Abstract
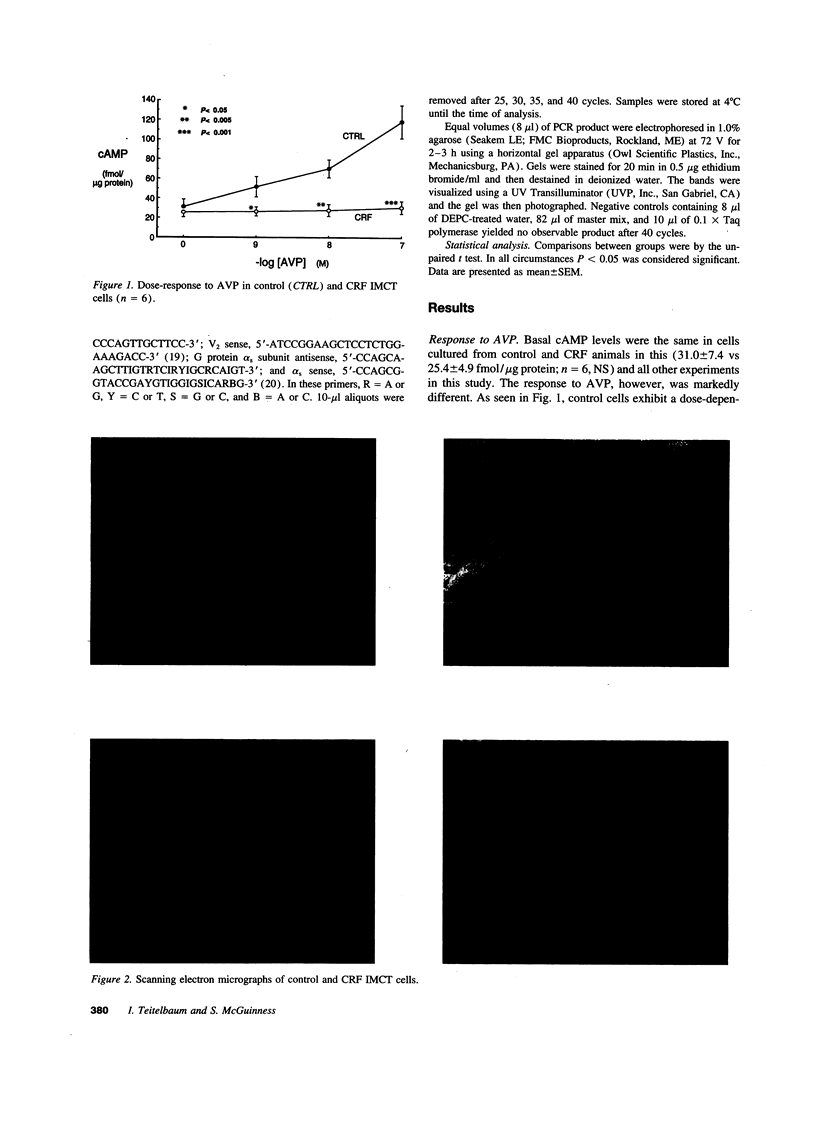
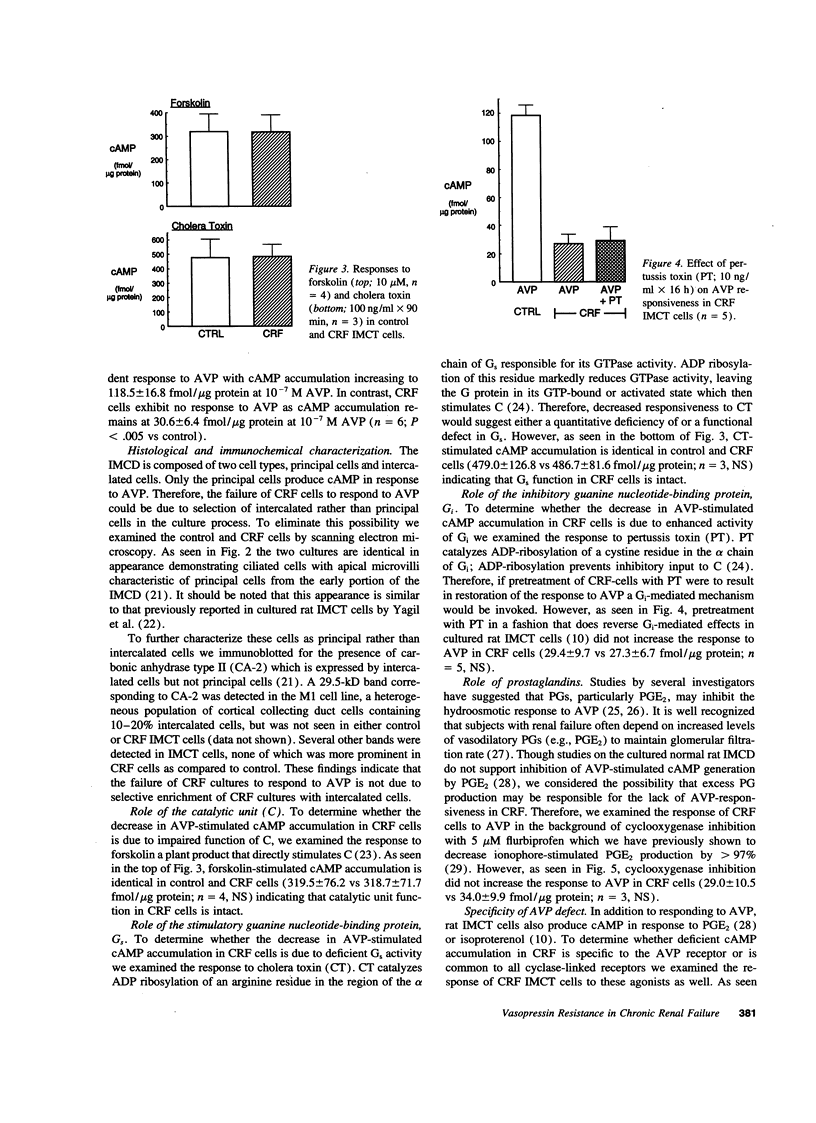
Studies were performed to determine the mechanism underlying deficient arginine vasopressin (AVP)-stimulated adenylyl cyclase activity in chronic renal failure (CRF). As compared to control, principal cells cultured from the inner medullary collecting tubule of rats previously made uremic by 5/6 nephrectomy fail to accumulate cAMP when stimulated with AVP. CRF cells do respond normally to forskolin or cholera toxin and the defect in AVP responsiveness is not prevented by treatment with pertussis toxin, by cyclooxygenase inhibition, or by inhibition or down-regulation of protein kinase C. In contrast to their lack of responsiveness to AVP, CRF cells respond normally to other agonists of adenylyl cyclase such as isoproterenol or prostaglandin E2. Plasma membranes prepared from the inner medullae of CRF rats exhibit a marked decrease in apparent AVP receptor number but no change in the apparent number of beta adrenergic receptors. Reverse transcriptase PCR of total RNA from the inner medullae of CRF animals reveals virtual absence of V2 receptor mRNA; mRNA for alpha s is present in normal abundance. These studies indicate that AVP resistance in CRF is due, at least in part, to selective down-regulation of the V2 receptor as a consequence of decreased V2 receptor mRNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amico J. A., Silver M. R., Finn F. M., Robinson A. G. High-performance liquid chromatographic characterization of neurophysins in chronic renal failure. J Lab Clin Med. 1987 Oct;110(4):439–447. [PubMed] [Google Scholar]
- Anderson R. J., Berl T., McDonald K. D., Schrier R. W. Evidence for an in vivo antagonism between vasopressin and prostaglandin in the mammalian kidney. J Clin Invest. 1975 Aug;56(2):420–426. doi: 10.1172/JCI108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando Y., Tabei K., Asano Y. Luminal vasopressin modulates transport in the rabbit cortical collecting duct. J Clin Invest. 1991 Sep;88(3):952–959. doi: 10.1172/JCI115398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRICKER N. S., DEWEY R. R., LUBOWITZ H., STOKES J., KIRKENSGAARD T. Observations on the concentrating and diluting mechanisms of the diseased kidney. J Clin Invest. 1959 Mar;38(3):516–523. doi: 10.1172/JCI103829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla-Felix M., Hamm L. L., Herndon J., Vehaskari V. M. Response of cortical collecting ducts from remnant kidneys to arginine vasopressin. Kidney Int. 1992 May;41(5):1150–1154. doi: 10.1038/ki.1992.175. [DOI] [PubMed] [Google Scholar]
