Abstract
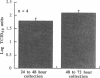
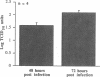
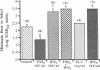
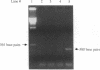
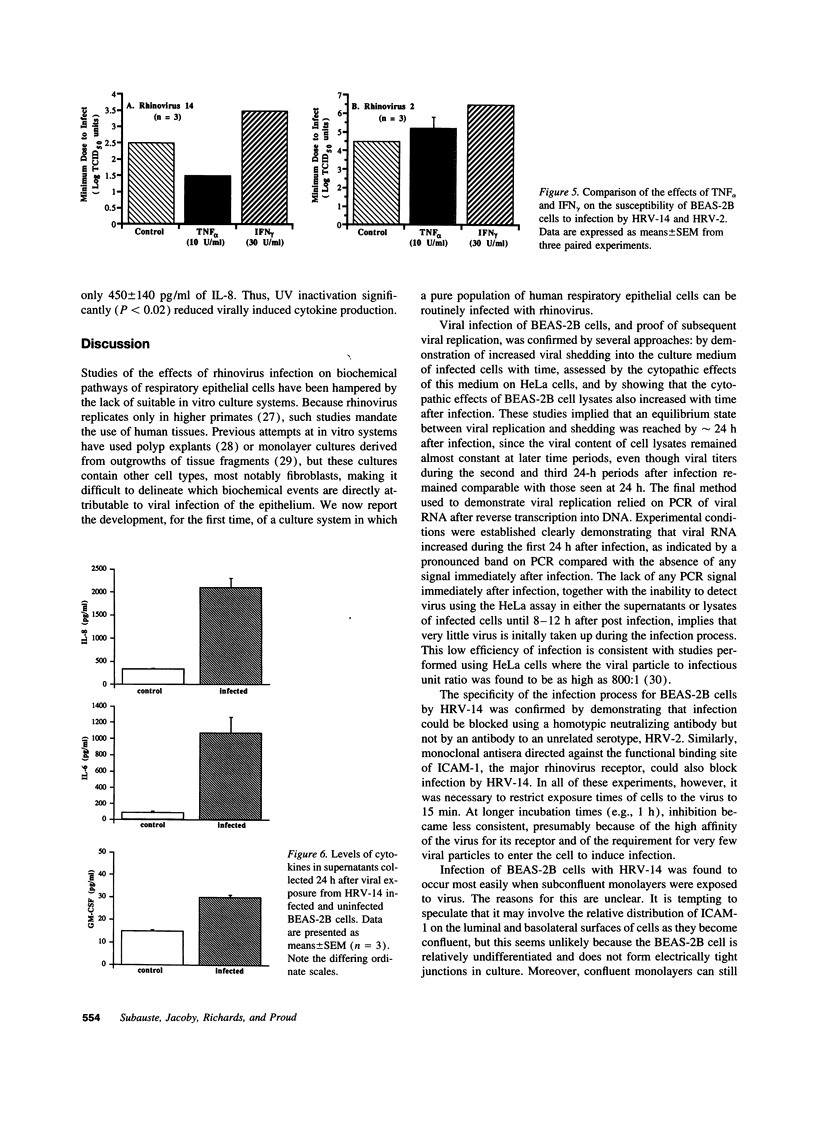
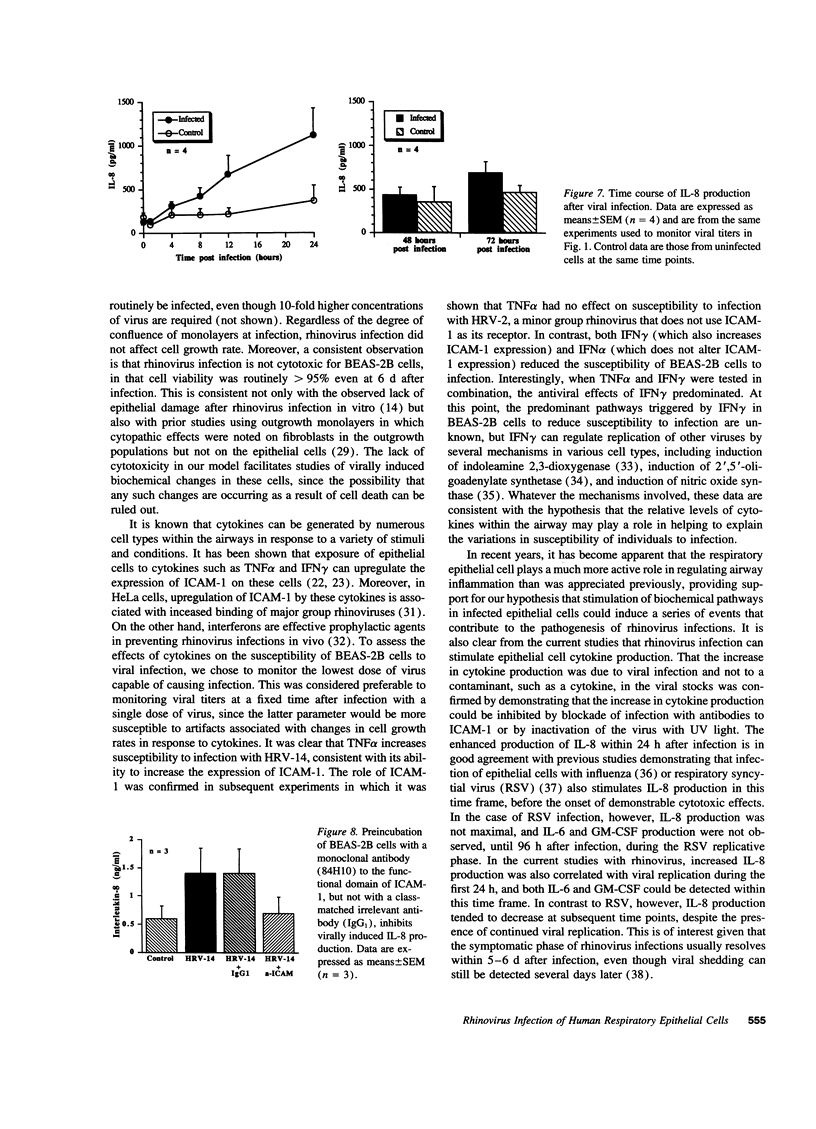
Rhinovirus infections cause over one third of all colds and are a contributing factor to exacerbations of asthma. To gain insights into the early biochemical events that occur in infected epithelial cells, we develop, for the first time, a model in which a pure respiratory epithelial cell population can be routinely infected by rhinovirus. Viral infection was confirmed by demonstrating that viral titers of supernatants and lysates from infected cell increased with time and by PCR. Infection by rhinovirus 14 was inhibited by homotypic antiserum and by antibodies to intercellular adhesion molecule-1 (ICAM-1), the receptor for this virus. Susceptibility of epithelial cells to infection by rhinovirus 14 (but not rhinovirus 2, an ICAM-1 independent strain) can be increased by preexposure of cells to TNF alpha, whereas IFN gamma reduces susceptibility to infection by both rhinovirus strains. Rhinovirus infection per se does not markedly alter ICAM-1 expression on epithelial cells. Finally, we demonstrate that rhinovirus infection induced increased production of IL-8, IL-6, and GM-CSF from epithelial cells. Production of IL-8 correlated with viral replication during the first 24 h after infection. This model should provide useful insights into the pathogenesis of rhinovirus infections.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Hirano T., Taga T., Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 1990 Aug;4(11):2860–2867. [PubMed] [Google Scholar]
- Albelda S. M. Endothelial and epithelial cell adhesion molecules. Am J Respir Cell Mol Biol. 1991 Mar;4(3):195–203. doi: 10.1165/ajrcmb/4.3.195. [DOI] [PubMed] [Google Scholar]
- Arola M., Ziegler T., Puhakka H., Lehtonen O. P., Ruuskanen O. Rhinovirus in otitis media with effusion. Ann Otol Rhinol Laryngol. 1990 Jun;99(6 Pt 1):451–453. doi: 10.1177/000348949009900607. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin P. G., Johnston S. L., Sanderson G., Robinson B. S., Pickett M. A., Fraenkel D. J., Holgate S. T. Detection of rhinovirus infection of the nasal mucosa by oligonucleotide in situ hybridization. Am J Respir Cell Mol Biol. 1994 Feb;10(2):207–213. doi: 10.1165/ajrcmb.10.2.8110476. [DOI] [PubMed] [Google Scholar]
