Abstract
Phosphate (Pi) deficiency limits plant growth and development, resulting in adaptive stress responses. Among the molecular determinants of Pi stress responses, transcription factors play a critical role in regulating adaptive mechanisms. WRKY75 is one of several transcription factors induced during Pi deprivation. In this study, we evaluated the role of the WRKY75 transcription factor in regulating Pi starvation responses in Arabidopsis (Arabidopsis thaliana). WRKY75 was found to be nuclear localized and induced differentially in the plant during Pi deficiency. Suppression of WRKY75 expression through RNAi silencing resulted in early accumulation of anthocyanin, indicating that the RNAi plants were more susceptible to Pi stress. Further analysis revealed that the expression of several genes involved in Pi starvation responses, including phosphatases, Mt4/TPS1-like genes, and high-affinity Pi transporters, was decreased when WRKY75 was suppressed. Consequently, Pi uptake of the mutant plant was also decreased during Pi starvation. In addition, when WRKY75 expression was suppressed, lateral root length and number, as well as root hair number, were significantly increased. However, changes in the root architecture were obvious under both Pi-sufficient and Pi-deficient conditions. This indicates that the regulatory effect of WRKY75 on root architecture could be independent of the Pi status of the plant. Together, these results suggest that WRKY75 is a modulator of Pi starvation responses as well as root development. WRKY75 is the first member of the WRKY transcription factor family reported to be involved in regulating a nutrient starvation response and root development.
Phosphate (Pi) is one among the least-available macronutrients required by plants. It is a constituent of key molecules such as ATP, nucleic acids, and phospholipids. It plays a crucial role in energy transfer, metabolic regulation, and protein activation (Marschner, 1995). Although abundant phosphorus (P) is present in many soils, very little of it is present in ionic forms that are available to plants. Consequently, plants have evolved several adaptive mechanisms by way of altered morphology, physiology, and biochemical processes that help them cope with Pi deficiency (Bucher et al., 2001; Raghothama and Karthikeyan, 2005). These biochemical and physiological adaptations, such as elevated phosphatase activity (Lipton et al., 1987) and secretion of organic acids (Marschner, 1995), help augment the availability of both endogenous and exogenous Pi. In addition, developmental responses involving changes in root growth and architecture enhance the exploration of soil Pi resources (López-Bucio et al., 2003).
Over the past decade, many genes, including Pi transporters, phosphatases, RNases, and others of unknown function that help plants adapt to Pi stress, have been characterized. It is also becoming clear that the acquisition, allocation, and metabolism of Pi are highly regulated processes that directly affect plant performance (Franco-Zorilla et al., 2004). Microarray analysis of 22,810 Arabidopsis (Arabidopsis thaliana) genes revealed a coordinated induction and suppression of 612 and 254 Pi-responsive genes, respectively (Misson et al., 2005). While details of the adaptive mechanisms in response to Pi starvation are emerging, the molecular determinants that regulate these adaptive processes have yet to be clearly elucidated. Among the known regulators, microRNA miR399, representing a class of noncoding small RNAs that generally function as posttranscriptional negative regulators, has been implicated in regulating Pi homeostasis (Bari et al., 2006; Chiou et al., 2006). SIZ1, a SUMO E3 ligase, is also known to control Pi homeostasis at the posttranslational level through sumoylation (Miura et al., 2005). At the transcriptional level, the spatiotemporal regulation of transcription factors responsive to Pi starvation has been described by microarray analysis in rice (Oryza sativa) and Arabidopsis (Wasaki et al., 2003; Wu et al., 2003; Misson et al., 2005). A potential transcription factor candidate, Phi-2, coding for a bZIP transcription factor in tobacco (Nicotiana tabacum), was reported to be induced during Pi starvation earlier (Sano and Nagata, 2002). Despite these developments, to date only two transcription factors involved in regulating Pi starvation responses in higher plants have been examined in detail. The Myb transcription factor PHR1 was first reported to play a regulatory role in Pi starvation responses in Arabidopsis (Rubio et al., 2001). More recently, OsPTF1, a bHLH transcription factor, has been shown to provide tolerance to Pi starvation in rice (Yi et al., 2005). However, PHR1 expression is not responsive to Pi starvation and it controls only a small subset of Pi starvation responses, whereas OsPTF1 does not regulate any known high-affinity Pi transporter. A transcriptional regulatory model for the Pi starvation responses in plants has been proposed on the basis of microarray analysis (Hammond et al., 2004). Thus, the regulation of the global Pi starvation responses by transcription factors is an interesting area of study that needs further attention.
Previously, we analyzed the expression patterns of Pi stress-responsive genes, including transcription factors, in Arabidopsis through microarray analysis (Misson et al., 2005). Among the putative candidates, WRKY75, a transcription factor, was strongly induced during Pi deprivation. Members of the plant-specific WRKY transcription factor family are known to be involved in regulating many plant processes, including biotic and abiotic stresses. An analysis of the expression profiles of all the members of the WRKY gene family suggested that the expression of WRKY75 is also induced during pathogen infection (Dong et al., 2003). Although most WRKY proteins studied thus far have been implicated in regulating biotic stress responses, several of them play a role in the regulation of abiotic stresses. In barley (Hordeum vulgare), a transcription factor belonging to the WRKY family, SUSIBA2 (a sugar-responsive element binding factor), was shown to be involved in sugar signaling (Sun et al., 2003). Interestingly, sugar signaling has been implicated in the regulation of Pi starvation responses and root architecture in Arabidopsis (Müller et al., 2005; Karthikeyan et al., 2007). Another WRKY gene in barley, Hv-WRKY38, is involved in regulating cold and drought stress response (Mare et al., 2004). In Arabidopsis, microarray studies have suggested a role for WRKY transcription factors in drought, cold, or high salinity stress (Seki et al., 2002). Several WRKY transcription factors, such as WRKY4, 6, 11, and 53, of Arabidopsis have also been shown to play a role in leaf senescence (Eulgem et al., 2000; Robatzek and Somssich, 2001; Miao et al., 2004). The involvement of WRKY75 during senescence has also been suggested based on a microarray analysis (Guo et al., 2004) as well as unpublished data (Y. Guo and S. Gan). This is particularly interesting since leaf senescence and anthocyanin accumulation are often observed during nutrient stress (Buchanan-Wollaston et al., 2003).
In this study, the function of WRKY75 is investigated and its characterization as a regulator of Pi stress responses is reported. We demonstrate that WRKY75 is responsive to Pi stress and is nuclear localized. RNAi suppression of WRKY75 resulted in impaired Pi starvation responses. In contrast, the same mutant shows increased root growth independent of the Pi status of the plant. These results indicate that WRKY75 is a positive regulator of Pi starvation responses besides having an impact on root development. To our knowledge, this is the first report showing a WRKY transcription factor involved in regulation of nutrient starvation responses in plants.
RESULTS
WRKY75 Responds to Pi Starvation and Is Nuclear Localized
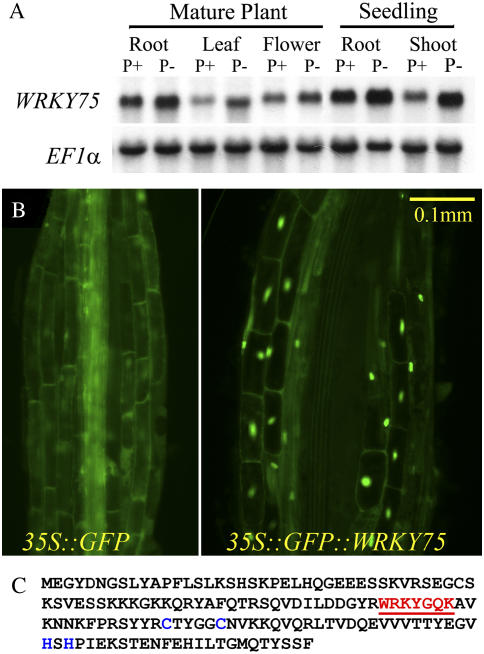
The relative transcript abundance of WRKY75 in mature plants as well as young seedlings grown under Pi-sufficient (P+) or Pi-deficient (P−) conditions was evaluated (Fig. 1A). Although a basal level of WRKY75 transcripts was observed under Pi-sufficient conditions, during Pi deprivation there was a distinct but differential increase in the transcript's abundance in different part of the plants. This highlights the spatial regulation of WRKY75 by Pi. Upon transferring Pi-deprived plants into medium with sufficient Pi, a decrease in the elevated transcript levels was observed within 3 h (Supplemental Fig. S1). These results suggest that WRKY75 is among the early induced genes that respond to altered Pi status of the plant.
Figure 1.
