensory, functional (9)] are represented in distinct brain regions. There is evidence supporting the view that semantic organization is characterized by both domain-specific supramodal (verbal and nonverbal) and unimodal feature-specific regions (8, 10). Patients with neurodegeneration of the anterior temporal lobe (11) demonstrate not only verbal but also nonverbal semantic impairments, which lead to the conclusion that this region represents supramodal semantic knowledge (12–15).
Contrary to concepts that are expressed by names for living things (e.g., “dog”), social concepts (e.g., “loyal”) are names for social behavior or properties of living things [e.g., “acting in a loyal way” or “being loyal” (16)]. Therefore, as the most suitable class of concepts for comparison with social concepts, we chose concepts that are names for animal behavior or properties (e.g., “being nutritious,” “useful,” “trainable,” and “healthy”).
Social concepts can apply to humans as well as other animals (e.g., “a loyal dog”), and they rely on abstract functional, more than on sensory, knowledge (16). In some instances, we associate social concepts with socially relevant sensory cues, such as biological motion, which depend on posterior temporal regions (17), and social judgments based on such sensory cues also involve prefrontal areas (18). Grasping the meaning of social concepts, such as “honorable” or “tactless,” however, most prominently requires abstract functional (i.e., nonsensory) knowledge, which entails descriptions of social behavior rather than sensory detail (16). For example, this knowledge enables us to understand a person's social behavior as tactless even when sensory cues (e.g., a friendly facial expression and body posture) would indicate otherwise. The neuroanatomical basis of this abstract social conceptual knowledge is elusive.
There have been several neuroimaging studies using socially relevant words as stimuli. Most studies contrasted either a social and nonsocial task condition (3) or two different social conditions [e.g., self- vs. nonself-related (5, 19–23)] containing the same set of words, thereby subtracting activations evoked by the words and their respective semantic representations. It is well established that the automatic activation of semantic representations can be elicited by the mere presentation of words even when the task to be performed on the words does not require explicit semantic knowledge [e.g., word/nonword decision (24)]. Two studies by Mitchell et al. (1, 2) used a different approach by contrasting social and nonsocial words. They first looked at activation for word pairs containing first names versus word pairs containing fruit or clothing names (1). The authors concluded that the medial prefrontal cortex represents social semantic information about characteristics of persons and confined the role of the detected left anterior temporal cortex activation to sensory identification of socially relevant stimuli. It is not clear, however, whether activity was related to the uniqueness of first names in comparison to nonunique object names, as proposed by Grabowski et al. (25). In a subsequent study, Mitchell et al. (2) reproduced medial prefrontal, but not anterior temporal activations for person-descriptive words when compared with body part words; this finding supported their earlier conclusion that semantic knowledge of psychological states is bound to the medial prefrontal cortex. We argue, however, that none of the above studies critically tested that prediction because when socially relevant stimuli are categorically compared with less socially relevant stimuli, activations can be equally well explained by social cognitive processes other than domain-specific semantic processing, such as mental state attribution.
Here, we use functional MRI (fMRI) to record brain activity when participants make judgments about the meaning relatedness of social concepts (e.g., honor–brave or tactless–impolite) as compared with other animal function concepts (e.g., nutritious–useful, presented as word pairs), a task that requires access to detailed conceptual knowledge. Animal function concepts describe behaviors related to animal use and biological function and can in principle apply to humans as well. Using this categorical comparison, we are able to reproduce the network of social cognition regions found in previous studies, including medial prefrontal and anterior temporal cortex when comparing socially relevant with less socially relevant words. However, as the critical test of which of these regions is representing abstract conceptual information, we applied two key measures of conceptual knowledge as parameters in a separate regression analysis: (i) descriptiveness, and (ii) meaning relatedness of presented word pairs. By using these regression analyses, we provide evidence that a superior sector of the anterior temporal lobe is the only region in the brain to be selectively associated with conceptual knowledge of social behaviors.
Descriptiveness of concepts is the richness of conceptual knowledge detail (26). The more general a concept, the less detail of description it conveys. A general concept (e.g., unfriendly) is less descriptive than a more specific one (e.g., tactless) (27). More descriptive concepts require more detailed conceptual knowledge and are thus predicted to increase neural activity in conceptual brain regions. Because we specifically investigated the detail of behavior descriptions (not sensory detail) and adjusted for the effects of word imageability (highly correlated with concreteness; Pearson r = 0.91, P < 0.0001), we were able to determine whether regions code for abstract functional (i.e., nonsensory) knowledge. Meaning relatedness is an established measure of the degree to which two concepts are similar in meaning, and the organization of conceptual knowledge critically depends upon such information (14, 28).