- Burnatowska-Hledin M. A., Spielman W. S. Vasopressin V1 receptors on the principal cells of the rabbit cortical collecting tubule. Stimulation of cytosolic free calcium and inositol phosphate production via coupling to a pertussis toxin substrate. J Clin Invest. 1989 Jan;83(1):84–89. doi: 10.1172/JCI113888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramelo C., Tsai P., Okada K., Schrier R. W. Protein kinase C activity in compensatory kidney growth. Biochem Biophys Res Commun. 1988 Apr 15;152(1):315–321. doi: 10.1016/s0006-291x(88)80716-2. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clapp W. L., Madsen K. M., Verlander J. W., Tisher C. C. Morphologic heterogeneity along the rat inner medullary collecting duct. Lab Invest. 1989 Feb;60(2):219–230. [PubMed] [Google Scholar]
- Donoghue M. J., Merlie J. P., Rosenthal N., Sanes J. R. Rostrocaudal gradient of transgene expression in adult skeletal muscle. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5847–5851. doi: 10.1073/pnas.88.13.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue M. J., Morris-Valero R., Johnson Y. R., Merlie J. P., Sanes J. R. Mammalian muscle cells bear a cell-autonomous, heritable memory of their rostrocaudal position. Cell. 1992 Apr 3;69(1):67–77. doi: 10.1016/0092-8674(92)90119-w. [DOI] [PubMed] [Google Scholar]
- Fine L. G., Schlondorff D., Trizna W., Gilbert R. M., Bricker N. S. Functional profile of the isolated uremic nephron. Impaired water permeability and adenylate cyclase responsiveness of the cortical collecting tubule to vasopressin. J Clin Invest. 1978 Jun;61(6):1519–1527. doi: 10.1172/JCI109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Harris D. C., Hammond W. S., Burke T. J., Schrier R. W. Verapamil protects against progression of experimental chronic renal failure. Kidney Int. 1987 Jan;31(1):41–46. doi: 10.1038/ki.1987.6. [DOI] [PubMed] [Google Scholar]
- Holliday R. DNA methylation and epigenetic inheritance. Philos Trans R Soc Lond B Biol Sci. 1990 Jan 30;326(1235):329–338. doi: 10.1098/rstb.1990.0015. [DOI] [PubMed] [Google Scholar]
- Hughes A. K., Cline R. C., Kohan D. E. Alterations in renal endothelin-1 production in the spontaneously hypertensive rat. Hypertension. 1992 Nov;20(5):666–673. doi: 10.1161/01.hyp.20.5.666. [DOI] [PubMed] [Google Scholar]
- Ishizuka T., Cooper D. R., Arnold T., Hernandez H., Farese R. V. Downregulation of protein kinase C and insulin-stimulated 2-deoxyglucose uptake in rat adipocytes by phorbol esters, glucose, and insulin. Diabetes. 1991 Oct;40(10):1274–1281. doi: 10.2337/diab.40.10.1274. [DOI] [PubMed] [Google Scholar]