WRKY75 expression during Pi deprivation and its subcellular localization. A, RNA-blot analysis of WRKY75 gene expression. Arabidopsis plants were grown either hydroponically or in liquid culture conditions for 7 d and then transferred to medium containing Pi (P+) or lacking Pi (P−), where they were grown for an additional 7 d. Roots, rosette leaves, and flowers were collected from mature plants grown hydroponically, whereas roots and shoots were collected from young seedlings grown in liquid culture. Ten micrograms of total RNA from these samples was separated electrophoretically and blotted onto a nylon membrane that was then probed with a 32P-labeled WRKY75 cDNA. The membrane was subsequently stripped and rehybridized with an elongation factor (EF1α) gene probe used as a loading control. B, Subcellular localization of a GFP∷WRKY75 fusion protein. Microscopic images of root cells from Arabidopsis plants transformed with a control gene, 35S∷GFP (left), or a 35S∷GFP∷WRKY75 fusion gene (right). C, Amino acid sequence of WRKY75 showing the highly conserved WRKY domain WRKYGQK and the novel C2H2 zinc finger motif in red and blue letters, respectively.
It is known that nutrient starvation responses could be regulated by controlling the subcellular localization of a transcription factor (Beck and Hall, 1999). Therefore, to identify the subcellular localization of WRKY75, its coding region was fused with the 3′ end of an ENHANCED GREEN FLUORESCENT PROTEIN (EGFP) reporter gene and expressed constitutively under the control of a strong cauliflower mosaic virus (CaMV) 35S promoter. The EGFP gene alone under the control of the CaMV 35S promoter served as a control. Transgenic Arabidopsis plants expressing the control EGFP gene and the chimeric WRKY∷GFP gene were analyzed under both Pi-sufficient and Pi-deficient conditions. In the control transgenic plant, GFP fluorescence was uniformly distributed all over the cell, whereas, in the plants with the WRKY75-GFP protein, fluorescence was localized in the nucleus (Fig. 1B). The WRKY75-GFP fusion protein was localized to the nucleus irrespective of the Pi regimen under which the plants were grown. Further, WRKY transcription factors are defined by the presence of a highly conserved WRKY domain and a novel zinc finger motif in their amino acid sequence. An examination of the amino acid sequence of WRKY75 revealed the presence of a single WRKY domain as well as the characteristic C2H2 zinc finger motif located at the C-terminal end (Fig. 1C). Together, these data suggest that WRKY75 is a Pi starvation-responsive transcription factor localized in the nucleus.
Suppression of WRKY75 Expression through RNAi-Mediated Silencing
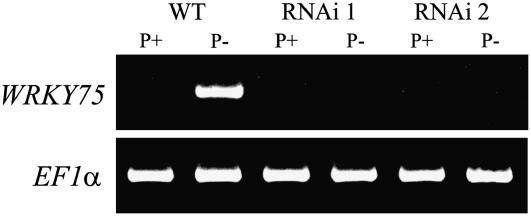
To characterize the role of WRKY75 during Pi starvation, RNAi mutant plants harboring inverted repeats of a WRKY75 fragment under the control of a CaMV 35S promoter were generated. Silencing of WRKY75 in the RNAi plants was confirmed by semiquantitative reverse transcription (RT)-PCR analysis of the wild-type control and two independent RNAi lines grown under different Pi regimens (Fig. 2). Amplifying the first-strand cDNA through 25 cycles of PCR indicated the absence of any WRKY75 transcripts in the RNAi plants, suggesting the silencing of the gene. However, when 2 μL of the product from this PCR was used as the template for a second PCR with an additional 25 cycles, faint DNA bands were observed in the lanes representing the RNAi samples. Strong DNA bands were also observed in lanes representing the wild-type plants grown under Pi-sufficient conditions after the second set of PCR cycles (data not shown). The presence of WRKY75 transcripts in the wild-type plants during Pi-sufficient conditions was consistent with the results of northern analysis (Fig. 1A). On the other hand, the presence of a few copies of the WRKY75 transcripts in the RNAi plants suggests that the expression of WRKY75 is drastically reduced, but not completely silenced, in the RNAi lines. A representative RNAi line showing almost complete suppression of WRKY75 was selected from among 10 lines for subsequent characterization. In addition, a SALK T-DNA insertion mutant for WRKY75 (kindly provided by Dr. Z. Chen, Department of Botany and Plant Pathology, Purdue University) was used as an internal control for most of the experiments and showed similar results as the RNAi line (data not shown).
Figure 2.
RT-PCR analysis of WRKY75 RNAi plants. Semiquantitative RT-PCR was performed using RNA extracted from wild type (WT) and two independent RNAi lines grown in liquid culture for 7 d under Pi-sufficient (P+) and Pi-deficient (P−) conditions. Gene-specific primers were used for WRKY75 cDNA. EF1α was used as a constitutive control gene. The figure indicates the product from 25 PCR cycles.
Suppression of WRKY75 Leads to Early Accumulation of Anthocyanin during Pi Deprivation
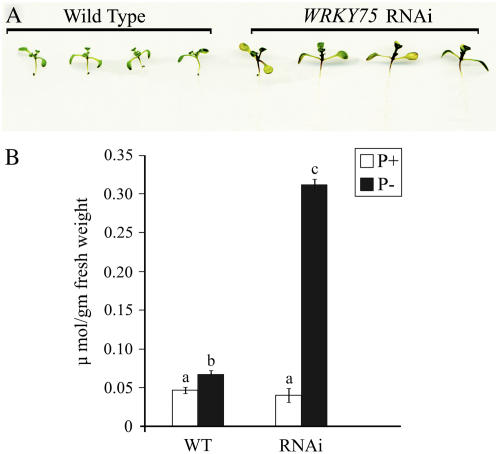
One of the most striking symptoms of Pi starvation in plants is the accumulation of anthocyanin (Marschner, 1995). When WRKY75 RNAi and the wild-type plants were grown in petri dishes on medium without Pi, it was observed that WRKY75 RNAi plants turned purple by the third day of Pi starvation, whereas the wild-type plants were still green (Fig. 3A). Comparative analysis of the anthocyanin content in 10-d-old WRKY75 RNAi and the wild-type seedlings was done after growing them for 3 d on medium with (1 mm) or without Pi (Fig. 3B). The results indicated that the anthocyanin content in the RNAi seedlings was approximately 5-fold higher than in the wild-type seedlings after 3 d of Pi deprivation. The anthocyanin content in the RNAi seedlings grown under Pi-sufficient conditions did not vary significantly in comparison to the wild type. The wild-type plants accumulated similar levels of anthocyanin after three more days of Pi deprivation (data not shown). The data indicate that the suppression of WRKY75 accelerates the process of anthocyanin accumulation during Pi starvation. Further, it was observed that the accelerated accumulation of anthocyanin in RNAi seedlings did not occur during nitrogen, potassium, or iron deficiency, indicating that it was specific to Pi starvation (data not shown). This suggested that some of the adaptive mechanisms to Pi stress could be impaired by the suppression of WRKY75 in the plant.
Figure 3.
Anthocyanin accumulation in WRKY75 RNAi plants during Pi deprivation. Seven-day-old seedlings of wild-type and WRKY75 RNAi plants grown on half-strength MS medium were transferred into MS medium in petri dishes with Pi (1 mm) or without Pi (P−). Images and anthocyanin content were recorded on the third day of treatment. A, The seedlings grown in P− conditions were scanned at 600 dpi, showing the early accumulation of anthocyanin in the hypocotyl and cotyledonary leaves of the WRKY75 RNAi plants as compared to the wild type (WT). The samples are representative of 15 seedlings of each genotype. B, Anthocyanin content was determined in wild-type and WRKY75 RNAi plants grown with Pi and without Pi on the third day of Pi starvation. Values are mean ± se (n = 5), and different letters above the bars indicate that the means are statistically different (P < 0.05). [See online article for color version of this figure.]