Although there is no direct evidence on the anatomical locus of abstract social concept knowledge, indirect evidence suggests that anterior temporal lobe regions might be involved. Some patients with penetrating head injuries to the temporal lobes incurred during World War I were selectively unable to give examples of social behaviors to define concepts that describe character attributes (29). In addition, patients with anterior temporal lobe neurodegeneration not only exhibit gross conceptual impairments (12), but also display changes in social behavior (30). Functional imaging studies have revealed anterior temporal lobe activations in such diverse tasks as moral cognition (31), understanding others' mental states (4) or emotions (32), when tasks used persons' first names (1) or famous faces and names (33), as well as retrieval of famous name–face associations (34). Despite this indirect evidence for the importance of the anterior temporal lobe in social cognition, its exact contribution remains obscure because the common cognitive component across these different tasks has not been identified. In a recent model (31), we therefore hypothesized that specific anterior temporal lobe regions represent conceptual knowledge of social behaviors, which would be an essential underlying cognitive component shared by these social cognition tasks. A critical test of this prediction is whether there are distinct anterior temporal lobe regions selective for social concepts, and whether activity in these regions correlates with (i) the degree of detail with which concepts describe social behavior and (ii) the relatedness in meaning of two concepts in a word pair.
Results
The number of concept pairs judged as being related or unrelated in meaning during fMRI was equal across conditions [see supporting information (SI) Fig. 3]. Participants responded more quickly to related word pairs in all conditions. Overall response time was significantly slower for social than animal function concepts (see SI Fig. 3). Therefore, we tested the effects of response time on the observed brain activations for social concepts. There was no association of increased response time with temporal lobe activation for social concepts, ruling out effects of task difficulty.
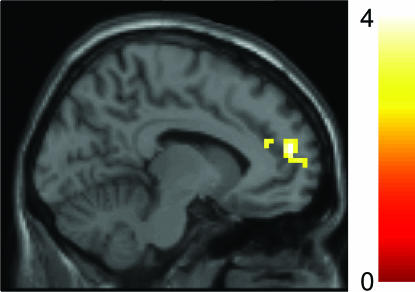
The categorical subtraction analysis for social compared with animal function concepts revealed a cluster of activation (significant at P = 0.05, family-wise error-corrected for multiple comparisons; Table 1) within right superior temporal [Brodmann's area (BA)38] and lateral orbitofrontal/inferior frontal cortex (BA47/45). In this categorical comparison, additional regions implicated in social cognition (4, 7, 31) were also activated, including the dorsomedial prefrontal cortex (BA8) and the left parietotemporal junction (BA22/40; Table 1). In addition, lateral posterior fusiform activations comparable with those reported in studies of sensory social semantics and face recognition were detected (17, 35, 36). Although temporal lobe activation did not differ between positive and negative social concepts, positive social concepts engaged a more anterior sector of the medial prefrontal cortex (BA10/32; see Fig. 1 and SI Fig. 4), indicating that anterior temporal lobe activation is independent of emotional valence.
Table 1.
Social vs. animal function concepts
| Hemisphere | Area | MNI |
BA | Z score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| R | Lateral orbitofrontal/anterior temporal cluster** | 48 | 21 | −9 | 47 | 4.29 |
| R | Anterior superior temporal gyrus** | 57 | 12 | 0 | 38 | 3.82 |
| R | Lateral orbitofrontal/inferior frontal gyrus** | 54 | 33 | 6 | 47/45 | 3.94 |
| L and R | Dorsomedial prefrontal cortex* | −6 | 21 | 54 | 8 | 3.55 |
| L | Middle frontal gyrus* | −36 | 33 | 24 | 46 | 3.55 |
| L | Inferior frontal gyrus* | −48 | 15 | 9 | 45 | 3.48 |
| L | Parieto-temporal junction* | −57 | −45 | 30 | 40/22 | 3.18 |
| L | Lateral inferior temporal gyrus* | −63 | −39 | −12 | 20/21 | 3.28 |
| L | Lateral fusiform gyrus* | −42 | −51 | −30 | 37 | 3.99 |
| L | Medial occipital gyrus* | −33 | −84 | 12 | 19 | 3.71 |
| L | Subthalamic nucleus* | −12 | −15 | −3 | — | 3.66 |
Social concepts (sum of all effects of interest: i + ii + iii) vs. animal function concepts (sum of all effects of interest: i + ii + iii) inclusively masked with social concepts (all effects of interest: i + ii + iii) vs. fixation. Effects of interest: (i) partial regression effect of event-related hemodynamic response function (HRF), (ii) partial regression effect of descriptiveness on HRF, and (iii) partial regression effect of meaning relatedness on HRF. All areas surviving P = 0.005, uncorrected voxel-level threshold (minimum cluster size = 10 voxels) in the whole-brain analysis are reported. Subclusters >8 mm apart are italic. ∗, Areas surviving FDR-corrected threshold at P = 0.05; ∗∗, areas surviving the most stringent correction for multiple comparisons (family-wise error) at P = 0.05. Only areas surviving stringent correction (family-wise error) or those predicted by an a priori anatomical hypothesis are discussed in the text (see SI Methods). MNI, Montreal Neurological Institute Standard Brain coordinates. L, left; R, right.