- Bloemen P. G., van den Tweel M. C., Henricks P. A., Engels F., Wagenaar S. S., Rutten A. A., Nijkamp F. P. Expression and modulation of adhesion molecules on human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1993 Dec;9(6):586–593. doi: 10.1165/ajrcmb/9.6.586. [DOI] [PubMed] [Google Scholar]
- Bochner B. S., McKelvey A. A., Schleimer R. P., Hildreth J. E., MacGlashan D. W., Jr Flow cytometric methods for the analysis of human basophil surface antigens and viability. J Immunol Methods. 1989 Dec 20;125(1-2):265–271. doi: 10.1016/0022-1759(89)90102-6. [DOI] [PubMed] [Google Scholar]
- Choi A. M., Jacoby D. B. Influenza virus A infection induces interleukin-8 gene expression in human airway epithelial cells. FEBS Lett. 1992 Sep 14;309(3):327–329. doi: 10.1016/0014-5793(92)80799-m. [DOI] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Gwaltney J. M., Jr, Hendley J. O., Simon G., Jordan W. S., Jr Rhinovirus infections in an industrial population. I. The occurrence of illness. N Engl J Med. 1966 Dec 8;275(23):1261–1268. doi: 10.1056/NEJM196612082752301. [DOI] [PubMed] [Google Scholar]
- Gwaltney J. M., Jr, Phillips C. D., Miller R. D., Riker D. K. Computed tomographic study of the common cold. N Engl J Med. 1994 Jan 6;330(1):25–30. doi: 10.1056/NEJM199401063300105. [DOI] [PubMed] [Google Scholar]
- Hamory B. H., Hendley J. O., Gwaltney J. M., Jr Rhinovirus growth in nasal polyp organ culture. Proc Soc Exp Biol Med. 1977 Sep;155(4):577–582. doi: 10.3181/00379727-155-39854. [DOI] [PubMed] [Google Scholar]
- Hayden F. G., Albrecht J. K., Kaiser D. L., Gwaltney J. M., Jr Prevention of natural colds by contact prophylaxis with intranasal alpha 2-interferon. N Engl J Med. 1986 Jan 9;314(2):71–75. doi: 10.1056/NEJM198601093140202. [DOI] [PubMed] [Google Scholar]
- Hughes J. H., Mitchell M., Hamparian V. V. Rhinoviruses: kinetics of ultraviolet inactivation and effects of UV and heat on immunogenicity. Arch Virol. 1979;61(4):313–319. doi: 10.1007/BF01315018. [DOI] [PubMed] [Google Scholar]