Pi Starvation-Induced Genes Are Attenuated upon Suppression of WRKY75
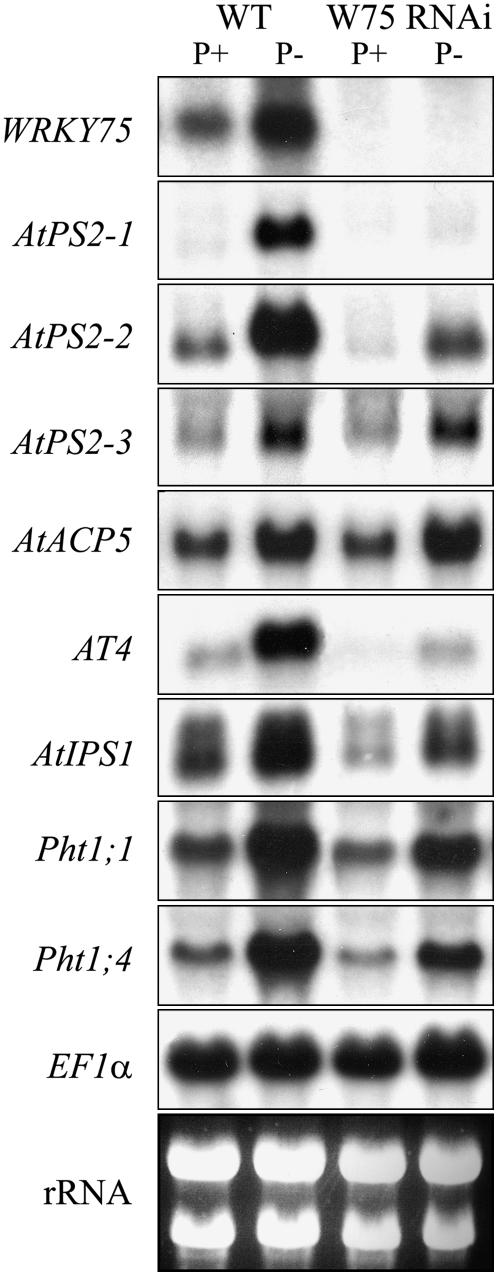
Global gene expression analysis using microarray techniques has shown an array of spatiotemporally regulated Pi-responsive genes involved in Pi mobilization and acquisition from the soil and its remobilization within the plant (Misson et al., 2005). Therefore, to elucidate the molecular components involved in triggering the early Pi stress response in WRKY75 RNAi plants, expression of some of the well-characterized Pi-responsive genes involved in maintaining Pi homeostasis was evaluated in both the wild-type and WRKY75 RNAi plants grown under Pi-sufficient and Pi-deficient conditions (Fig. 4). The absence of WRKY75 transcripts in the mutant as compared to the wild-type plants confirmed the suppression of WRKY75 in the mutant. To find if defective mobilization of Pi from the media was the cause of early Pi stress symptoms in the mutant, the expression of AtPS2-1, AtPS2-2, and AtPS2-3, encoding three members of a phosphatase family specifically induced by Pi stress (K.G. Raghothama, unpublished data), which are orthologs of the LePS2 gene family from tomato (Solanum lycopersicum; Baldwin et al., 2001), was evaluated. In addition, the expression of AtACP5, encoding an acid phosphatase involved in Pi stress responses (Del Pozo et al., 1999), was also evaluated. There was a complete suppression of AtPS2-1 expression, significant reduction in the transcript levels of AtPS2-2, and no change in the AtPS2-3 and AtACP5 transcripts in the mutant as compared to the wild-type plants. These results showed that suppression of WRKY75 had differential effects on the genes involved in Pi mobilization. Recent studies on at4, a loss-of-function mutant, revealed the role of At4, a member of the Mt4/TPSI1 gene family, in Pi distribution between roots and shoots (Shin et al., 2006). We therefore evaluated the expression profiles of At4 and AtIPS1, another member of the Mt4/TPSI1 gene family that is involved in cytokinin signaling during Pi starvation (Martín et al., 2000). Interestingly, there was a marked reduction in the transcripts of both At4 and AtIPS1 in the mutant as compared to the wild-type plants during Pi deprivation. WRKY75 suppression also resulted in the reduced expression of Pht1;1 and Pht1;4, which encode high-affinity Pi transporters (Muchhal et al., 1996; Shin et al., 2004). Together, these results reveal the global effect of WRKY75 suppression on an array of Pi deficiency-induced genes involved in Pi mobilization, translocation, and acquisition. The novel C2H2 zinc finger of plant WRKY proteins serves as a DNA-binding domain that specifically recognizes TTGAC/T (W box) elements (Ulker and Somssich, 2004). Several studies have suggested that WRKY transcription factors regulate their target genes by binding to the W boxes on their promoters (Yu et al., 2001; Kim et al., 2006). An in silico analysis of the promoter regions of known Pi stress-responsive genes, including the ones analyzed here, indicated that most of them have one or more W boxes on their promoters (Table I).
Figure 4.
RNA-blot analysis showing the effect of WRKY75 RNAi knockdown on the expression of Pi starvation-responsive genes. Wild-type (WT) and WRKY75 RNAi (W75 RNAi) plants grown in liquid culture under Pi-sufficient (P+) and Pi-deficient (P−) conditions for 7 d were used for RNA extraction. Total RNA (15 μg) was electrophoretically separated, blotted onto a nylon membrane, and hybridized with a 32P-labeled WRKY75 probe. The membrane was stripped and subsequently rehybridized with probes corresponding to the following genes consecutively: AtPS2-1, AtPS2-2, AtPS2-3, AtACP5, At4, AtIPS1, Pht1;1, and Pht1;4. EF1α was used as the loading control. Ethidium bromide-stained rRNA prior to blotting demonstrates the RNA integrity.
Table I.
In silico analysis of the cis-regulatory regions of Pi starvation-responsive genes for predicted WRKY-binding W boxes (ttgacc/t)
| Gene | No. of W Box Promoter Motifs
|
Gene Function during Pi Starvation | Reference | |
|---|---|---|---|---|
| ttgacc | ttgact | |||
| At4 | 2 | – | Internal Pi allocation | Shin et al. (2006) |
| AtIPS1 | – | 1 | Cytokinin signaling | Martín et al. (2000) |
| Pht1;1 | 1 | 4 | Pi transporter | Muchhal et al. (1996) |
| Pht1;3 | 1 | 3 | Pi transporter | Okumura et al. (1998) |
| Pht1;4 | 1 | 3 | Pi transporter | Muchhal et al. (1996) |
| Pht1;5 | – | 1 | Pi transporter | Okumura et al. (1998) |
| Pht1;7 | – | 3 | Pi transporter | Mudge et al. (2002) |
| Pht1;8 | – | 1 | Pi transporter | Mudge et al. (2002) |
| Pht1;9 | – | 2 | Pi transporter | Mudge et al. (2002) |
| AtPS2-1 | 3 | 3 | Protein phosphatase | K.G. Raghothama (unpublished data) |
| AtPS2-2 | – | 1 | Protein phosphatase | K.G. Raghothama (unpublished data) |
| AtPS2-3 | – | – | Protein phosphatase | K.G. Raghothama (unpublished data) |
| PHR1 | – | – | Transcription factor | Rubio et al. (2001) |
| ACP5 | – | – | Type 5 acid Phosphatase | Del Pozo et al. (1999) |
| PAP11 | 1 | – | Purple acid phosphatase | Li et al. (2002) |
| PLDζ2 | – | – | Phosphate recycling | Cruz-Ramírez et al. (2006) |
| RNS1 | 1 | 1 | Ribonuclease RNS1 | Bariola et al. (1994) |
| SQD1 | 1 | – | Sulfolipid biosynthesis gene SQD1 | Essigmann et al. (1998) |
| SQD2 | 1 | 2 | Sulfolipid biosynthesis gene SQD2 | Yu et al. (2002) |
| Vsp2 | 3 | 1 | Vegetative storage protein | Utsugi et al. (1998) |
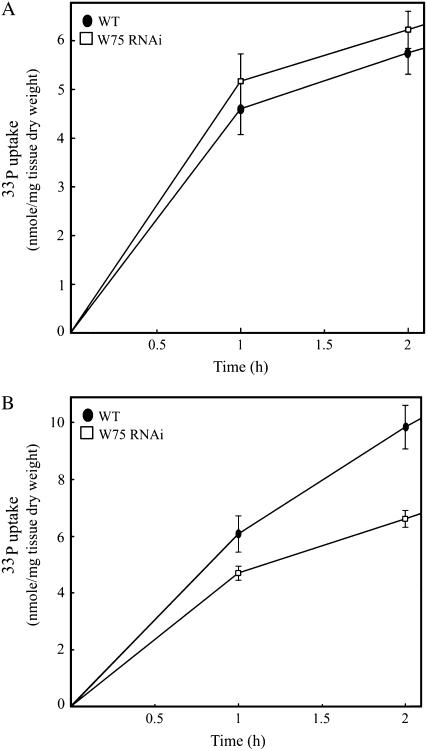
Reduced Pi Uptake by the WRKY75 RNAi Mutant during Pi Deprivation
Pi uptake experiments were carried out to further decipher the effect of an impaired Pi starvation response caused by the suppression of WRKY75. The wild-type plants and WRKY75 RNAi mutants were grown under Pi-sufficient or Pi-deficient conditions. Ten-day-old seedlings were transferred into a Pi uptake solution containing 50 μm Pi supplemented with 33P, a radiotracer. Pi uptake over a 2-h period was measured (Fig. 5). There was no significant difference in Pi uptake of the wild-type and WRKY75 RNAi plants grown under Pi-sufficient conditions (Fig. 5A). In contrast, WRKY75 RNAi plants showed a substantial reduction in Pi uptake relative to the wild type when the plants were grown under Pi-deficient conditions (Fig. 5B). The suppression of WRKY75 thus appears to have an effect on Pi uptake rate during Pi deprivation, but not during normal conditions, when the Pi starvation response mechanism is not active. This result was consistent with the reduced expression of several genes involved in Pi homeostasis observed in the WRKY75 mutant (Fig. 4). Together, these data suggest that WRKY75 functions as a positive regulator of the Pi starvation response mechanism, possibly through the control of various genes involved in the process. In an in planta labeling experiment, 10-d-old wild-type and WRKY75 RNAi seedlings were allowed to absorb 33P in the uptake solution for 4 h and the pattern of distribution of the radiotracer was monitored after 10 d (Supplemental Fig. S2). The results indicated no difference in the pattern of movement of 33P within the WRKY75 mutant as compared to the wild-type plants. This suggests that the acquisition of Pi, but not its internal movement, is affected by the suppression of WRKY75.
Figure 5.
Decreased Pi uptake in WRKY75 RNAi plants during Pi starvation. Wild-type plants (black circles) and WRKY75 RNAi plants (white squares) were grown on 0.5× MS medium for 7 d and then transferred as groups of 10 seedlings into Pi-sufficient or Pi-deficient medium for 3 d. The Pi uptake of these 10-d-old seedlings was monitored over a 2-h period. A, Pi uptake in plants from Pi-sufficient conditions. B, Pi uptake in plants from Pi-deficient conditions. Error bars represent se (n = 3).