Fig. 1.
Positive vs. negative social concepts. Whole-brain analysis at a voxel-level threshold of P = 0.005 (minimum cluster size = 10 voxels). The peak coordinate of the displayed anterior medial prefrontal (BA10/32) region is −12, 54, 18, Z = 3.64. Regions not displayed here, which were additionally detected: left lateral orbitofrontal cortex (BA47/11), −42, 30, −15, Z = 4.44; dorsal anterior cingulate (BA24), −3, 0, 24, Z = 3.51. No temporal lobe differences emerged on a whole-brain and anterior temporal ROI basis, also at P = 0.05 uncorrected. See also SI Fig. 4 for parameter estimates for positive and negative social concepts.
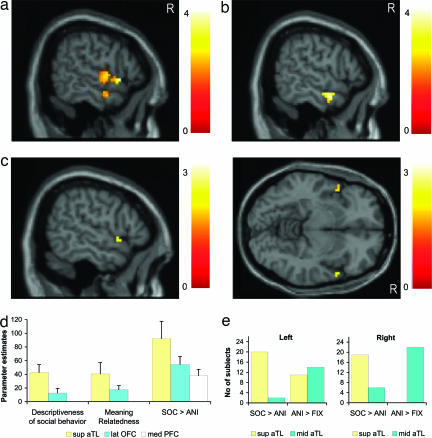
The anterior temporal region of interest (ROI) analysis comparing social vs. animal concepts revealed bilateral activation of the superior anterior temporal lobe (BA38) and less pronounced signal increases in the anterior middle temporal cortex (BA21; Fig. 2a). The reverse comparison (animal vs. social concepts) revealed no significant effects. Animal function concepts compared with fixation engaged anterior middle temporal cortex (BA21; Fig. 2b). The same area was activated by social concepts vs. fixation, indicating that the anterior middle temporal areas are shared by both classes of concepts.
Fig. 2.
(a) Social vs. animal function concepts (ROI analysis, section at x = 59) showed activation of a superior anterior temporal region [BA38; right hemisphere activation greater than left hemisphere activation (right > left); 51, 18, −12, Z = 3.5, surviving P = 0.05 corrected for 360 anterior temporal voxel volume activated by social concepts vs. fixation] and less strongly anterior middle temporal cortex (BA21; right > left; 57, −3, −21, Z = 2.17). (b) Animal function concepts vs. fixation engaged the same anterior middle temporal area (BA21, right > left; 57, −3, −21; Z = 3.62, section at x = 60). This region was also activated by social concepts vs. fixation (BA21, right > left; 54, −3, −21; Z = 4.83, corrected P = 0.001 by using the total 914 voxel volume of the bilateral anterior temporal ROI mask; data not shown). (Note that Z scores for clusters with P values that are < 0.05 and were small-volume-corrected by using a 12-mm sphere around the peak voxel in the whole-brain analysis are bold.) (c) Regions in which activity was higher for social than for animal concepts and that were independently correlated with descriptiveness of social behavior and meaning relatedness: conjunction null analysis for all of these three effects (ROI for display, sagittal at x = 57, and axial views at z = −6) revealed a selective right superior anterior temporal region on a whole-brain basis (BA38; right vs. left; 51, 15, −12; Z = 2.9). (d) Parameter estimates and SEs for descriptiveness of social behavior, meaning relatedness, and domain-specificity (social vs. animal) at right superior anterior temporal, orbitofrontal, and medial prefrontal peak coordinates. (e) Number of individual subjects with activation within middle and superior anterior temporal lobe (aTL) at P = 0.05 for social vs. animal function concepts (right superior aTL, n = 19; right middle aTL, n = 6; other, n = 1) and animal concepts vs. fixation (right superior aTL, n = 0; right middle aTL, n = 22; other, n = 4). Fisher's two-sided exact tests showed that type of contrast (social vs. animal or animal vs. fixation) influenced how many subjects had activations located within the superior aTL or middle aTL, respectively (right, P < 0.0001; left, P < 0.001; see SI Methods).
