Abstract
We have used apolipoprotein genes to investigate the signal transduction mechanisms involved in the control of intestinal specific gene expression. The human apoAI, apoCIII, and apoAIV genes are tandemly organized within a 15-kb DNA segment and are expressed predominantly in the liver and intestine. Transient transfection of various human apoAI gene plasmid constructs into human hepatoma (HepG2) and colon carcinoma (Caco-2) cells showed that apoAI gene transcription is under the control of two separate and distinct cell-specific promoters. The region between nucleotides -192 and -41 is essential for expression in HepG2 cells, whereas the region from -595 to -192 is essential for expression in Caco-2 cells. A third 0.6 kb DNA fragment in the apoCIII gene promoter region, approximately 5 kb down-stream from the human apoAI gene, enhances transcription mediated by either of these two tissue-specific apoAI promoters. In Caco-2 cells, expression of the apoAI gene and activation by the distal enhancer required the presence of a nuclear hormone receptor response element (NHRRE) located in the -214 to -192 apoAI promoter region. Overexpression of the orphan receptor hepatocyte nuclear factor 4 (HNF-4), which binds to the NHRRE, dramatically stimulates apoAI gene expression in Caco-2 cells but not in HepG2 cells. Maximal stimulation of transcription by HNF-4 in Caco-2 cells required the presence of both the intestinal specific promoter, the NHRRE, and distal enhancer elements. Transactivation by HNF-4 thus appears to result from functional synergy between the NHRRE binding HNF-4 and distal DNA elements containing intestinal-specific DNA binding activities. The apoAI gene provides a model system to define the mechanism(s) governing intestinal cell specific gene regulation and the role of nuclear hormone receptors in the establishment and regulation of enterocytic gene transcription.
Full text
PDF