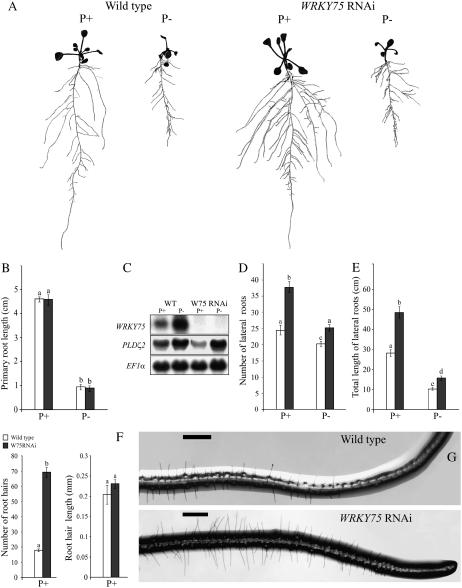
Suppression of WRKY75 Affects the Development of Lateral Roots and Root Hair
Modulation of the root system architecture (RSA) is one of the adaptive responses to Pi starvation in Arabidopsis (López-Bucio et al., 2003). Since the suppression of WRKY75 caused the early accumulation of anthocyanin, reduced expression of some of the Pi-responsive genes, and reduced 33P uptake (Figs. 3–5), it was logical to investigate its effect on the RSA as well. We therefore examined the RSA of the wild type and the WRKY75 RNAi mutant grown in vertically oriented agar plates under Pi-sufficient and Pi-deficient conditions for 7 d (Fig. 6). The Pi deprivation resulted in a significant reduction of primary root growth in both the wild type and the mutant (Fig. 6B). This result was in agreement with an earlier study showing that Pi deficiency induced progressive loss of meristematic cells in the primary root, thereby causing determinate growth (Sánchez-Calderón et al., 2005). However, the effect of WRKY75 suppression in the mutant was not evident on the primary root growth both under Pi-sufficient and Pi-deficient conditions and was comparable to that of the wild-type plants (Fig. 6B). This result indicated that localized Pi deficiency-induced inhibition of primary root growth (Ticconi et al., 2004) is not influenced by the suppression of WRKY75. A recent study on the analysis of the pldz2 mutant suggested the role of Pi deficiency-induced PLDZ2 in the maintenance of RSA (Cruz-Ramírez et al., 2006). We therefore compared the expression of PLDZ2 in the wild type and the mutant grown under Pi-sufficient and Pi-deficient conditions (Fig. 6C). No difference was observed in the levels of PLDZ2 transcripts in Pi-deficient mutants as compared to the wild-type plants, thus substantiating the data on primary root growth. On the other hand, there was a significant increase (P < 0.05) in the number (Fig. 6D) and total length (Fig. 6E) of the lateral roots of the mutant under both Pi-sufficient and Pi-deficient conditions as compared to the wild-type plants. This suggested that the effect of WRKY75 suppression on the RSA may be independent of the Pi status of the plant. A similar accentuated effect of WRKY75 suppression was also evident on the number of root hairs in the region spanning 5 mm from the root tip of the mutant grown under Pi-sufficient condition (Fig. 6, F and G). However, the root hair length was comparable to the wild type (Fig. 6F). Together, the results suggest that WRKY75 plays an important role in root development by negatively regulating lateral root and root hair growth in a process that might be independent of the Pi status of the plant.
Figure 6.
Root architecture of WRKY75 RNAi plants. Wild-type and WRKY75 RNAi plants were grown under Pi-sufficient (P+) and Pi-deficient (P−) conditions for 7 d on vertically oriented petri plates. A, Lateral roots were spread to reveal the architectural details and scanned at 600 dpi. The seedlings shown are representative of 20 seedlings of the wild type and WRKY75 RNAi mutants grown under P+ and P− conditions. B, D, E, and F show comparative histograms of wild-type (white bars) and WRKY75 RNAi plants (black bars) with regard to various components of their root architecture under P+ or P− conditions. Different letters on the bars represent means that are statistically different (P < 0.02). Values are means ± se (n = 16) of each genotype per treatment. B, Primary root length. C, RNA-blot analysis showing the effect of WRKY75 RNAi knockdown on the expression of PLDζ2. Total RNA (15 μg) from wild-type (WT) and WRKY75 RNAi (W75 RNAi) plants grown under P+ and P− conditions was electrophoretically separated and blotted onto a nylon membrane. The membrane was initially hybridized with a 32P-labeled WRKY75 probe and later rehybridized with a probe for PLDζ2. EF1α was used as the loading control. D, Total length of all first-order lateral roots per plant. E, Total number of lateral roots per plant. F, Root hair length and number of root hair under P+ conditions. The data were recorded in an area spanning 5 mm from the root tip. Values are means ± se (n = 5) per genotype. G, Microscopic images of root tips with intact root hair from plants grown under P+ conditions. The scale bars in the panels represent 0.5 mm.
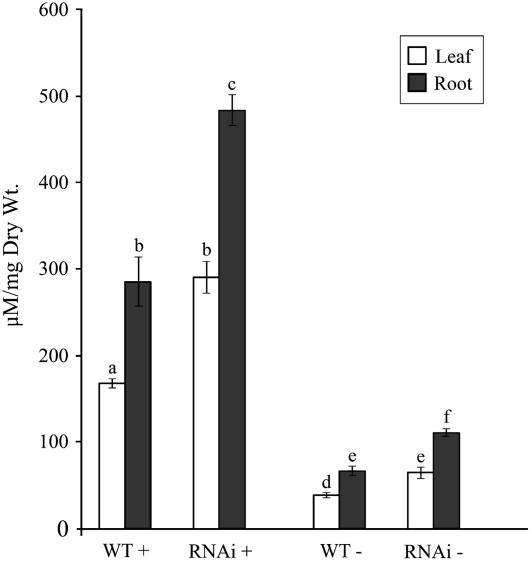
Silencing WRKY75 Influences Accumulation of Total P and Reduces Phosphatase Activity
An increase in the total root surface of a plant improves its ability to explore a greater soil volume and thus acquire more P (López-Bucio et al., 2003). To further analyze the effect of increased root surface area in the WRKY75 RNAi mutants, total P concentration was estimated in the leaves and the roots of 4-week-old wild-type and mutant plants grown hydroponically in media with (P+) or without (P−) Pi (Fig. 7). A significant increase (P < 0.05) in the total Pi concentration was observed in the leaves and the roots of the WRKY75 RNAi plants as compared to the wild-type plants under both Pi-sufficient and Pi-deficient conditions (Fig. 7). However, relative to plants grown with Pi, the increase in total P concentration under Pi-deficient conditions was significantly lower. These results were in contrast to the Pi uptake measurements where lower Pi uptake in the mutant seedlings was observed (Fig. 5B). To resolve this discrepancy, we estimated the total P concentration of the wild-type and the WRKY75 RNAi mutant seedlings grown in agar plates on media with Pi or without Pi for 7 d (Supplemental Fig. S3). The age of these seedlings is closer to those used for Pi uptake analysis. The Pi concentrations in the leaves and roots of the WRKY75 RNAi and wild-type seedlings grown under Pi-sufficient conditions did not show any significant differences. In seedlings grown under Pi-deficient conditions, there was no significant difference in the Pi concentration of the roots, whereas the leaves of the mutant seedling had significantly lower (P < 0.05) Pi content relative to the wild-type leaves (Supplemental Fig. S3). This result is in agreement with the results from Pi uptake measurements (Fig. 5) and the accelerated accumulation of anthocyanin observed in young mutant seedlings during Pi deprivation (Fig. 3). Together, these results suggest that, despite an impaired Pi uptake mechanism, the increased root surface area of the WRKY75 RNAi plants may help the mutants accumulate more P over a period of time.
Figure 7.
Increased total P concentration in WRKY75 RNAi plants. Pi concentration in leaves (white bars) and roots (black bars) of wild-type and WRKY75 RNAi plants grown hydroponically with Pi (P+: 250 μm) and without Pi (P−) for 7 d. Error bars indicate se (n = 4), and different letters above the bars represent means that are statistically different (P < 0.05).
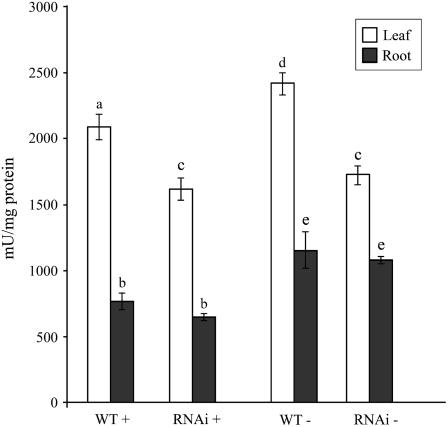
An increase in the production of extracellular and intracellular phosphatases is a distinct and universal response of higher plants to Pi starvation. Consequently, phosphatase activity has been used as a potential marker of the Pi status of plants (Ascencio, 1994). A comparative analysis of the total acid phosphatase activity in the leaves and the roots of wild-type and WRKY75 RNAi plants grown hydroponically under Pi-sufficient or Pi-deficient conditions was performed (Fig. 8). Phosphatase activity was significantly reduced (P < 0.05) in the leaves of the mutant plants grown under both Pi-sufficient and Pi-deficient conditions as compared to the wild-type plants. However, no significant variation was observed in the roots of the WRKY75 RNAi plants relative to the wild-type plants under both Pi regimens.