The prior demonstration of regional activity for social concepts by categorical subtraction of activation for less socially relevant concepts, however, does not reveal whether activity specific to social concepts is elicited by conceptual knowledge of social behaviors or by other social cognitive processes (e.g., attribution of mental states or self-reflection). The critical test of this hypothesis was an independent analysis that searched for regions in which activity was not only higher for social than for animal concepts, but was independently correlated with the degree of descriptiveness of social behavior and with meaning relatedness (conjunction analysis for these three effects). The right superior anterior temporal region (BA38) was the only region surviving this conjunction analysis on a whole-brain basis. ROI analysis revealed additional activation in homologous left-hemispheric cortex (BA38; Fig. 2c). In remarkably close agreement with our predictions, activity in the right superior anterior temporal region (BA38) showed a significantly stronger correlation with descriptiveness of social behavior than activity in orbitofrontal and medial prefrontal regions (Fig. 2d and SI Table 2). There was no correlation of neural activity with descriptiveness of social behavior in the nonspecific right anterior middle temporal region (BA21; data not shown). Furthermore, individual case analyses confirmed consistent anatomical separation of activations related to social concepts (superior temporal) and animal function concepts (middle temporal) within the anterior temporal cortex, particularly within the right hemisphere (Fig. 2e; right hemisphere, P < 0.0001; left hemisphere, P < 0.001; Fisher's two-sided exact test; see SI Methods).
Discussion
In summary, social concepts consistently activated a selective superior anterior temporal lobe region (BA38), and both animal and social concepts shared a nonspecific anterior middle temporal region (BA21). Social concepts also activated other regions (orbitofrontal, medial prefrontal cortex, and temporoparietal junction) known to be crucial for social cognition (1, 4, 31). Only activity in the superior anterior temporal cortex, however, robustly correlated with the richness of detail with which social concepts describe social behavior. This finding corroborates our prediction that specific anterior temporal lobe regions represent conceptual knowledge of social behaviors (31). Activity in the superior temporal pole (BA38) agrees with selective connections between this area and medial prefrontal cortex (37), a known key region for social cognition (4, 6, 7, 31, 38). On the contrary, middle and inferior temporal lobes are primarily connected to the orbital network, which integrates information from sensory systems and rewards (37). The exact anatomical location of our superior anterior temporal lobe region according to recent human anatomical studies (39) is at the posterior border of the temporal pole (BA38) reaching into the anterior superior temporal gyrus (BA22), which is highly connected to the superior temporal pole [BA38 (37)].
The independence of temporal lobe activation from emotional valence is in line with our prediction that abstract social conceptual representations in the anterior temporal lobe are valence-independent and can be dynamically associated with different emotionally relevant contexts encoded in frontolimbic circuits (31). This independence from emotional valence may explain why neuroimaging investigations of emotional word connotations did not find consistent anterior temporal lobe activation (40, 41).
These results cannot be attributed to confounding differences between social and animal function words because we meticulously controlled for all relevant psycholinguistic differences (including lexical frequency and familiarity) in the categorical subtractions and confirmed our results by independent parametric regression analyses. Higher frequency of adjectives in the social concept condition cannot explain effects within the anterior temporal lobe because lesions in this region lead to conceptual impairment irrespective of word class (12, 14). Also, there was no association of increased response time with temporal lobe activation for social concepts, ruling out effects of task difficulty.
Our results are in agreement with the central role of the anterior temporal lobes for representing abstract conceptual knowledge (12–15, 28, 42), concepts denoted by composite expressions (43–45), and the importance of the right temporal lobe for social cognition (30). Subdivisions for different semantic domains (e.g., tools, animals, and faces) were demonstrated in modality-specific posterior temporal regions (35, 36, 46). This study demonstrates that specialized subregions for different conceptual domains also exist within the anterior temporal lobe. It has been argued that subdivisions for different object categories in the posterior temporal cortex do not necessarily reflect modular specialization for a given category, and that for most categories of objects category effects can be explained by a continuous topographical representation of attributes (i.e., features) according to feature similarity (35, 46). The same logic can be applied to our finding of topographic differences within the anterior temporal lobe for conceptual representations of social and general animal behavior. They could be equally well explained by domain-specific as well as conceptual similarity-based topographic organization of underlying cortical representations.
Further studies are necessary to address the exact role of more inferior anterior temporal lobe regions that were reported in addition to superior anterior temporal cortex in neuroimaging studies of social cognition (31–33) and in what way famous face naming relates to conceptual knowledge of social behaviors studied here. Famous face and proper name processing were used as measures of person-specific semantic knowledge in patient lesion and functional imaging studies (33, 47–52). Neuropsychological cases exhibited extensive lesions of the left (49) or right (50) anterior temporal lobe, with relative sparing of the superior sector (51). The specificity of famous face-naming impairment after temporal pole lesions has been questioned by findings of equal impairments for other unique entities [e.g., famous buildings (25, 48, 52)]. Tranel (48) concluded that the left temporal pole is involved in lexical retrieval of unique object names. Other authors have stressed the distributed nature of lesions leading to impairments of retrieving proper names (53). In any case, our findings cannot be attributed to lexical (i.e., word-form) retrieval of proper names because we used nonunique concepts as stimuli, and our task did not involve lexical retrieval.