- Johnston S. L., Sanderson G., Pattemore P. K., Smith S., Bardin P. G., Bruce C. B., Lambden P. R., Tyrrell D. A., Holgate S. T. Use of polymerase chain reaction for diagnosis of picornavirus infection in subjects with and without respiratory symptoms. J Clin Microbiol. 1993 Jan;31(1):111–117. doi: 10.1128/jcm.31.1.111-117.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karupiah G., Xie Q. W., Buller R. M., Nathan C., Duarte C., MacMicking J. D. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993 Sep 10;261(5127):1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- Lambert H. P., Stern H. Infective factors in exacerbations of bronchitis and asthma. Br Med J. 1972 Aug 5;3(5822):323–327. doi: 10.1136/bmj.3.5822.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Lee W. M., Monroe S. S., Rueckert R. R. Role of maturation cleavage in infectivity of picornaviruses: activation of an infectosome. J Virol. 1993 Apr;67(4):2110–2122. doi: 10.1128/jvi.67.4.2110-2122.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levandowski R. A., Weaver C. W., Jackson G. G. Nasal-secretion leukocyte populations determined by flow cytometry during acute rhinovirus infection. J Med Virol. 1988 Aug;25(4):423–432. doi: 10.1002/jmv.1890250406. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Williamson D. J., Gamble J. R., Begley C. G., Harlan J. M., Klebanoff S. J., Waltersdorph A., Wong G., Clark S. C., Vadas M. A. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986 Nov;78(5):1220–1228. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini M., Vittori E., Hollemborg J., Mattoli S. Expression of the potent inflammatory cytokines, granulocyte-macrophage-colony-stimulating factor and interleukin-6 and interleukin-8, in bronchial epithelial cells of patients with asthma. J Allergy Clin Immunol. 1992 May;89(5):1001–1009. doi: 10.1016/0091-6749(92)90223-o. [DOI] [PubMed] [Google Scholar]
- McHardy V. U., Inglis J. M., Calder M. A., Crofton J. W., Gregg I., Ryland D. A., Taylor P., Chadwick M., Coombs D., Riddell R. W. A study of infective and other factors in exacerbations of chronic bronchitis. Br J Dis Chest. 1980 Jul;74(3):228–238. doi: 10.1016/0007-0971(80)90048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor T. E., Dick E. C., Baker J. W., Ouellette J. J., Cohen M., Reed C. E. Rhinovirus and influenza type A infections as precipitants of asthma. Am Rev Respir Dis. 1976 Feb;113(2):149–153. doi: 10.1164/arrd.1976.113.2.149. [DOI] [PubMed] [Google Scholar]