Figure 8.
Decreased phosphatase activity in WRKY75 RNAi plants. Total acid phosphatase activity in leaves (white bars) and roots (black bars) of wild-type and WRKY75 RNAi plants grown hydroponically with Pi (P+: 250 μm) and without Pi (P−) for 7 d. Error bars indicate se (n = 4), and different letters above the bars represent means that are statistically different (P < 0.05).
DISCUSSION
Proteins belonging to the WRKY superfamily of transcription factors are known to regulate a multiplicity of biotic and abiotic stress responses. Members of the plant-specific WRKY transcription factor family have been implicated in the regulation of genes involved in pathogen-induced stress, as well as drought, cold, and salinity stresses (Seki et al., 2002; Dong et al., 2003). They are also believed to play an important role during senescence (Miao et al., 2004). Their regulatory effect is primarily through their binding with conserved W box elements on the promoters of specific genes (Ulker and Somssich, 2004). We report here the functional characterization of WRKY75, a member of this family. This study shows that WRKY75 is likely to be involved in modulating Pi stress responses and root development. The involvement of WRKY75 in the modification of the root system in addition to regulating Pi starvation responses highlights the intricate cross talk between these biological processes.
WRKY75 Has a Role in Regulating Pi Starvation Responses
The response of higher plants to Pi starvation is a highly regulated process involving the transcriptional activation of numerous genes that help in establishing an adaptive mechanism (Franco-Zorilla et al., 2004). However, information about the transcription factors involved in this complex process is limited. In this study we demonstrate that WRKY75, encoding a WRKY family transcription factor, is induced differentially in different parts of the plant during Pi stress, suggesting its involvement in regulating Pi starvation responses. WRKY75 was also found to be localized to the nucleus irrespective of the Pi status of the plant. This pattern of localization is similar to that of PHR1, a Myb transcription factor from Arabidopsis that has been shown to be involved in regulating several Pi starvation responses (Rubio et al., 2001). Since the expression of PHR1 is not induced upon Pi starvation and it is localized to the nucleus independent of the Pi status of the plant, it was suggested that PHR1 is regulated posttranslationally. In support of this idea, it was recently found that PHR1 is a sumoylation target of SIZ1, a SUMO E3 ligase, and that sumoylation is one of the control mechanisms of Pi deficiency responses (Miura et al., 2005). However, considering that the expression of WRKY75 is strongly induced upon Pi starvation (Fig. 1A) and is rapidly suppressed by Pi resupply (Supplemental Fig. S1), as well as its possible involvement in regulating root growth independent of the Pi status of the plant, sumoylation may not be involved in the regulation of WRKY75.
Gene silencing is one of the effective ways to analyze the function of a gene. We used this approach to generate RNAi lines that showed significant reduction in the expression of the WRKY75 transcripts (Fig. 2). It was observed that the mutant demonstrated accelerated Pi starvation symptoms, as seen by the rapid accumulation of anthocyanin during Pi deprivation (Fig. 3A). Anthocyanin accumulation is a common response to several nutrient stresses. However, the accelerated accumulation of anthocyanin in the WRKY75 RNAi seedlings appears to be a specific response to Pi deprivation and was not observed when they were deprived of other nutrients, such as nitrogen, potassium, or iron. An investigation of the probable cause showed that the expression of a broad spectrum of genes, encoding different components of the Pi starvation response mechanism, was reduced (Fig. 4). These genes included phosphatases such as AtPS2-1 and AtPS2-2 (K.G. Raghothama, unpublished data), high-affinity transporters Pht1;1 and Pht1;4 (Muchhal et al., 1996), and components of the Pi signaling and allocation mechanisms (AtIPS1 and At4, respectively; Martín et al., 2000; Shin et al., 2006). This suggests that WRKY75 could be implicated in the regulation of genes involved in the acquisition of Pi during Pi starvation. Interestingly, the suppression of WRKY75 resulted in the reduced expression of several genes involved in Pi starvation responses but had no negative effect on the anthocyanin accumulation during Pi starvation. This was surprising considering that several genes in the anthocyanin biosynthetic pathway are induced during Pi deprivation (Misson et al., 2005). However, the constitutive accumulation of anthocyanin in the plants upon Pi deprivation, when WRKY75 transcripts are induced in wild-type plants but remain suppressed in WRKY75 RNAi plants, suggests that WRKY75 has a minor, if any, role in regulating anthocyanin biosynthesis. The accelerated accumulation of anthocyanin in WRKY75 RNAi plants during Pi stress could also be a response to the significant decrease in the leaf Pi concentration (Supplemental Fig. S3) due to the reduced Pi uptake in these plants (Fig. 5B). Thus, although the results presented here reinforce the notion that anthocyanin accumulation is a primary response to Pi starvation, more detailed studies are required to understand the role of WRKY75 in this process.
WRKY transcription factors are known to regulate specific genes by binding to the conserved TTGAC/T W boxes on their promoter regions (Ulker and Somssich, 2004). A differentially reduced transcript level of Pi stress-responsive genes involved in maintaining Pi homeostasis was observed in the WRKY75 RNAi plants (Fig. 4). The differential level of suppression appeared to be correlated to a combination of the number and type of W boxes predicted in the promoter regions of Pi-responsive genes by in silico analysis (Table I). Genes that were completely suppressed in the WRKY75 RNAi plants, such as AtPS2-1 and At4, had multiple predicted W boxes on their promoters, with at least two of them having the TTGACC motif. On the other hand, genes that were not affected by the suppression of WRKY75 (AtACP5, AtPS2-3, and PLDZ2) had no predicted W box motifs on their promoters. Genes that were partially suppressed, such as AtPS2-2 and AtIPS1, had only one predicted W box with the TTGACT motif. Partially suppressed genes with multiple predicted W boxes, Pht1;1 and Pht1;4, had more W boxes with the TTGACT motif, which could potentially lead to competition with the apparently more active TTGACC motif. Future studies with specific mutations in these conserved WRKY-binding sequences will provide confirmatory data on the role of these target sequences in gene activation during Pi stress. Our studies suggested that WRKY75 could be working as a positive regulator of several genes involved in the global Pi starvation response. These results are interesting considering that PHR1 and OsPTF1 (Rubio et al., 2001; Yi et al., 2005), the two transcription factors implicated in the regulation of Pi stress responses, did not appear to be regulating genes encoding high-affinity Pi transporters or phosphatases. Suppression of WRKY75 expression also led to decreased phosphatase activity (Fig. 8) in the leaves of the plants. This further confirms the role of WRKY75 in activating multiple responses associated with Pi starvation. In addition, the expression of AtPS2-1 and AtPS2-2, phosphatases responsive to Pi stress (K.G. Raghothama, unpublished data), was attenuated by the suppression of WRKY75 expression. These results further support the notion that WRKY75 is a positive regulator of Pi starvation responses. Considering the complex cross talk that occurs during plant stresses, it is often possible that genes induced by a specific stress response could also respond to other stresses as a secondary effect. For example, many of the genes involved in Pi starvation responses, such as RNases, ATPases, and purple acid phosphatases, are induced during senescence and vice versa (Buchanan-Wollaston et al., 2003). Similarly, LePS2, a Pi stress-responsive gene, is also induced during pathogen infection (Stenzel et al., 2003). However, the reduction in the expression of Pi stress-responsive genes in WRKY75 RNAi mutants appears to be rather specific to Pi stress as indicated by impaired Pi uptake during Pi deprivation (Fig. 5B). The lack of any change in the Pi remobilization pattern of the mutant as compared to the wild-type plants (Supplemental Fig. S2) suggests that WRKY75 may not be directly involved in the internal Pi allocation and remobilization. Thus, WRKY75 can be implicated in the regulation of genes involved in the acquisition of P during Pi starvation. Noticeably, when 4-week-old plants were analyzed, there was a significant increase in the total P concentration in all parts of the WRKY75 RNAi mutants (Fig. 7) despite reduced Pi uptake in these mutants during Pi starvation. Although other possibilities could be considered, this increase could be attributed to the significant (≈75%) increase in the root surface area caused by the suppression of WRKY75 (Fig. 6). In addition, the age of the plants and rapid diffusion of Pi when the plants were grown in a hydroponic system may have contributed to the increased Pi concentration in the WRKY75 RNAi plants. This notion is supported by the fact that total P concentration in young seedlings of WRKY75 RNAi plants grown on agar media does not vary significantly in most parts relative to the wild-type seedlings (Supplemental Fig. S3). The presence of a hypothetical intracellular Pi-sensing mechanism that mediates the induction of Pi starvation-inducible (PSI) genes at the cellular level has been proposed (Abel et al., 2002). Therefore, WRKY75 could be influencing the Pi status of the plant directly by activating PSI genes during Pi deprivation while indirectly regulating it through the accumulation of Pi by increasing root surface area. Although the details of the actual regulatory mechanism are yet to be dissected, it is clear that WRKY75 acts as a positive regulator of Pi acquisition during Pi stress conditions.