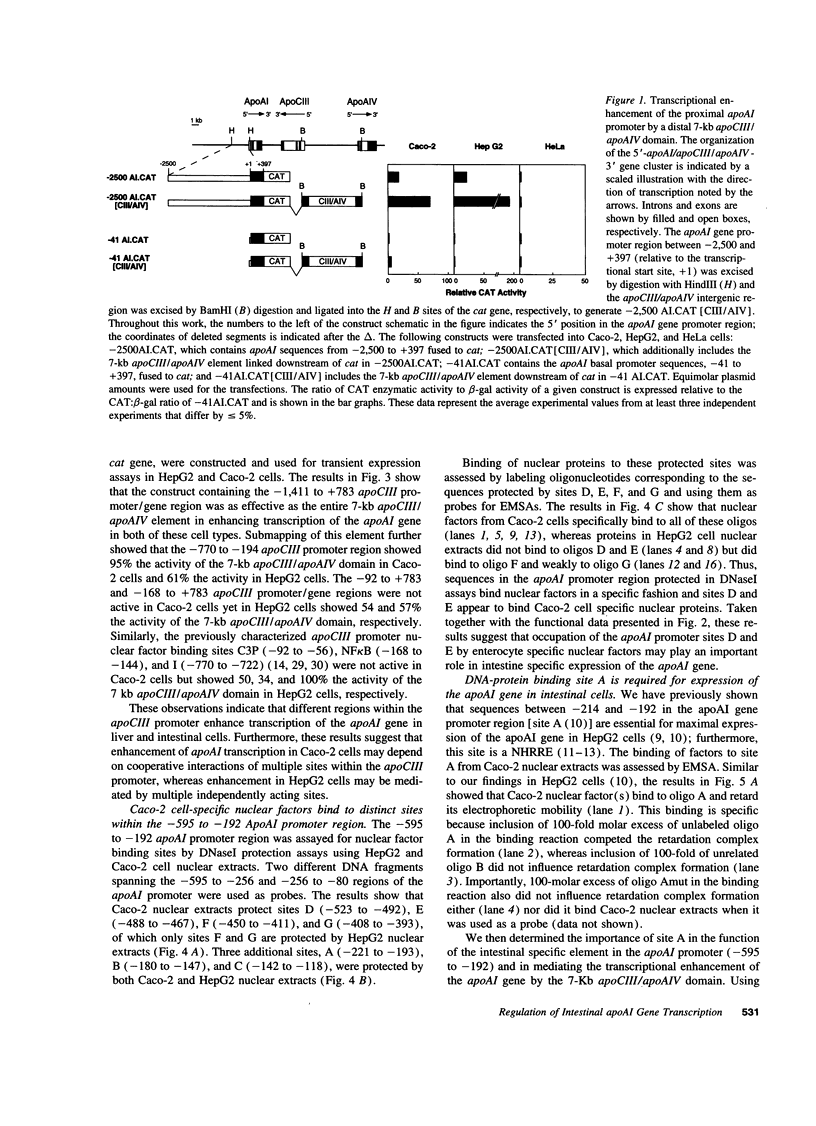

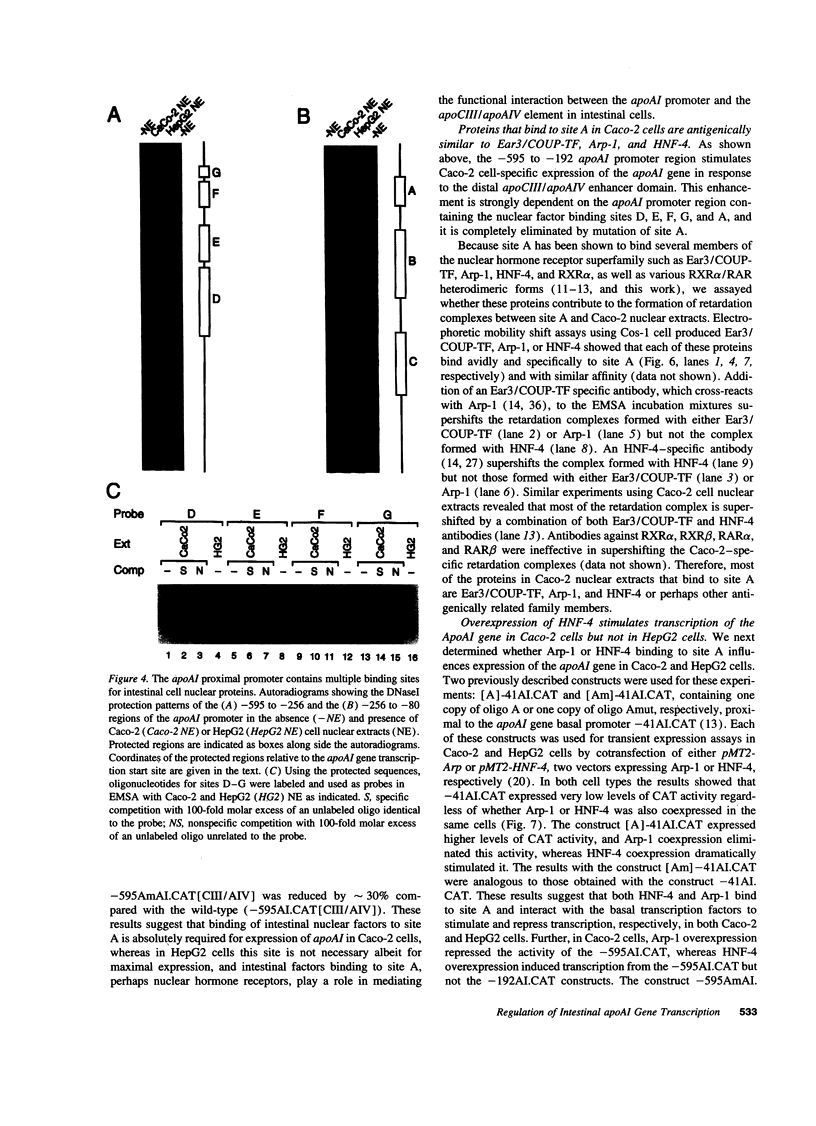

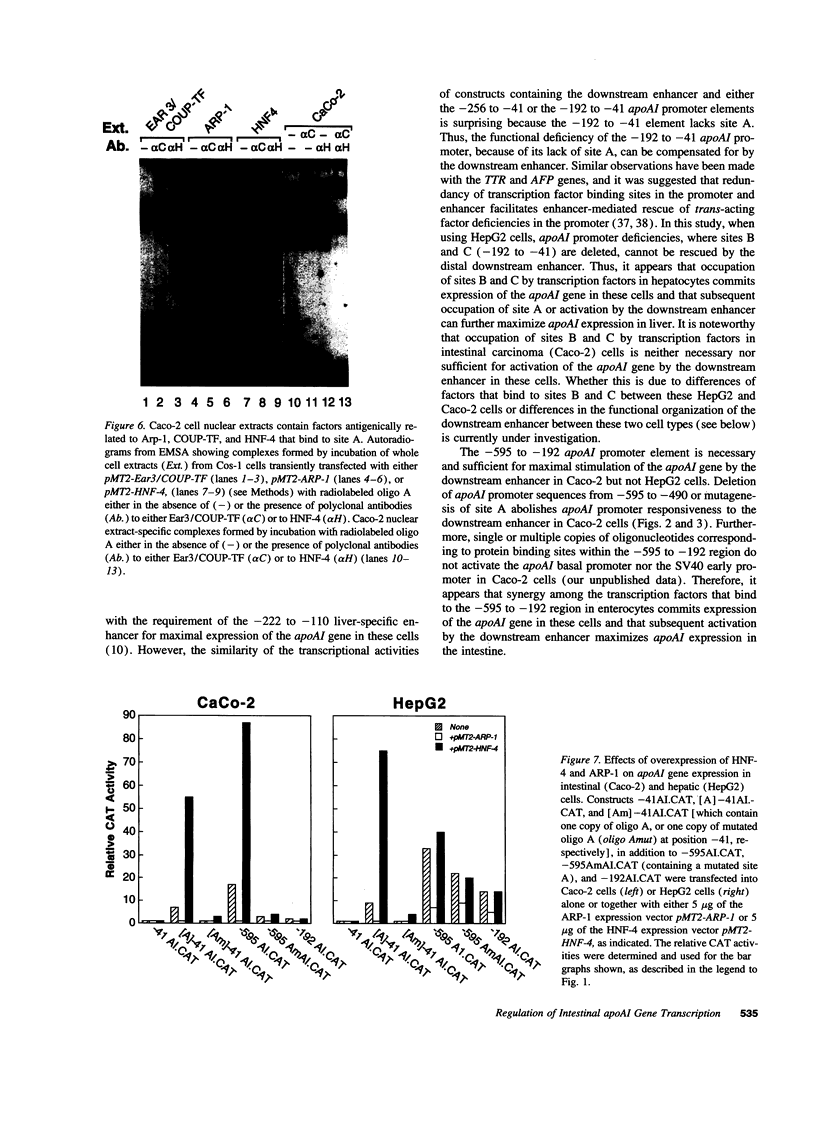
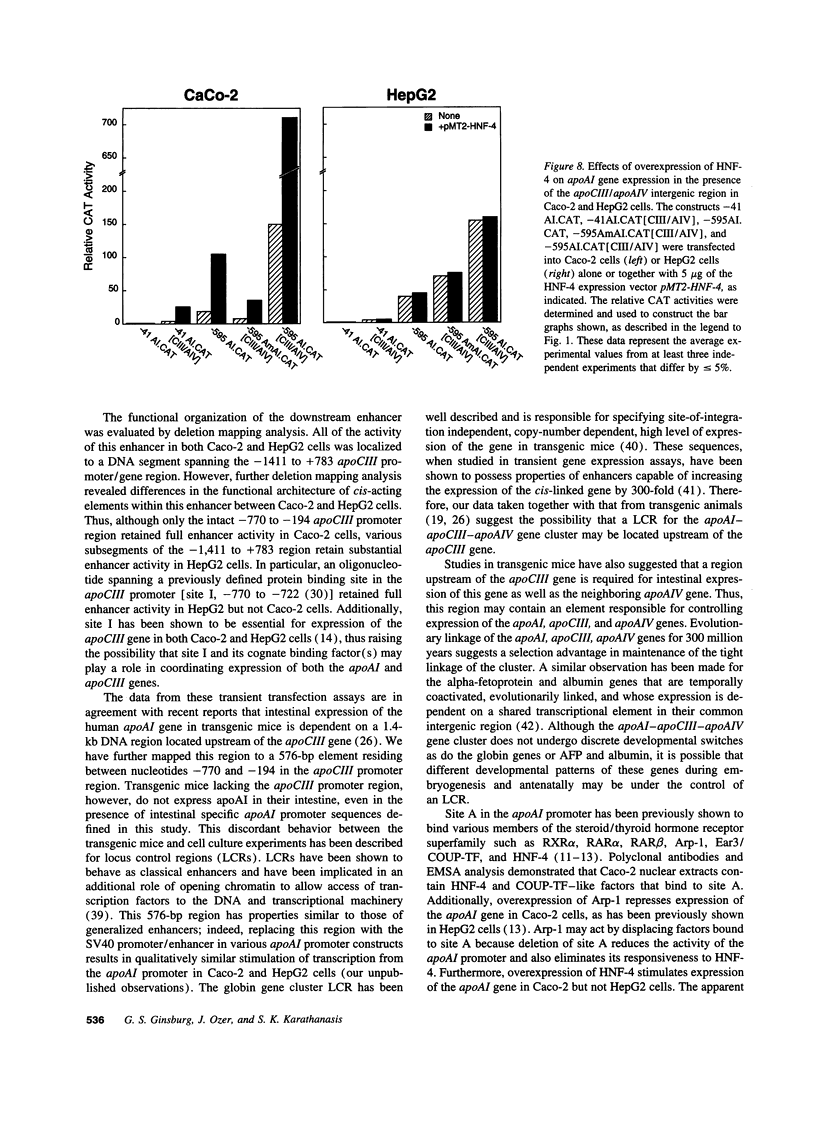


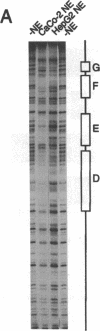
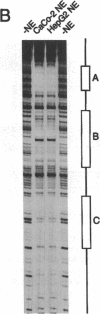
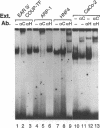
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chao Y. S., Ding X. H., Dai P. H., Wu T. J., Pan T. C., Hao Q. L., Yamin T. T. Identification of an enhancer-like element in the 5' flanking region of the rat apolipoprotein A-I gene. Nucleic Acids Res. 1988 Jul 25;16(14B):7061–7070. doi: 10.1093/nar/16.14.7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognet M., Lone Y. C., Vaulont S., Kahn A., Marie J. Structure of the rat L-type pyruvate kinase gene. J Mol Biol. 1987 Jul 5;196(1):11–25. doi: 10.1016/0022-2836(87)90507-9. [DOI] [PubMed] [Google Scholar]
- Cohn S. M., Simon T. C., Roth K. A., Birkenmeier E. H., Gordon J. I. Use of transgenic mice to map cis-acting elements in the intestinal fatty acid binding protein gene (Fabpi) that control its cell lineage-specific and regional patterns of expression along the duodenal-colonic and crypt-villus axes of the gut epithelium. J Cell Biol. 1992 Oct;119(1):27–44. doi: 10.1083/jcb.119.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney A. J., Leng X., Tsai S. Y., O'Malley B. W., Tsai M. J. Multiple mechanisms of chicken ovalbumin upstream promoter transcription factor-dependent repression of transactivation by the vitamin D, thyroid hormone, and retinoic acid receptors. J Biol Chem. 1993 Feb 25;268(6):4152–4160. [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R. Site-directed mutagenesis of hepatocyte nuclear factor (HNF) binding sites in the mouse transthyretin (TTR) promoter reveal synergistic interactions with its enhancer region. Nucleic Acids Res. 1991 Aug 11;19(15):4139–4145. doi: 10.1093/nar/19.15.