One possible relation between more sensory semantic information related to persons (as measured by famous face-naming tasks) and the abstract conceptual knowledge investigated here was proposed by Burton and colleagues (54). They suggest dissociable cognitive representations for multimodal information necessary to identify a person (e.g., face image, proper name) and more abstract information about a person (e.g., occupation). The anatomical locus of both systems has not been identified yet. Following this scheme, the abstract conceptual representations detected in our study would be part of the latter system devoted to describe a person's social behavior, but neither necessary nor sufficient to identify a person. Formal testing of more abstract social semantic knowledge is usually not reported in cases describing famous face-naming impairments in anterior temporal lobe lesions. In a patient with bilateral inferior temporal pole lesions, however, normal spontaneous use of abstract knowledge about social values was described, which contrasted with severe impairments on famous face naming (55). Together with the relative sparing of the superior anterior temporal lobe in another case of famous face-naming impairment (51), this evidence points to a possible inferior–superior gradient for multisensory versus abstract person-specific knowledge. The exact topographic relation of both types of person-related semantic systems needs to be addressed in future studies.
Previous functional neuroimaging studies comparing abstract with concrete concepts have, among other areas, reported activations in comparable superior temporal pole regions, as the one identified here (38, 56–58). In principle, one could derive two different conclusions from this anatomical convergence: (i) Our findings can be explained on the basis of the abstractness of our stimuli and are not specific to the social relevance of meaning representations, and (ii) the reported superior anterior temporal activations in studies on abstract concepts are due to the incidental use of socially relevant concepts as stimuli. We argue that the latter conclusion is strongly supported. Because in all our analyses partial effects of social concepts are adjusted for effects of imageability, which is highly correlated with concreteness, differences in abstractness/concreteness cannot explain the differences in activations between social and animal function concepts. Furthermore, our regression analyses demonstrated that the degree of activation in the superior anterior temporal region was increased with the degree to which concepts described social behavior. This effect was again independent of differences in imageability because it was adjusted for in the multiple-regression model. In summary, our experimental design carefully rules out a confounding effect of general abstractness to explain our data. Thus, the comparable activation sites within the superior anterior temporal lobe reported in previous studies on abstract concepts can be reinterpreted as due to the social relevance of used concepts. This conclusion is corroborated by looking at provided listings of stimuli, which, to a large proportion, contained socially relevant concepts (38, 56, 58). For example, in the study by Sabsevitz et al. (38), concepts such as “courage” and “disgrace” were mixed with less socially relevant abstract concepts such as “lesson” and “riddle.” The degree of social relevance, however, was not controlled.
Taken together, our findings indicate that a superior sector of the anterior temporal cortex plays a key role in social cognition by representing abstract conceptual knowledge of social behaviors, and that these representations are independent of emotional valence. Furthermore, we demonstrated that, although medial prefrontal cortex is involved in processing socially relevant information, it does not represent abstract social semantic knowledge. We further speculate that abstract conceptual representations in the anterior temporal lobe can be associated with different contexts of social actions and emotions through integration with frontolimbic circuits to enable flexible evaluations of social behavior (31).
Materials and Methods
Subjects.
Twenty-six healthy participants (13 men; age mean = 29.4 ± 9.0; years of education mean = 17.5 ± 2.5) took part in the fMRI experiment. Data from five additional participants had to be excluded before the statistical analysis (n = 2, no response times recorded; n = 1, MR-scanner failure; n = 1, head motion; n = 1, temporal lobe signal loss). All participants were right- handed (59) and native English speakers. All participants underwent a neurological examination by a board-certified neurologist and a clinical MRI during the previous 12 months, had normal or corrected-to-normal vision, had no history of psychiatric or neurological disorders, and were not taking centrally active medications. Informed consent was obtained according to procedures approved by the National Institute of Neurological Disorders and Stroke's Internal Review Board. Participants were compensated for their participation according to the National Institute of Neurological Disorders and Stroke's standards. Measures of self-esteem and trait affective style were collected before the fMRI experiment [Rosenberg Self-Esteem Scale (60), mean = 36.1 ± 3.7; PANAS (61) positive affect score, mean = 36.3 ± 6.8; negative affect score, mean = 13.6 ± 3.7].
fMRI Paradigm.
Participants decided whether written word pairs were related in meaning by pressing one of two response keys. Three different types of word pairs or a visual fixation pattern were presented: (i) animal function concepts [used with kind permission of the authors of ref. 62; e.g., nutritious–useful, n = 75], (ii) positive social concepts [used with kind permission of the authors of ref. 27; e.g., honor–brave, n = 75], and (iii) negative social concepts (27) (e.g., tactless–impolite, n = 75; see SI Fig. 5 and SI Methods). In two independent prestudies, we asked participants to rate the degree of detail with which each word described social behavior (social concepts) or animal behavior (animal function concepts) and how related in meaning both words within a pair were (i.e., meaning relatedness; see SI Methods). Relevant psycholinguistic variables were matched across conditions (word familiarity, frequency, difference in category breadth and social desirability within word pair, associativity, and meaning relatedness; see SI Methods).