WRKY75 Negatively Regulates Lateral Root and Root Hair Growth
The root architecture in Arabidopsis comprises three components, namely, the primary root, lateral root, and root hairs. The cell division at the primary root meristem enables indeterminate growth by adding new cells to the root. Lateral root formation increases the capacity of the root system to explore the rhizosphere. On the other hand, root hair formation increases the total surface area of primary and lateral roots. Changes in these components can influence root architecture and the ability of plants to grow in soils where nutrients are limiting. Typically, Pi deprivation results in proliferation of roots in the plants (López-Bucio et al., 2003). In this study, we show that WRKY75 negatively regulates lateral root and root hair development. In plants where WRKY75 expression was suppressed, a significant increase in lateral root length and number, as well as root hair number, was observed independent of the Pi status of the plant (Fig. 6). The suppression of WRKY75 resulted in increased root growth but attenuated expression of PSI genes. This supports the hypothesis that the effect of WRKY75 on the RSA is independent of its effect on PSI genes. Thus far, the phytohormone auxin is believed to be the critical factor influencing the components of the root architecture. Two distinct auxin transport mechanisms have been described in roots: acropetal transport from the shoot system to the root and basipetal transport from the root tip to the root base (Rashotte et al., 2000; Bhalerao et al., 2002). In Arabidopsis, acropetal movement of IAA has been implicated in the control of lateral root development (Reed et al., 1998). Auxin and the ethylene precursor ACC are known to regulate root hair formation in response to external stimuli such as nutrient stress (Masucci and Schiefelbein, 1994). It has also been reported that genetic and chemical reductions in protein phosphatase activity alter auxin transport and, thereby, lateral root growth (Rashotte et al., 2001). Therefore, a hypothetical model could be considered wherein WRKY75 transcriptionally regulates phosphatases, resulting in altered auxin transport and changes in lateral root growth. Future studies on WRKY75 could explore this hypothetical model to understand the mechanism by which WRKY75 regulates root architecture. In contrast to these results, the primary root development and expression of PLDζ2, a phospholipase gene known to be involved in root development (Cruz-Ramírez et al., 2006), were unaffected by the suppression of WRKY75 (Fig. 6, B and C) The localized Pi deficiency-induced inhibition of primary root growth was also unaffected in the WRKY75 RNAi plants. This could be attributed to the ontogenically distinct entity of the embryonic primary root from that of the postembryonically developed lateral roots. Evidences have been provided earlier showing that primary root growth is influenced by localized Pi deprivation (Linkohr et al., 2002; Ticconi et al., 2004), suggesting that lateral roots could be regulated systemically. Therefore, WRKY75 could be acting as a Pi-independent component of the systemic regulatory mechanism of the RSA. The presence of low levels of WRKY75 transcripts detected during Pi-sufficient condition (Fig. 1A) supports the notion of a biological function for WRKY75 even in the absence of Pi stress. These results suggest that WRKY75 may be negatively regulating lateral root and root hair development independent of the Pi status of the plant.
It has been suggested that two major transcriptional programs, one early and general while the other later and specific, are involved in response to Pi starvation (Hammond et al., 2004). The Pi-responsive transcription factor PHR1 (Rubio et al., 2001) is believed to be part of the second, more specific response to Pi starvation. The transcriptional program induced during the early stages of Pi stress is thought to involve genes with the characteristics of general stress response factors. A macroarray study involving mineral nutrition-related genes showed that many of the genes induced early by Pi stress are also activated by potassium and iron starvation (Wang et al., 2002). We found that WRKY75 is also induced during nitrogen, potassium, and iron starvation, but not by other abiotic stresses tested, such as cold or salt stress (data not shown). Interestingly, although WRKY75 is induced early during Pi stress, its expression persists. In this context, the importance of WRKY75 in maintaining the Pi homeostasis of the plant cannot be overemphasized. The control of root architecture by WRKY75 indirectly affects the Pi status of the plant during Pi-sufficient or Pi-deficient conditions as indicated by the increase in Pi accumulation upon its suppression (Fig. 7). On the other hand, its induction during Pi starvation conditions results in the coinduction of several genes that help the plant adapt to Pi stress.
In summary, this study shows that WRKY75 is a negative regulator of root development while it also acts as a positive regulator of Pi stress responses. This strengthens our understanding of transcriptional regulation during Pi starvation responses in higher plants and provides a base for future work in this area. WRKY75 could potentially be a useful tool in understanding multiple stress responses as it plays an important role in regulating root architecture, which is critical for many other nutrient stresses besides P.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia was used in this study. To confirm observations under varied conditions, as well as to meet the specific requirements of individual experiments, one of the following growth conditions was used.
Hydroponic Culture
Seeds were germinated in Premier ProMix PGX peat mix (Premier Horticulture). Plants were grown under greenhouse conditions under a 16-h-light/8-h-dark cycle at 1,000 μmol m−2 s−1 photosynthetically active radiation (PAR). Seedlings at the five- to seven-leaf stage were transferred to hydroponics after their roots were gently washed. After a recovery period of 7 d in 0.5× Hoagland solution, plants were transferred to hydroponic solutions containing 250 μm Pi (P+) or no Pi (P−) for 7 d before they were harvested (Karthikeyan et al., 2002).
Petri Dish Culture
Seeds were surface sterilized, stratified at 4°C, and germinated initially on 0.5× Murashige and Skoog (MS) medium. Seven-day-old seedlings were transferred to modified MS medium containing 2.06 mm NH4NO3, 1.88 mm KNO3, 0.31 mm MgSO4, 0.1 mm MnSO4, 0.03 mm ZnSO4, 0.1 μm CuSO4, 0.3 mm CaCl2, 5.0 μm KI, 0.1 μm CoCl2, 0.1 mm FeSO4; 0.1 mm EDTA, 0.1 mm H3BO3, and 1 μm Na2MoO4.2H20 supplemented with 1% (w/v) agar and 3% (w/v) Suc. Treatments with Pi-sufficient and Pi-deficient medium were supplemented with 1 mm KH2PO4 or 0.5 mm K2SO4, respectively. The seedlings were grown under a 16-h-light/8-h-dark cycle at 22°C with 75 μmol m−2 s−1 PAR. The plates were inclined at a 65° angle to allow the roots to grow along the agar surface.
Liquid Culture
This method was used to generate material for all gene expression analyses. Surface-sterilized seeds were dispensed into conical flasks containing one-half-strength MS medium without agar. The seedlings were grown under a 16-h-light (125 μmol m−2 s−1 PAR)/8-h-dark cycle at 22°C with constant shaking (85 rpm). Seven-day-old seedlings were rinsed three times with distilled water and transferred into MS liquid media with Pi (1 mm) or without Pi. Plants were grown for 7 d, harvested, blot dried, frozen immediately in liquid nitrogen, and stored at −70°C until being used for RNA extraction (Karthikeyan et al., 2002).
Plant Transformation
Two different gene constructs were used to transform Arabidopsis plants by use of the floral-dip method (Clough and Bent, 1998).
To generate the WRKY75 RNAi construct, a 225-bp fragment of the WRKY75 coding sequence—unique to WRKY75—was amplified using the primers 5′GGACTAGTCCATGGCCAGAGCTGCATCAAGG3′ and 5′CGGGATCCGGCGCGCCATCCTCCATATGTACC3′. The amplified fragment was initially digested with NcoI and AscI and cloned in sense orientation into the binary double-stranded RNA vector pGSA1131 immediately after the CaMV 35S promoter to generate an intermediary vector. The same WRKY75 fragment was then digested with SpeI and BamHI and cloned in antisense orientation next to the GUS intron spacer region of the intermediary vector. The vector was sequenced to confirm the authenticity and correct orientation of the two cloned inserts. The pGSA1131 binary vector confers Basta resistance in planta.
The second construct was used to generate a GFP∷WRKY75 translational fusion.
The full-length WRKY75 cDNA was amplified with the primers 5′CCCAAGCTTATGGAGGGATATGATAATGGG3′ and 5′GCGGATCCCTAGAAAGAAGAGTAGATTTGC3′. The amplified fragment was digested with HindIII and BamHI and cloned into the binary pEGAD expression vector with the EGFP as an N-terminal translational fusion. This construct and an empty vector control were stably transformed into Arabidopsis and transgenic seedlings were selected by spraying 50 μL L−1 Basta.
Visualization of GFP
Wide-field fluorescence imaging was done using a NIKON E800 compound microscope equipped with a SPOT RT-slider digital camera (Diagnostic Instruments) interfaced to a computer. GFP excitation was done with standard FITC filters. Images of the roots were taken through FITC filters under the 20× objective. To confirm the nuclear localization of WRKY75, roots as well as mesophyll protoplasts from 35S∷GFP∷WRKY75 transgenic and wild-type control plants were stained with 4′,6-diamino-phenylindole (DAPI). They were fixed in phosphate buffered saline, pH 7.2, containing 4% (w/v) paraformaldehyde, 50 mm EDTA, and 100 mm NaCl for 1 h and then washed three times over 40 min with DAPI stain solution (100 mm phosphate buffered saline, 50 mm EDTA, and 1 μg mL−1 DAPI). The nuclear-specific dye DAPI colocalized with GFP fluorescence in the cells (data not shown).