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- Elshourbagy N. A., Walker D. W., Paik Y. K., Boguski M. S., Freeman M., Gordon J. I., Taylor J. M. Structure and expression of the human apolipoprotein A-IV gene. J Biol Chem. 1987 Jun 15;262(17):7973–7981. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Haddad I. A., Ordovas J. M., Fitzpatrick T., Karathanasis S. K. Linkage, evolution, and expression of the rat apolipoprotein A-I, C-III, and A-IV genes. J Biol Chem. 1986 Oct 5;261(28):13268–13277. [PubMed] [Google Scholar]
- Hammer R. E., Krumlauf R., Camper S. A., Brinster R. L., Tilghman S. M. Diversity of alpha-fetoprotein gene expression in mice is generated by a combination of separate enhancer elements. Science. 1987 Jan 2;235(4784):53–58. doi: 10.1126/science.2432657. [DOI] [PubMed] [Google Scholar]
- Higuchi K., Law S. W., Hoeg J. M., Schumacher U. K., Meglin N., Brewer H. B., Jr Tissue-specific expression of apolipoprotein A-I (ApoA-I) is regulated by the 5'-flanking region of the human ApoA-I gene. J Biol Chem. 1988 Dec 5;263(34):18530–18536. [PubMed] [Google Scholar]
- Karathanasis S. K. Apolipoprotein multigene family: tandem organization of human apolipoprotein AI, CIII, and AIV genes. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6374–6378. doi: 10.1073/pnas.82.19.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J. Vectors used for expression in mammalian cells. Methods Enzymol. 1990;185:487–511. doi: 10.1016/0076-6879(90)85041-l. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ladias J. A., Karathanasis S. K. Regulation of the apolipoprotein AI gene by ARP-1, a novel member of the steroid receptor superfamily. Science. 1991 Feb 1;251(4993):561–565. doi: 10.1126/science.1899293. [DOI] [PubMed] [Google Scholar]
- Lamon-Fava S., Sastry R., Ferrari S., Rajavashisth T. B., Lusis A. J., Karathanasis S. K. Evolutionary distinct mechanisms regulate apolipoprotein A-I gene expression: differences between avian and mammalian apoA-I gene transcription control regions. J Lipid Res. 1992 Jun;33(6):831–842. [PubMed] [Google Scholar]