Image Acquisition.
Echo-planar T2*-weighted images with blood oxygenation-level-dependent contrast were acquired (311 volumes per run) on a 3 Tesla General Electric scanner (GE Healthcare, Milwaukee, WI) equipped with a standard head coil, high-order manual shimming to temporal and ventral frontal lobes [3-mm slice thickness, 64 × 64 matrix, 37 slices, repetiton time = 2.3 sec, field of view: 220 × 220, parallel to the anterior to posterior commissural line, whole-brain coverage (not cerebellum)]. The first five volumes were discarded to allow for T1 equilibration effects. The combination of high-field MRI, thinner slices, and high-order manual shimming optimized the signal in anterior temporal and ventral frontal lobes. All participants had full coverage of the anterior temporal lobes upon inspection of normalized images (see SI Fig. 6). One subject was excluded before statistical analysis because of signal dropout within predefined critical regions (anterior temporal lobe, BA38/22, BA21, BA20; ventromedial prefrontal, BA11, BA25, BA24, BA32; ventrolateral prefrontal, BA11/47; and frontopolar cortex, BA10). In addition, high-resolution (≈1 mm3) T1-weighted 3D magnetization-prepared rapid acquisition gradient echo structural images were collected (1-mm slice thickness, 128 slices, matrix: 224 × 224, field of view: 220 × 222). Head motion was restricted by using vacuum bags fitted to the participant's head.
Image Analysis.
Imaging data were analyzed by using statistical parametric mapping (SPM5; www.fil.ion.ucl.ac.uk/spm/software/spm5) and a general linear model (63). The mean degree of descriptiveness and meaning relatedness were modeled as parametric predictors of interest for each stimulus condition. Imageability, number of syllables, and social desirability for social concepts were modeled as covariates of no interest. A separate model was set up including all above variables, with the addition of response time for each stimulus condition to test whether domain-specific effects were due to response time effects.
Categorical contrasts were formed by summing up all effects of interest per condition: (i) condition-specific hemodynamic response function (HRF), (ii) effect of behavior descriptiveness convolved with HRF, and (iii) effect of meaning relatedness convolved with HRF. Reported statistics were performed on the second level by using a random-effects model.
To investigate whether there was a brain region where domain-specific effect (social vs. animal), descriptiveness of social behavior, and meaning relatedness of social concepts were detectable in conjunction (conjunction null analysis), we set up a separate factorial model at the second level. The factorial model included the following contrasts: (i) condition-specific HRF compared with fixation HRF, (ii) effect of behavior descriptiveness, and (iii) meaning relatedness of each condition convolved with the respective HRF.
We inclusively masked each reported categorical contrast (e.g., social vs. animal) with a contrast against the low-level control condition (e.g., social vs. fixation; see SI Methods). Whole-brain analyses were based on an uncorrected voxel level of P = 0.005 (10 voxels minimum cluster size) in a priori predicted regions known from the social and semantic neuroscience literature (see SI Methods). Results from anterior temporal lobe ROI analyses were displayed on an uncorrected voxel level of P = 0.05 (10 voxels minimum cluster size) to show the extent of activation and corroborate regional specificity. All reported coordinates are in Montreal Neurological Institute Space.
Supplementary Material
Acknowledgments
We thank Katherine O'Leary for help with data acquisition, John Bartko for statistical advice, Eric Wassermann for performing neurological exams, and Kris Knutson and several statistical parametric mapping experts from the discussion list for imaging analysis advice. This study was supported by German Academy of Natural Scientists Leopoldina Grant BMBF-LPD 9901/8–122 (to R.Z.); the National Institute of Neurological Disorders and Stroke Intramural Research Program (to J.G.); and Fundação de Amparo á Pesquisa do Estando de São Paulo Grant 03/11794-6 (to G.G.).
Abbreviations
- BA
Brodmann's area
- fMRI
functional MRI
- HRF
hemodynamic response function
- ROI
region of interest.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
Data deposition: The neuroimaging data have been deposited with the fMRI Data Left, www.fmrdc.org (accession no. 2-2007-12248).
This article contains supporting information online at www.pnas.org/cgi/content/full/0607061104/DC1.