RNA Gel-Blot Analysis and RT-PCR
Total RNA was extracted from plant samples using the TRIzol reagent (Invitrogen). Ten micrograms of total RNA was electrophoretically separated in a denaturing formaldehyde agarose gel and blotted onto nylon membranes. The nylon membranes were hybridized overnight with 32P-labeled DNA probes at 42°C.
For RT-PCR analysis, two micrograms of DNAse-treated (RQ1 DNAse; Promega) total RNA was used as a template for first-strand cDNA synthesis with Superscript II (Invitrogen) and an oligo(dT) primer. The EF1α gene was amplified with specific primers to calibrate equal quantities of first-strand cDNA per reaction. The following gene-specific primers were used to detect WRKY75 cDNA: 5′ATGGAGGGATATGATAATGGG3′ and 5′GAAAGAAGAGTAGATTTGC3′. Ten-microliter reactions were set up for each sample and amplified through 25 cycles. Eight microliters of the product was run on an agarose gel and the results were documented. The remaining 2 μL of the product was used as a template for a second 25-cycle PCR.
Measurements of Roots and Root Hairs
Seedlings were grown on petri dishes under Pi-sufficient or Pi-deficient conditions as described earlier. After 7 d of treatment, the primary root tips were photographed in a Nikon stereo microscope with a SPOT-RT digital camera attached to a computer. Root tip images were captured under the 0.5× objective. The total number of root hairs in the region 5 mm from the root tip as well as the length of the root hairs in this region were measured using the ImageJ program (Abramoff et al., 2004). Data were recorded from five individual plants from each line per treatment. For measurement of primary root length, lateral root number, and lateral root length, the roots from 20 individual plants of each line per treatment were spread out carefully with a fine brush, scanned at 600 dpi, and different root traits were evaluated using the ImageJ program.
Physiological Measurements
Anthocyanin Estimation
Plant material was raised through petri dish culture as described earlier. About 100 mg of frozen ground tissue from each treatment and line was used for the quantification of anthocyanins as described by Lange et al. (1971). The optical density was measured at A532 and A653. Subtraction of 0.24 A653 compensated for the small overlap in A532 by the chlorophylls. The concentration was determined by using the corrected absorbance and the molar extinction coefficient (ɛ) of 38,000 L mol−1 cm−1 for anthocyanin.
Quantification of Total Pi
Plant material was generated hydroponically. Total Pi concentration was quantified using a modification of the U.S. Environmental Protection Agency Method 365.2. About 50 mg of fresh sample was taken in a preweighed vial and oven dried. After recording their dry weight, the samples were flamed to ash and dissolved in 100 μL of concentrated HCl. Ten microliters of this sample was diluted in 790 μL of water. To a reaction containing 800 μL of diluted sample, 200 μL of assay solution (4.8 mm NH4MoO4, 2.5 n H2SO4, and 35 mm ascorbic acid) was added and incubated at 45°C for 20 min. Total Pi content was measured at A650 and expressed as total Pi/milligram tissue dry weight.
Quantification of Total Acid Phosphatase Activity
Plant material was generated hydroponically. Total acid phosphatase was measured as described earlier using the pNPP hydrolysis assay (Richardson et al., 2001). Samples were extracted from about 30 mg of finely ground frozen tissue. The enzyme activity was measured at A405. Total protein was estimated separately using Bradford's reagent and the total acid phosphatase activity expressed as milliunits/milligram protein.
Pi Uptake Assay
Wild-type and WRKY75 RNAi seedlings were grown as described earlier in Pi-sufficient or Pi-deficient medium for 3 d. Groups of 10 seedlings were used as one biological sample. The roots of the seedlings were incubated in a pretreatment solution (5 mm MES and 0.1 mm CaCl2, pH 5.7) for 20 min before moving them into 2 mL of uptake solution (5 mm MES, 0.1 mm CaCl2, 50 μm KH2PO4, pH 5.7) containing [33P]orthophosphate (0.15 μCi/mL−1). Samples were moved into ice-cold desorption solution (5 mm MES, 0.1 mm CaCl2, and 1 mm KH2PO4, pH 5.7) at the end of 1 and 2 h, respectively. After two washes with fresh desorption solution for 45 min, the samples were blot dried, placed in preweighed scintillation vials, oven dried overnight at 65°C, and their dry weight recorded. Four milliliters of scintillation cocktail was added into each vial, and radioactivity was measured with a scintillation counter (Beckman Coulter).
For in planta labeling experiments, wild-type plants and WRKY75 RNAi plants were grown for 10 d in Pi-sufficient (1 mm) and Pi-deficient conditions. Roots of these seedlings were incubated for 4 h in the [33P]orthophosphate uptake solution and subsequently desorbed for 2 h as described above. The seedlings were moved to one-half-strength Hoagland solution and grown for 10 d to allow emergence of new leaves. The nutrient solution was replaced every day. The apical meristem and pairs of leaves were harvested from the plants after 10 d and the radioactivity was measured.
Statistical Analysis
Statistical significance of difference between mean values was determined using Student's t test. Different letters on the error bars of histograms were used to indicate means that were statistically different at P ≤ 0.05.
In Silico Analysis of Promoter Sequences
W box elements on the promoter regions of Pi stress-responsive genes were collated from the Arabidopsis cis-regulatory element database (AtcisDB) maintained by the Arabidopsis Gene Regulatory Information Server, Ohio State University (http://Arabidopsis.med.ohio-state.edu).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Decrease in WRKY75 transcripts as Pi-deprived plants are replenished with Pi.
Supplemental Figure S2. Comparative distribution pattern of Pi in WRKY75 RNAi plants.
Supplemental Figure S3. Total Pi concentration in WRKY75 RNAi and wild-type seedlings.
Supplementary Material
Acknowledgments
We thank Ajay Jain, Mike Poling, and Madhuvanthi Ramaiah for their valuable help in preparing and editing this manuscript. We also thank the Arabidopsis Resource Center at The Ohio State University for providing the vectors used in this study and Dr. Zhixiang Chen, Department of Botany and Plant Pathology, Purdue University, for the homozygous SALK mutant line of WRKY75.
This work was supported by grants from the U.S. Department of Agriculture.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Kashchandra G. Raghothama (kraghoth@purdue.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Abel S, Ticconi AC, Delatorre CA (2002) Phosphate sensing in higher plants. Physiol Plant 115 1–8 [DOI] [PubMed] [Google Scholar]
- Abramoff MD, Magelhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics International 11 36–42 [Google Scholar]
- Ascencio J (1994) Acid phosphatase as a diagnostic tool. Commun Soil Sci Plant Anal 25 1553–1564 [Google Scholar]
- Baldwin JC, Karthikeyan AS, Raghothama KG (2001) LEPS2, a phosphorus starvation-induced novel acid phosphatase from tomato. Plant Physiol 125 728–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari R, Pant BD, Stitt M, Scheible W-R (2006) PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol 141 988–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariola PA, Howard CJ, Taylor CB, Verburg MT, Jaglan VD, Green PJ (1994) The Arabidopsis ribonuclease gene RNS1 is tightly controlled in response to phosphate limitation. Plant J 6 673–685 [DOI] [PubMed] [Google Scholar]
- Beck T, Hall MN (1999) The TOR signaling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature 402 689–692 [DOI] [PubMed] [Google Scholar]
- Bhalerao RP, Eklöf J, Ljung K, Marchant A, Bennett M, Sandberg G (2002) Shoot derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29 325–332 [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T, Pink D (2003) The molecular analysis of leaf senescence—a genomics approach. Plant Biotechnol J 1 3–22 [DOI] [PubMed] [Google Scholar]
- Bucher M, Rausch C, Daram P (2001) Molecular and biochemical mechanisms of phosphorus uptake into plants. J Plant Nutr Soil Sci 164 209–217 [Google Scholar]
- Chiou T-J, Aung K, Lin S-I, Wu C-C, Chiang S-F, Su C-L (2006) Regulation of phosphate homeostasis by microRNA in Arabidopsis. Plant Cell 18 412–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Cruz-Ramírez A, Oropeza-Aburto A, Razo-Hernández F, Ramírez-Chavez E, Herrera-Estrella L (2006) Phospholipase DZ2 plays an important role in extraplastidic galactolipid biosynthesis and phosphate recycling in Arabidopsis roots. Proc Natl Acad Sci USA 103 6765–6770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pozo JC, Allona I, Rubio V, Leyva A, de la Pena A, Aragoncillo C, Paz-Ares J (1999) A type 5 acid phosphatase gene from Arabidopsis thaliana is induced by phosphate starvation and by some other types of phosphate mobilizing/oxidative stress conditions. Plant J 19 579–589 [DOI] [PubMed] [Google Scholar]
- Dong J, Chen C, Chen Z (2003) Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol 51 21–37 [DOI] [PubMed] [Google Scholar]