- Leff T., Reue K., Melian A., Culver H., Breslow J. L. A regulatory element in the ApoCIII promoter that directs hepatic specific transcription binds to proteins in expressing and nonexpressing cell types. J Biol Chem. 1989 Sep 25;264(27):16132–16137. [PubMed] [Google Scholar]
- Mietus-Snyder M., Sladek F. M., Ginsburg G. S., Kuo C. F., Ladias J. A., Darnell J. E., Jr, Karathanasis S. K. Antagonism between apolipoprotein AI regulatory protein 1, Ear3/COUP-TF, and hepatocyte nuclear factor 4 modulates apolipoprotein CIII gene expression in liver and intestinal cells. Mol Cell Biol. 1992 Apr;12(4):1708–1718. doi: 10.1128/mcb.12.4.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millán J. L. Promoter structure of the human intestinal alkaline phosphatase gene. Nucleic Acids Res. 1987 Dec 23;15(24):10599–10599. doi: 10.1093/nar/15.24.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogami K., Hadzopoulou-Cladaras M., Cladaras C., Zannis V. I. Promoter elements and factors required for hepatic and intestinal transcription of the human ApoCIII gene. J Biol Chem. 1990 Jun 15;265(17):9808–9815. [PubMed] [Google Scholar]
- Papazafiri P., Ogami K., Ramji D. P., Nicosia A., Monaci P., Cladaras C., Zannis V. I. Promoter elements and factors involved in hepatic transcription of the human ApoA-I gene positive and negative regulators bind to overlapping sites. J Biol Chem. 1991 Mar 25;266(9):5790–5797. [PubMed] [Google Scholar]
- Robine S., Sahuquillo-Merino C., Louvard D., Pringault E. Regulatory sequences on the human villin gene trigger the expression of a reporter gene in a differentiating HT29 intestinal cell line. J Biol Chem. 1993 May 25;268(15):11426–11434. [PubMed] [Google Scholar]
