Abstract
Polymyxin B nonapeptide, a polymyxin B derivative which lacks the fatty acyl part and the bactericidal activity of polymyxin, was shown to sensitize smooth encapsulated Escherichia coli (O18:K1) and smooth Salmonella typhimurium to hydrophobic antibiotics (novobiocin, fusidic acid, erythromycin, clindamycin, nafcillin, and cloxacillin). The polymyxin B nonapeptide-treated bacteria were as sensitive to these antibiotics as are deep rough mutants. A lysine polymer with 20 lysine residues (lysine 20) had a largely similar effect. Larger lysine polymers and the protamine salmine were bactericidal but, at sublethal concentrations, sensitized the strains to the antibiotics mentioned above, whereas lysine4, streptomycin, cytochrome c, lysozyme, and the polyamines cadaverine, spermidine, and spermine had neither bactericidal nor sensitizing activity.
Full text
PDF

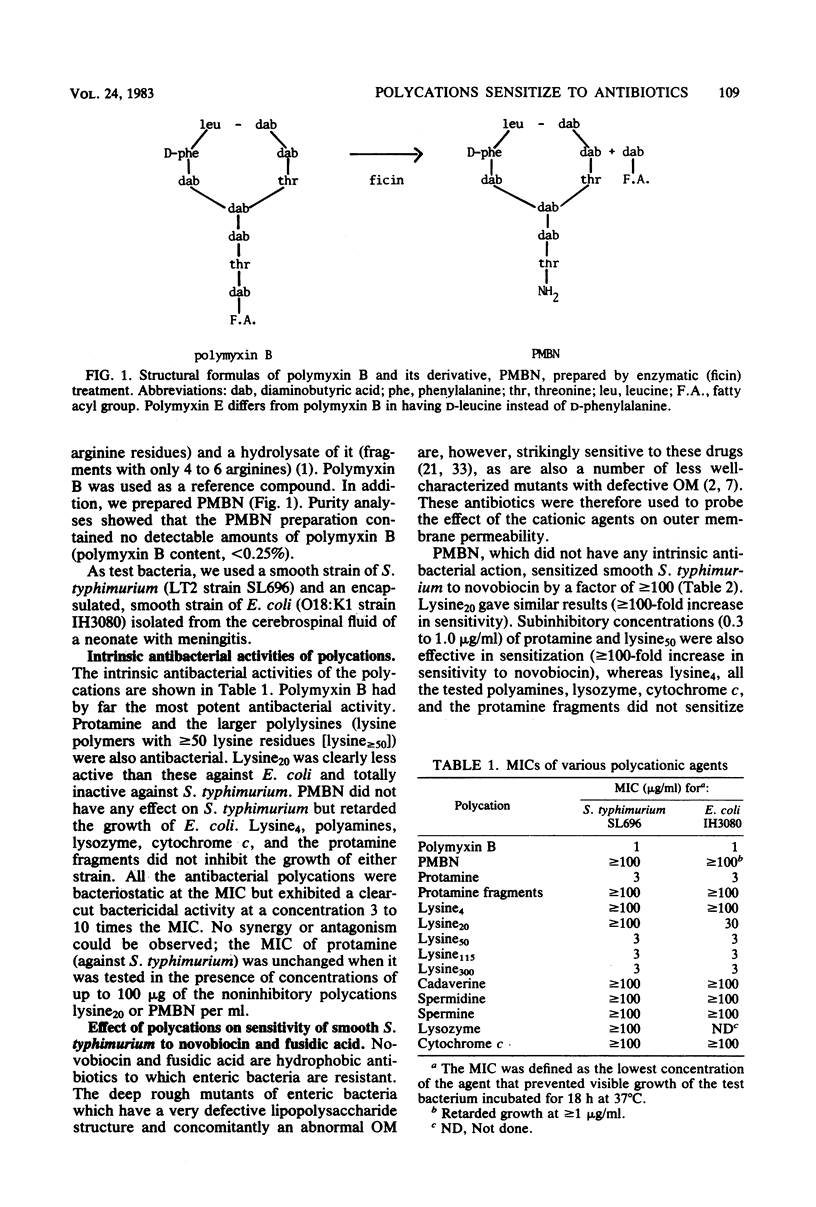
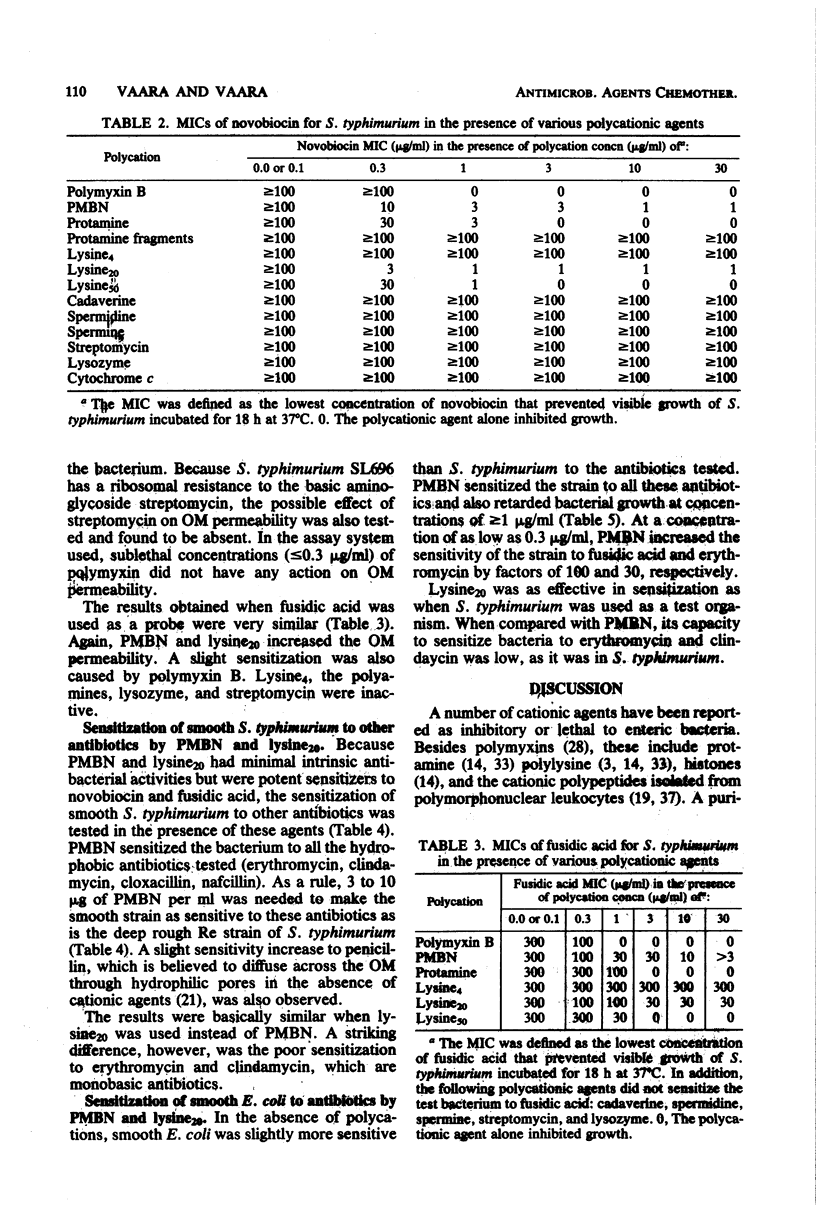



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T., Suzuki K. The amino acid sequence of the third component of clupeine. Biochim Biophys Acta. 1967 Jun 27;140(2):375–377. doi: 10.1016/0005-2795(67)90481-3. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Nordström K., Normark S. Penicillin resistance in Escherichia coli K12: synergism between penicillinases and a barrier in the outer part of the envelope. Ann N Y Acad Sci. 1974 May 10;235(0):569–586. doi: 10.1111/j.1749-6632.1974.tb43291.x. [DOI] [PubMed] [Google Scholar]
- Buchanan-Davidson D. J., Seastone C. V., Stahmann M. A. ACTION OF SYNTHETIC POLYLYSINE ON THE GROWTH AND PHAGOCYTOSIS OF BACTERIA IN VITRO. J Bacteriol. 1960 Nov;80(5):590–594. doi: 10.1128/jb.80.5.590-594.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. F., Martinez R. J. Antibacterial peptide from normal rat serum. 1. Isolation from whole serum, activity, and microbicidal spectrum. Biochemistry. 1981 Oct 13;20(21):5973–5981. doi: 10.1021/bi00524a008. [DOI] [PubMed] [Google Scholar]
- Coleman W. G., Jr, Leive L. Two mutations which affect the barrier function of the Escherichia coli K-12 outer membrane. J Bacteriol. 1979 Sep;139(3):899–910. doi: 10.1128/jb.139.3.899-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. F., Marceau-Day M. L., Ingram J. M. Protein-lipopolysaccharide interactions. 1. The reaction of lysozyme with Pseudomonas aeruginosa LPS. Can J Microbiol. 1978 Feb;24(2):196–199. doi: 10.1139/m78-035. [DOI] [PubMed] [Google Scholar]