- Jamison R. L., Oliver R. E. Disorders of urinary concentration and dilution. Am J Med. 1982 Feb;72(2):308–322. doi: 10.1016/0002-9343(82)90823-3. [DOI] [PubMed] [Google Scholar]
- Jawadi M. H., Ho L. S., Dipette D., Ross D. L. Regulation of plasma arginine vasopressin in patients with chronic renal failure maintained on hemodialysis. Am J Nephrol. 1986;6(3):175–181. doi: 10.1159/000167108. [DOI] [PubMed] [Google Scholar]
- Kim J., Tisher C. C., Linser P. J., Madsen K. M. Ultrastructural localization of carbonic anhydrase II in subpopulations of intercalated cells of the rat kidney. J Am Soc Nephrol. 1990 Sep;1(3):245–256. doi: 10.1681/ASN.V13245. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linser P. J., Perkins M. S., Fitch F. W., Moscona A. A. Comparative characterization of monoclonal antibodies to carbonic anhydrase. Biochem Biophys Res Commun. 1984 Dec 14;125(2):690–697. doi: 10.1016/0006-291x(84)90594-1. [DOI] [PubMed] [Google Scholar]
- Lumlertgul D., Burke T. J., Gillum D. M., Alfrey A. C., Harris D. C., Hammond W. S., Schrier R. W. Phosphate depletion arrests progression of chronic renal failure independent of protein intake. Kidney Int. 1986 Mar;29(3):658–666. doi: 10.1038/ki.1986.49. [DOI] [PubMed] [Google Scholar]
- Nadler S. P., Hebert S. C., Brenner B. M. PGE2, forskolin, and cholera toxin interactions in rabbit cortical collecting tubule. Am J Physiol. 1986 Jan;250(1 Pt 2):F127–F135. doi: 10.1152/ajprenal.1986.250.1.F127. [DOI] [PubMed] [Google Scholar]
- Sato M., Dunn M. J. Interactions of vasopressin, prostaglandins, and cAMP in rat renal papillary collecting tubule cells in culture. Am J Physiol. 1984 Sep;247(3 Pt 2):F423–F433. doi: 10.1152/ajprenal.1984.247.3.F423. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkfor S. I., Johnson G. L., Berl T. A molecular map of G protein alpha chains in microdissected rat nephron segments. J Clin Invest. 1993 Aug;92(2):786–790. doi: 10.1172/JCI116651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes J. B., Grupp C., Kinne R. K. Purification of rat papillary collecting duct cells: functional and metabolic assessment. Am J Physiol. 1987 Aug;253(2 Pt 2):F251–F262. doi: 10.1152/ajprenal.1987.253.2.F251. [DOI] [PubMed] [Google Scholar]
- Tannen R. L., Regal E. M., Dunn M. J., Schrier R. W. Vasopressin-resistant hyposthenuria in advanced chronic renal disease. N Engl J Med. 1969 May 22;280(21):1135–1141. doi: 10.1056/NEJM196905222802101. [DOI] [PubMed] [Google Scholar]