References
- 1.Mitchell JP, Heatherton TF, Macrae CN. Proc Natl Acad Sci USA. 2002;99:15238–15243. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell JP, Banaji MR, Macrae CN. NeuroImage. 2005;28:757–762. doi: 10.1016/j.neuroimage.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Mason MF, Banfield JF, Macrae CN. Cereb Cortex. 2004;14:209–214. doi: 10.1093/cercor/bhg120. [DOI] [PubMed] [Google Scholar]
- 4.Blakemore SJ, Winston J, Frith U. Trends Cogn Sci. 2004;8:216–222. doi: 10.1016/j.tics.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Gusnard D, Akbudak E, Shulman G, Raichle M. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amodio DM, Frith CD. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 8.Caramazza A, Mahon BZ. Trends Cogn Sci. 2003;7:354–361. doi: 10.1016/s1364-6613(03)00159-1. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy RA, Warrington EK. Nature. 1988;334:428–430. doi: 10.1038/334428a0. [DOI] [PubMed] [Google Scholar]
- 10.Zahn R, Garrard P, Talazko J, Gondan M, Bubrowski P, Juengling F, Slawik H, Dykierek P, Koester B, Huell M. J Cogn Neurosci. 2006;18:2138–2151. doi: 10.1162/jocn.2006.18.12.2138. [DOI] [PubMed] [Google Scholar]
- 11.Davies RR, Hodges JR, Kril JJ, Patterson K, Halliday GM, Xuereb JH. Brain. 2005;128:1984–1995. doi: 10.1093/brain/awh582. [DOI] [PubMed] [Google Scholar]
- 12.Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Neuropsychologia. 2000;38:1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- 13.Garrard P, Carroll E. Brain. 2006;129:1152–1163. doi: 10.1093/brain/awl069. [DOI] [PubMed] [Google Scholar]
- 14.Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K. Psychol Rev. 2004;111:205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- 15.Jefferies E, Lambon Ralph MA. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- 16.Hampson S, John O, Goldberg L. J Pers Soc Psychol. 1986;51:37–54. doi: 10.1037/0022-3514.51.1.37. [DOI] [PubMed] [Google Scholar]
- 17.Martin A, Weisberg J. Cogn Neuropsychol. 2003;20:575–587. doi: 10.1080/02643290342000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heberlein AS, Saxe RR. NeuroImage. 2005;28:770–777. doi: 10.1016/j.neuroimage.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 19.Kircher TT, Senior C, Phillips ML, Benson PJ, Bullmore ET, Brammer M, Simmons A, Williams SC, Bartels M, David AS. Brain Res Cogn Brain Res. 2000;10:133–144. doi: 10.1016/s0926-6410(00)00036-7. [DOI] [PubMed] [Google Scholar]
- 20.Kircher TT, Brammer M, Bullmore E, Simmons A, Bartels M, David AS. Neuropsychologia. 2002;40:683–692. doi: 10.1016/s0028-3932(01)00138-5. [DOI] [PubMed] [Google Scholar]
- 21.Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H. Am J Psychiatry. 2003;160:1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- 22.Johnson S, Baxter L, Wilder L, Pipe J, Heiserman J, Prigatano G. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz T, Kawahara Baccus T, Johnson S. NeuroImage. 2004;22:941–947. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Mummery CJ, Shallice T, Price CJ. NeuroImage. 1999;9:516–525. doi: 10.1006/nimg.1999.0434. [DOI] [PubMed] [Google Scholar]
- 25.Grabowski TJ, Damasio H, Tranel D, Ponto LL, Hichwa RD, Damasio AR. Hum Brain Mapp. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosch E. J Exper Psychol Gen. 1975;104:192–233. [Google Scholar]
- 27.John OP, Hampson SE, Goldberg LR. J Pers Soc Psychol. 1991;60:348–361. doi: 10.1037//0022-3514.60.3.348. [DOI] [PubMed] [Google Scholar]
- 28.McClelland JL, Rogers TT. Nat Rev Neurosci. 2003;4:310–322. doi: 10.1038/nrn1076. [DOI] [PubMed] [Google Scholar]
- 29.Von Kleist K. In: Geistes und Nervenkrankheiten. Bonhoeffer K, editor. Leipzig: Verlag von Johann Ambrosius Barth; 1922. [Google Scholar]
- 30.Liu W, Miller BL, Kramer JH, Rankin K, Wyss-Coray C, Gearhart R, Phengrasamy L, Weiner M, Rosen HJ. Neurology. 2004;62:742–748. doi: 10.1212/01.wnl.0000113729.77161.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. Nat Rev Neurosci. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- 32.Wicker B, Perrett DI, Baron-Cohen S, Decety J. Neuropsychologia. 2003;41:139–146. doi: 10.1016/s0028-3932(02)00144-6. [DOI] [PubMed] [Google Scholar]