- Rottman J. N., Gordon J. I. Comparison of the patterns of expression of rat intestinal fatty acid binding protein/human growth hormone fusion genes in cultured intestinal epithelial cell lines and in the gut epithelium of transgenic mice. J Biol Chem. 1993 Jun 5;268(16):11994–12002. [PubMed] [Google Scholar]
- Rottman J. N., Widom R. L., Nadal-Ginard B., Mahdavi V., Karathanasis S. K. A retinoic acid-responsive element in the apolipoprotein AI gene distinguishes between two different retinoic acid response pathways. Mol Cell Biol. 1991 Jul;11(7):3814–3820. doi: 10.1128/mcb.11.7.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin E. M., Ishida B. Y., Clift S. M., Krauss R. M. Expression of human apolipoprotein A-I in transgenic mice results in reduced plasma levels of murine apolipoprotein A-I and the appearance of two new high density lipoprotein size subclasses. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):434–438. doi: 10.1073/pnas.88.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry K. N., Seedorf U., Karathanasis S. K. Different cis-acting DNA elements control expression of the human apolipoprotein AI gene in different cell types. Mol Cell Biol. 1988 Feb;8(2):605–614. doi: 10.1128/mcb.8.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek F. M., Zhong W. M., Lai E., Darnell J. E., Jr Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 1990 Dec;4(12B):2353–2365. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- Sweetser D. A., Birkenmeier E. H., Klisak I. J., Zollman S., Sparkes R. S., Mohandas T., Lusis A. J., Gordon J. I. The human and rodent intestinal fatty acid binding protein genes. A comparative analysis of their structure, expression, and linkage relationships. J Biol Chem. 1987 Nov 25;262(33):16060–16071. [PubMed] [Google Scholar]
- Townes T. M., Behringer R. R. Human globin locus activation region (LAR): role in temporal control. Trends Genet. 1990 Jul;6(7):219–223. doi: 10.1016/0168-9525(90)90182-6. [DOI] [PubMed] [Google Scholar]