- Essigmann B, Guler S, Narang RA, Linke D, Benning C (1998) Phosphate availability affects the thylakoid lipid composition and the expression of SQD1, a gene required for sulfolipid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 95 1950–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5 199–206 [DOI] [PubMed] [Google Scholar]
- Franco-Zorilla JM, González E, Bustos R, Linhares F, Leyva A, Paz-Ares J (2004) The transcriptional control of plant responses to phosphate limitation. J Exp Bot 55 285–293 [DOI] [PubMed] [Google Scholar]
- Guo Y, Cai Z, Gan S (2004) Transcriptome of Arabidopsis leaf senescence. Plant Cell Environ 27 521–549 [Google Scholar]
- Hammond JP, Broadley MR, White PJ (2004) Genetic responses to phosphorus deficiency. Ann Bot (Lond) 94 323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan AS, Varadarajan DK, Jain A, Held MA, Carpita NC, Raghothama KG (2007) Phosphate starvation responses are mediated by sugar signaling in Arabidopsis. Planta 225 907–918 [DOI] [PubMed] [Google Scholar]
- Karthikeyan AS, Varadarajan DK, Mukatira UT, D'Urzo MP, Damz B, Raghothama KG (2002) Regulated expression of Arabidopsis phosphate transporters. Plant Physiol 130 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KC, Fan B, Chen Z (2006) Pathogen-induced Arabidopsis WRKY7 is a transcriptional repressor and enhances plant susceptibility to Pseudomonas syringae. Plant Physiol 142 1180–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange H, Shropshire W Jr, Mohr H (1971) An analysis of phytochrome-mediated anthocyanin synthesis. Plant Physiol 47 649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Zhu H, Liu K, Liu X, Leggewie G, Udvardi M, Wang D (2002) Purple acid phosphatases of Arabidopsis thaliana. Comparative analysis and differential regulation by phosphate deprivation. J Biol Chem 277 27772–27781 [DOI] [PubMed] [Google Scholar]
- Linkohr BI, Williamson LC, Fitter AH, Leyser HMO (2002) Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J 29 751–760 [DOI] [PubMed] [Google Scholar]
- Lipton DS, Lanchar RW, Blevins DG (1987) Citrate, malate and succinate concentration in exudates from P-sufficient and P-stressed Medicago sativa L. seedlings. Plant Physiol 85 315–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L (2003) The role nutrient availability in regulating root architecture. Curr Opin Plant Biol 6 280–287 [DOI] [PubMed] [Google Scholar]
- Mare C, Mazzucotelli E, Crosatti C, Francia E, Stanca AM, Cattivelli L (2004) Hv-WRKY38: a new transcription factor involved in cold- and drought-response in barley. Plant Mol Biol 55 399–416 [DOI] [PubMed] [Google Scholar]
- Marschner H (1995) Mineral Nutrition of Higher Plants. Academic Press, London
- Martín AC, del Pozo JC, Iglesias J, Rubio V, Solano R, De La Peña A, Leyva A, Paz-Ares J (2000) Influence of cytokinins on the expression of phosphate starvation responsive genes in Arabidopsis. Plant J 24 1–11 [DOI] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW (1994) The rhd6 mutation of Arabidopsis thaliana alters root-hair initiation through an auxin- and ethylene-associated process. Plant Physiol 106 1335–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Laun T, Zimmermann P, Zentgraf U (2004) Targets of the WRKY53 transcription factor and its role during leaf senescence in Arabidopsis. Plant Mol Biol 55 853–867 [DOI] [PubMed] [Google Scholar]
- Misson J, Raghothama KG, Jain A, Jouhet J, Block MA, Bligny R, Ortet P, Creff A, Somerville S, Rolland N, et al (2005) A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc Natl Acad Sci USA 102 11934–11939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Rus A, Sharkhuu A, Yokoi S, Karthikeyan AS, Raghothama KG, Baek D, Koo YD, Jin JB, Bressan RA, et al (2005) The Arabidopsis SUMO E3 ligase SIZ1 controls phosphate deficiency responses. Proc Natl Acad Sci USA 102 7760–7765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchhal US, Pardo JM, Raghothama KG (1996) Phosphate transporters from the higher plant Arabidopsis thaliana. Proc Natl Acad Sci USA 93 10519–10523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge SR, Rae AL, Diatloff E, Smith FW (2002) Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J 31 341–353 [DOI] [PubMed] [Google Scholar]
- Müller R, Nilsson L, Nielsen LK, Nielsen TH (2005) Interaction between phosphate starvation signalling and hexokinase-independent sugar sensing in Arabidopsis leaves. Physiol Plant 124 81–90 [Google Scholar]
- Okumura S, Mitsukawa N, Shirano Y, Shibata D (1998) Phosphate transporter gene family of Arabidopsis thaliana. DNA Res 5 261–269 [DOI] [PubMed] [Google Scholar]
- Raghothama KG, Karthikeyan AS (2005) Phosphate acquisition. Plant Soil 274 37–49 [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday G (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 122 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, DeLong A, Muday GK (2001) Genetic and chemical reductions in the protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell 13 1683–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday GK (1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118 1369–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AE, Hadobas PA, Hayes JE (2001) Extracellular secretion of Aspergillus phytase from Arabidopsis roots enables plants to obtain phosphorus from phytate. Plant J 25 641–649 [DOI] [PubMed] [Google Scholar]
- Robatzek S, Somssich I (2001) A new member of the Arabidopsis WRKY transcription factor family, AtWRKY6, is associated with both senescence and defence-related processes. Plant J 28 123–133 [DOI] [PubMed] [Google Scholar]
- Rubio V, Linhares F, Solano R, Martín AC, Iglesias J, Leyva A, Paz-Ares J (2001) A conserved MYB transcription factor involved in phosphate starvation signalling both in vascular plants and in unicellular algae. Genes Dev 15 2122–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Calderón L, López-Bucio J, Chacón-López A, Cruz-Ramírez A, Nieto-Jacobo F, Dubrovsky JG, Herrera-Estrella L (2005) Phosphate starvation induces a determinate developmental program in the roots of Arabidopsis thaliana. Plant Cell Physiol 46 174–184 [DOI] [PubMed] [Google Scholar]
- Sano T, Nagata T (2002) The possible involvement of a phosphate-induced transcription factor encoded by Phi-2 gene from tobacco in ABA-signaling pathways. Plant Cell Physiol 43 12–20 [DOI] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Ishida J, Nanjo T, Fujita M, Oono Y, Kamiya A, Nakajima M, Enju A, Sakurai T, et al (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J 31 279–292 [DOI] [PubMed] [Google Scholar]
- Shin H, Shin H-S, Chen R, Harrison MJ (2006) Loss of At4 function impacts phosphate distribution between the roots and the shoots during phosphate starvation. Plant J 45 712–726 [DOI] [PubMed] [Google Scholar]
- Shin H, Shin H-S, Dewbre GR, Harrison MJ (2004) Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J 39 629–642 [DOI] [PubMed] [Google Scholar]
- Stenzel I, Ziethe K, Schurath J, Hertel SC, Bosse D, Köck M (2003) Differential expression of the LePS2 phosphatase gene family in response to phosphate availability, pathogen infection and during development. Physiol Plant 118 138–146 [DOI] [PubMed] [Google Scholar]
- Sun C, Palmqvist S, Olsson H, Boren M, Ahlandsberg S, Jansson C (2003) A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to sugar-responsive elements of the iso1 promoter. Plant Cell 15 2076–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticconi CA, Delatorre CA, Lahner B, Salt DE, Abel S (2004) Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. Plant J 37 801–814 [DOI] [PubMed] [Google Scholar]
- Ulker B, Somssich IE (2004) WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol 7 491–498 [DOI] [PubMed] [Google Scholar]
- Utsugi S, Sakamoto W, Murata M, Motoyoshi F (1998) Arabidopsis thaliana vegetative storage protein (VSP) genes: gene organization and tissue specific expression. Plant Mol Biol 38 565–576 [DOI] [PubMed] [Google Scholar]
- Wang YH, Garvin DF, Kochian LV (2002) Rapid induction of regulatory and transporter genes in response to phosphorus, potassium, and iron deficiencies in tomato roots. Evidence for cross talk and root/rhizosphere-mediated signals. Plant Physiol 130 1361–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasaki J, Yonetani R, Kuroda S, Shinano T, Yazaki J, Fujii F, Shimbo K, Yamamoto K, Sakata K, Sasaki T (2003) Transcriptomic analysis of metabolic changes by phosphorus stress in rice plant roots. Plant Cell Environ 26 1515–1523 [Google Scholar]
- Wu P, Ma L, Hou X, Wang M, Wu Y, Liu F, Deng XW (2003) Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiol 132 1260–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K, Wu Z, Zhou J, Du L, Guo L, Wu Y, Wu P (2005) OsPTF1, a novel transcription factor involved in tolerance to phosphate starvation in rice. Plant Physiol 138 2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Chen C, Chen Z (2001) Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 13 1527–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Xu C, Benning C (2002) Arabidopsis disrupted in SQD2 encoding sulfolipid synthase is impaired in phosphate-limited growth. Proc Natl Acad Sci USA 99 5732–5737 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.