- 33.Gorno-Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, Frackowiak RS. Brain. 1998;121:2103–2118. doi: 10.1093/brain/121.11.2103. [DOI] [PubMed] [Google Scholar]
- 34.Kikyo H, Miyashita Y. NeuroImage. 2004;23:1348–1357. doi: 10.1016/j.neuroimage.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Chao LL, Haxby JV, Martin A. Nat Neurosci. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- 36.Kanwisher N, McDermott J, Chun MM. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondo H, Saleem KS, Price JL. J Comp Neurol. 2003;465:499–523. doi: 10.1002/cne.10842. [DOI] [PubMed] [Google Scholar]
- 38.Sabsevitz DS, Medler DA, Seidenberg M, Binder JR. NeuroImage. 2005;27:188–200. doi: 10.1016/j.neuroimage.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkanen A. AJNR Am J Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- 40.Cato MA, Crosson B, Gokcay D, Soltysik D, Wierenga C, Gopinath K, Himes N, Belanger H, Bauer RM, Fischler IS, et al. J Cogn Neurosci. 2004;16:167–177. doi: 10.1162/089892904322984481. [DOI] [PubMed] [Google Scholar]
- 41.Beauregard M, Chertkow H, Bub D, Murtha S, Dixon R, Evans A. J Cogn Neurosci. 1997;9:441–461. doi: 10.1162/jocn.1997.9.4.441. [DOI] [PubMed] [Google Scholar]
- 42.Spitsyna G, Warren JE, Scott SK, Turkheimer FE, Wise RJ. J Neurosci. 2006;26:7328–7336. doi: 10.1523/JNEUROSCI.0559-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J, Kemeny S, Park G, Frattali C, Braun A. NeuroImage. 2005;25:1002–1015. doi: 10.1016/j.neuroimage.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 44.Vandenberghe R, Nobre AC, Price CJ. J Cogn Neurosci. 2002;14:550–560. doi: 10.1162/08989290260045800. [DOI] [PubMed] [Google Scholar]
- 45.Sharp DJ, Scott SK, Wise RJ. Ann Neurol. 2004;56:836–846. doi: 10.1002/ana.20294. [DOI] [PubMed] [Google Scholar]
- 46.Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Proc Natl Acad Sci USA. 1999;96:9379–9384. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Damasio H, Grabowski T, Tranel D, Hichwa R, Damasio A. Nature. 1996;380:485–486. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- 48.Tranel D. Neuropsychology. 2006;20:1–10. doi: 10.1037/0894-4105.20.1.1. [DOI] [PubMed] [Google Scholar]
- 49.Giovanello KS, Alexander M, Verfaellie M. Neurocase. 2003;9:15–26. doi: 10.1076/neur.9.1.15.14369. [DOI] [PubMed] [Google Scholar]
- 50.Thompson SA, Graham KS, Williams G, Patterson K, Kapur N, Hodges JR. Neuropsychologia. 2004;42:359–370. doi: 10.1016/j.neuropsychologia.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Evans JJ, Heggs AJ, Antoun N, Hodges JR. Brain. 1995;118:1–13. doi: 10.1093/brain/118.1.1. [DOI] [PubMed] [Google Scholar]
- 52.Ellis AW, Young AW, Critchley EM. Brain. 1989;112:1469–1483. doi: 10.1093/brain/112.6.1469. [DOI] [PubMed] [Google Scholar]
- 53.Semenza C, Mondini S, Zettin M. Neurocase. 1995;1:183–188. [Google Scholar]
- 54.Burton AM, Bruce V, Johnston RA. Br J Psychol. 1990;81:361–380. doi: 10.1111/j.2044-8295.1990.tb02367.x. [DOI] [PubMed] [Google Scholar]
- 55.Sirigu A, Duhamel JR, Poncet M. Brain. 1991;114:2555–2573. doi: 10.1093/brain/114.6.2555. [DOI] [PubMed] [Google Scholar]
- 56.Perani D, Cappa SF, Schnur T, Tettamanti M, Collina S, Rosa MM, Fazio F. Brain. 1999;122:2337–2344. doi: 10.1093/brain/122.12.2337. [DOI] [PubMed] [Google Scholar]
- 57.Kiehl KA, Liddle PF, Smith AM, Mendrek A, Forster BB, Hare RD. Hum Brain Mapp. 1999;7:225–233. doi: 10.1002/(SICI)1097-0193(1999)7:4<225::AID-HBM1>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noppeney U, Price CJ. NeuroImage. 2004;22:164–170. doi: 10.1016/j.neuroimage.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 59.Oldfield RC. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 60.Rosenberg M. Society and the Adolescent Self-Image. Middleton, CT: Wesley Univ Press; 1989. [Google Scholar]
- 61.Watson D, Clark LA, Tellegen A. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 62.McRae K, Cree GS, Seidenberg MS, McNorgan C. Behav Res Methods. 2005;37:547–559. doi: 10.3758/bf03192726. [DOI] [PubMed] [Google Scholar]
- 63.Friston KJ, Frith CD, Turner R, Frackowiak RS. NeuroImage. 1995;2:157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.