- Tuan D. Y., Solomon W. B., London I. M., Lee D. P. An erythroid-specific, developmental-stage-independent enhancer far upstream of the human "beta-like globin" genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2554–2558. doi: 10.1073/pnas.86.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh A., Azrolan N., Wang K., Marcigliano A., O'Connell A., Breslow J. L. Intestinal expression of the human apoA-I gene in transgenic mice is controlled by a DNA region 3' to the gene in the promoter of the adjacent convergently transcribed apoC-III gene. J Lipid Res. 1993 Apr;34(4):617–623. [PubMed] [Google Scholar]
- Walsh A., Ito Y., Breslow J. L. High levels of human apolipoprotein A-I in transgenic mice result in increased plasma levels of small high density lipoprotein (HDL) particles comparable to human HDL3. J Biol Chem. 1989 Apr 15;264(11):6488–6494. [PubMed] [Google Scholar]
- Widom R. L., Ladias J. A., Kouidou S., Karathanasis S. K. Synergistic interactions between transcription factors control expression of the apolipoprotein AI gene in liver cells. Mol Cell Biol. 1991 Feb;11(2):677–687. doi: 10.1128/mcb.11.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom R. L., Rhee M., Karathanasis S. K. Repression by ARP-1 sensitizes apolipoprotein AI gene responsiveness to RXR alpha and retinoic acid. Mol Cell Biol. 1992 Aug;12(8):3380–3389. doi: 10.1128/mcb.12.8.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. D., Wang W., Traber P. G. Isolation and characterization of the human sucrase-isomaltase gene and demonstration of intestine-specific transcriptional elements. J Biol Chem. 1992 Apr 15;267(11):7863–7870. [PubMed] [Google Scholar]
- Zhang D. E., Ge X., Rabek J. P., Papaconstantinou J. Functional analysis of the trans-acting factor binding sites of the mouse alpha-fetoprotein proximal promoter by site-directed mutagenesis. J Biol Chem. 1991 Nov 5;266(31):21179–21185. [PubMed] [Google Scholar]