- Naclerio R. M., Proud D., Lichtenstein L. M., Kagey-Sobotka A., Hendley J. O., Sorrentino J., Gwaltney J. M. Kinins are generated during experimental rhinovirus colds. J Infect Dis. 1988 Jan;157(1):133–142. doi: 10.1093/infdis/157.1.133. [DOI] [PubMed] [Google Scholar]
- Noah T. L., Becker S. Respiratory syncytial virus-induced cytokine production by a human bronchial epithelial cell line. Am J Physiol. 1993 Nov;265(5 Pt 1):L472–L478. doi: 10.1152/ajplung.1993.265.5.L472. [DOI] [PubMed] [Google Scholar]
- Pattemore P. K., Johnston S. L., Bardin P. G. Viruses as precipitants of asthma symptoms. I. Epidemiology. Clin Exp Allergy. 1992 Mar;22(3):325–336. doi: 10.1111/j.1365-2222.1992.tb03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud D., Gwaltney J. M., Jr, Hendley J. O., Dinarello C. A., Gillis S., Schleimer R. P. Increased levels of interleukin-1 are detected in nasal secretions of volunteers during experimental rhinovirus colds. J Infect Dis. 1994 May;169(5):1007–1013. doi: 10.1093/infdis/169.5.1007. [DOI] [PubMed] [Google Scholar]
- Proud D., Naclerio R. M., Gwaltney J. M., Hendley J. O. Kinins are generated in nasal secretions during natural rhinovirus colds. J Infect Dis. 1990 Jan;161(1):120–123. doi: 10.1093/infdis/161.1.120. [DOI] [PubMed] [Google Scholar]
- Proud D., Subauste M. C., Ward P. E. Glucocorticoids do not alter peptidase expression on a human bronchial epithelial cell line. Am J Respir Cell Mol Biol. 1994 Jul;11(1):57–65. doi: 10.1165/ajrcmb.11.1.7517143. [DOI] [PubMed] [Google Scholar]
- Reddel R. R., Ke Y., Gerwin B. I., McMenamin M. G., Lechner J. F., Su R. T., Brash D. E., Park J. B., Rhim J. S., Harris C. C. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988 Apr 1;48(7):1904–1909. [PubMed] [Google Scholar]
- Rose R. M., Kobzik L., Dushay K., Wolfthal S., Hondalus M., Metzger M., Stoudemire J., Brain J. D., Garnick M., O'Donnell C. The effect of aerosolized recombinant human granulocyte macrophage colony-stimulating factor on lung leukocytes in nonhuman primates. Am Rev Respir Dis. 1992 Nov;146(5 Pt 1):1279–1286. doi: 10.1164/ajrccm/146.5_Pt_1.1279. [DOI] [PubMed] [Google Scholar]
- Schleimer R. P., Rutledge B. K. Cultured human vascular endothelial cells acquire adhesiveness for neutrophils after stimulation with interleukin 1, endotoxin, and tumor-promoting phorbol diesters. J Immunol. 1986 Jan;136(2):649–654. [PubMed] [Google Scholar]