- Teitelbaum I., Berl T. Effects of calcium on vasopressin-mediated cyclic adenosine monophosphate formation in cultured rat inner medullary collecting tubule cells. Evidence for the role of intracellular calcium. J Clin Invest. 1986 May;77(5):1574–1583. doi: 10.1172/JCI112473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum I. Cyclic adenosine monophosphate and diacylglycerol. Mutually inhibitory second messengers in cultured rat inner medullary collecting duct cells. J Clin Invest. 1990 Jul;86(1):46–51. doi: 10.1172/JCI114713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum I. Protein kinase C inhibits arginine vasopressin-stimulated cAMP accumulation via a Gi-dependent mechanism. Am J Physiol. 1993 Feb;264(2 Pt 2):F216–F220. doi: 10.1152/ajprenal.1993.264.2.F216. [DOI] [PubMed] [Google Scholar]
- Teitelbaum I., Strasheim A., Berl T. Adrenergic control of cAMP generation in rat inner medullary collecting tubule cells. Kidney Int. 1989 Feb;35(2):647–653. doi: 10.1038/ki.1989.34. [DOI] [PubMed] [Google Scholar]
- Teitelbaum I., Strasheim A., Berl T. Epidermal growth factor-stimulated phosphoinositide hydrolysis in cultured rat inner medullary collecting tubule cells. Regulation by G protein, calcium, and protein kinase C. J Clin Invest. 1990 Apr;85(4):1044–1050. doi: 10.1172/JCI114534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada Y., Tomita K., Nonoguchi H., Yang T., Marumo F. Different localization and regulation of two types of vasopressin receptor messenger RNA in microdissected rat nephron segments using reverse transcription polymerase chain reaction. J Clin Invest. 1993 Nov;92(5):2339–2345. doi: 10.1172/JCI116838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Berton M. T., Burger C., Kepron M., Lee W. T., Yin X. M. Memory B and T cells. Annu Rev Immunol. 1991;9:193–217. doi: 10.1146/annurev.iy.09.040191.001205. [DOI] [PubMed] [Google Scholar]
- Wilson S. P. Regulation of chromaffin cell secretion and protein kinase C activity by chronic phorbol ester treatment. J Biol Chem. 1990 Jan 15;265(2):648–651. [PubMed] [Google Scholar]
- Yagil Y. Interaction of adenosine with vasopressin in the inner medullary collecting duct. Am J Physiol. 1990 Oct;259(4 Pt 2):F679–F687. doi: 10.1152/ajprenal.1990.259.4.F679. [DOI] [PubMed] [Google Scholar]
- Zahniser N. R., Parker D. C., Bier-Laning C. M., Miller J. A., Gerber J. G., Nies A. S. Comparison between the effects of aging on antagonist and agonist interactions with beta-adrenergic receptors on human mononuclear and polymorphonuclear leukocyte membranes. J Gerontol. 1988 Nov;43(6):M151–M157. doi: 10.1093/geronj/43.6.m151. [DOI] [PubMed] [Google Scholar]