- Galanos C. Physical state and biological activity of lipopolysaccharides. Toxicity and immunogenicity of the lipid A component. Z Immunitatsforsch Exp Klin Immunol. 1975 Jul;149(2-4):214–229. [PubMed] [Google Scholar]
- Geyer R., Galanos C., Westphal O., Golecki J. R. A lipopolysaccharide-binding cell-surface protein from Salmonella minnesota. Isolation, partial characterization and occurrence in different Enterobacteriaceae. Eur J Biochem. 1979 Jul;98(1):27–38. doi: 10.1111/j.1432-1033.1979.tb13156.x. [DOI] [PubMed] [Google Scholar]
- Gross R. J., Cheasty T., Rowe B. Isolation of bacteriophages specific for the K1 polysaccharide antigen of Escherichia coli. J Clin Microbiol. 1977 Dec;6(6):548–550. doi: 10.1128/jcm.6.6.548-550.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Raffle V. J., Nicas T. I. Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1981 May;19(5):777–785. doi: 10.1128/aac.19.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D., Steiner H., Rasmuson T., Boman H. G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem. 1980 May;106(1):7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Modrzakowski M. C., Cooney M. H., Martin L. E., Spitznagel J. K. Bactericidal activity of fractionated granule contents from human polymorphonuclear leukocytes. Infect Immun. 1979 Mar;23(3):587–591. doi: 10.1128/iai.23.3.587-591.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Bavoil P., Hirota Y. Outer membranes of gram-negative bacteria. XV. Transmembrane diffusion rates in lipoprotein-deficient mutants of Escherichia coli. J Bacteriol. 1977 Dec;132(3):1045–1047. doi: 10.1128/jb.132.3.1045-1047.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Song S. A., Shaltiel L., Nurminen M. Outer membrane of Salmonella XIV. Reduced transmembrane diffusion rates in porin-deficient mutants. Biochem Biophys Res Commun. 1976 May 23;76(2):324–330. doi: 10.1016/0006-291x(77)90728-8. [DOI] [PubMed] [Google Scholar]
- Paakkanen J., Gotschlich E. C., Mäkelä P. H. Protein K: a new major outer membrane protein found in encapsulated Escherichia coli. J Bacteriol. 1979 Sep;139(3):835–841. doi: 10.1128/jb.139.3.835-841.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Wotton S. Comparative study of seven cephalosporins: susceptibility to beta-lactamases and ability to penetrate the surface layers of Escherichia coli. Antimicrob Agents Chemother. 1976 Aug;10(2):219–222. doi: 10.1128/aac.10.