- Staunton D. E., Merluzzi V. J., Rothlein R., Barton R., Marlin S. D., Springer T. A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989 Mar 10;56(5):849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- Tomassini J. E., Graham D., DeWitt C. M., Lineberger D. W., Rodkey J. A., Colonno R. J. cDNA cloning reveals that the major group rhinovirus receptor on HeLa cells is intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4907–4911. doi: 10.1073/pnas.86.13.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B. W., Cail W. S., Hendley J. O., Hayden F. G., Doyle W. J., Sorrentino J. V., Gwaltney J. M., Jr Physiologic abnormalities in the paranasal sinuses during experimental rhinovirus colds. J Allergy Clin Immunol. 1992 Sep;90(3 Pt 2):474–478. doi: 10.1016/0091-6749(92)90172-x. [DOI] [PubMed] [Google Scholar]
- Turner R. B., Hendley J. O., Gwaltney J. M., Jr Shedding of infected ciliated epithelial cells in rhinovirus colds. J Infect Dis. 1982 Jun;145(6):849–853. doi: 10.1093/infdis/145.6.849. [DOI] [PubMed] [Google Scholar]
- Winther B., Farr B., Turner R. B., Hendley J. O., Gwaltney J. M., Jr, Mygind N. Histopathologic examination and enumeration of polymorphonuclear leukocytes in the nasal mucosa during experimental rhinovirus colds. Acta Otolaryngol Suppl. 1984;413:19–24. doi: 10.3109/00016488409128537. [DOI] [PubMed] [Google Scholar]
- Winther B., Gwaltney J. M., Hendley J. O. Respiratory virus infection of monolayer cultures of human nasal epithelial cells. Am Rev Respir Dis. 1990 Apr;141(4 Pt 1):839–845. doi: 10.1164/ajrccm/141.4_Pt_1.839. [DOI] [PubMed] [Google Scholar]
- Winther B., Gwaltney J. M., Jr, Mygind N., Turner R. B., Hendley J. O. Sites of rhinovirus recovery after point inoculation of the upper airway. JAMA. 1986 Oct 3;256(13):1763–1767. [PubMed] [Google Scholar]
- Yoshida R., Imanishi J., Oku T., Kishida T., Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc Natl Acad Sci U S A. 1981 Jan;78(1):129–132. doi: 10.1073/pnas.78.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein A., Kimchi A., Schmidt A., Revel M. Isolation of two interferon-induced translational inhibitors: a protein kinase and an oligo-isoadenylate synthetase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4734–4738. doi: 10.1073/pnas.75.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]