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Ross H., Ziegler L., Mäkelä P. H. F + , Hfr, and F' strains of Salmonella typhimurium and Salmonella abony. Bacteriol Rev. 1972 Dec;36(4):608–637. doi: 10.1128/br.36.4.608-637.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Osborn M. J. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry. 1979 Oct 2;18(20):4425–4430. doi: 10.1021/bi00587a024. [DOI] [PubMed] [Google Scholar]
- Stan-Lotter H., Gupta M., Sanderson K. E. The influence of cations on the permeability of the outer membrane of Salmonella typhimurium and other gram-negative bacteria. Can J Microbiol. 1979 Apr;25(4):475–485. doi: 10.1139/m79-070. [DOI] [PubMed] [Google Scholar]
- Storm D. R., Rosenthal K. S., Swanson P. E. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–763. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- Tabor C. W. STABILIZATION OF PROTOPLASTS AND SPHEROPLASTS BY SPERMINE AND OTHER POLYAMINES. J Bacteriol. 1962 May;83(5):1101–1111. doi: 10.1128/jb.83.5.1101-1111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuber M., Bader J. Action of polymyxin B on bacterial membranes. Binding capacities for polymyxin B of inner and outer membranes isolated from Salmonella typhimurium G30. Arch Microbiol. 1976 Aug;109(1-2):51–58. doi: 10.1007/BF00425112. [DOI] [PubMed] [Google Scholar]
- Vaara M. Increased outer membrane resistance to ethylenediaminetetraacetate and cations in novel lipid A mutants. J Bacteriol. 1981 Nov;148(2):426–434. doi: 10.1128/jb.148.2.426-434.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T., Jensen M., Helander I., Nurminen M., Rietschel E. T., Mäkelä P. H. Characterization of the lipopolysaccharide from the polymyxin-resistant pmrA mutants of Salmonella typhimurium. FEBS Lett. 1981 Jun 29;129(1):145–149. doi: 10.1016/0014-5793(81)80777-6. [DOI] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations as outer membrane-disorganizing agents. Antimicrob Agents Chemother. 1983 Jul;24(1):114–122. doi: 10.1128/aac.24.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T., Sarvas M. Decreased binding of polymyxin by polymyxin-resistant mutants of Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):664–667. doi: 10.1128/jb.139.2.664-667.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Elsbach P., Olsson I., Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978 Apr 25;253(8):2664–2672. [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]


