Abstract
Seizures induce profound plastic changes in the brain, including altered expression of neuropeptide Y (NPY) and its receptors. Here I discuss a potential role of NPY plasticity in the developmental brain: In a rat model of febrile seizures (FS), the most common type of seizures in infants and young children, NPY expression was up-regulated in hippocampus after experimentally-induced FS. Interestingly, NPY up-regulation was associated with an increased seizure threshold for additional (recurrent) FS, and this effect was abolished when an antagonist against NPY receptor type 2 was applied. These findings suggest that inhibitory actions of NPY, released after seizures, exert a protective effect that reduces the risk of seizure recurrence in the developing brain.
Keywords: neuropeptide Y, NPY, febrile seizures, rat, hyperthermia, epilepsy, hippocampus
1. Introduction
Neuropeptide Y (NPY) is an important modulator of hippocampal synaptic transmission[27, 49, 50, 58, 79, 81]. Increasing attention has therefore been devoted to assessing whether seizures induce changes in NPY-mediated neurotransmission, and whether these changes are involved in the underlying pathophysiology of seizure disorders or compensatory mechanisms. Indeed, elevated NPY release after seizures and in epileptic tissue has been reported in brain regions that are crucial for the initiation and propagation of epileptic discharges, such as the amygdala, hippocampus, piriform and entorhinal cortices [13, 54, 61, 65, 67, 74, 75, 77]. In these limbic regions, up-regulation of NPY may be part of an adaptive response aimed at counteracting hyperexcitability and thus recurrence of seizures [78]. Recent findings in an animal model of developmental febrile seizures, which I shall review here, suggest that such an adaptive role of NPY release may also be critical in the developing brain, and may protect the highly plastic, immature brain from undergoing recurrent seizures.
2. Febrile seizures and epileptogenesis
A frequent hallmark in the histories of patients with temporal lobe epilepsy (TLE), particularly if associated with hippocampal sclerosis (mesial TLE), is the occurrence of prolonged febrile seizures (FS) early in life [21, 38, 44, 72], prompting suggestions that the FS are a causal factor in the etiology of this epileptic disorder [6, 52]. However, most FS (affecting 2–14% of all children worldwide; [46, 71 for review]) do not lead to epilepsy, and certain risk factors have been distinguished. These include: 1) FS that are prolonged, i.e., >10 to 15 min [3, 16, 56], 2) FS that are recurrent, i.e., several seizures within a 24h period [3, 55], and 3) FS that are focal. A seizure that has one or more of the previous features is referred to as complex FS [28].
In order to study the consequences of complex FS, and thus to gain a better understanding of their potential contribution to human epilepsy, an animal model of FS was established in our laboratory [10, 18, 19, 32, 33, 73]. In this model, experimental FS are induced in rat pups on postnatal days 10 or 11, an age that corresponds to the hippocampal developmental stage at which human infants are most susceptible to febrile seizures (see comparison of developmental milestones in humans and rodent hippocampus in [4]). The majority of FS in humans occur between 6 months and 5 years of age with a peak of incidence at 18 months [46, 57].
Experimental FS are induced by hyperthermia [30] which triggers processes in the brain that are similar to those evoked by fever (e.g., release of cytokines, [34, 41, 63, 64]). For seizure generation, rat pups are placed in a glass jar and exposed to a constant stream of mildly heated air [32, 73]. Seizures reliably occur when core and brain temperatures reach a threshold temperature of ~40.8°C. The threshold temperatures generating experimental FS are close to those required for FS in normal children [14]. As indicated by seizure behavior and confirmed with electrophysiological recordings from multiple brain sites, these seizures are limbic and the behavior during the seizures is reproducible and stereotyped [10, 18, 32]. Seizure duration can be tightly controlled in the model. In addition, mortality (<1%) and morbidity after seizures are low. Taken together, the similarities to the human situation and the controllability of seizure conditions render this animal model suitable for studying the impact of developmental “febrile” seizures on the immature brain.
We first used this model to generate experimental FS of prolonged duration, the type of complex FS most frequently associated with mesial TLE [22, 69 for review]. For this purpose, seizure duration was controlled to last ~24 minutes (i.e., not status epilepticus). Rats were then returned to their mothers and further investigated weeks or months later. The major findings of these studies are that prolonged FS do not lead to cell death nor to other neuroanatomical abnormalities, although transient neuronal injury occurs [10, 11, 33, 35, 73]. Nevertheless, rats that had experienced prolonged FS early in life have a reduced seizure threshold later in life and ~35% develop spontaneous seizures, i.e. limbic (temporal lobe) epilepsy, as adults [32, 33]. This may be due in part to profound and long-lasting changes in the expression of the hyperpolarization-activated cyclic nucleotide-gated cation (HCN) channel genes after FS [12, 18, 19, 66]. These data together suggest that prolonged experimental FS induce plastic processes in the brain that render the neuronal network hyperexcitable [18, 19, 23, 24, 32, 33].
3. The problem of seizure recurrence
We used this animal model to study recurrent “FS”, i.e., those occurring during the subsequent 24 hours. The rationale for these studies derives from observations on human FS. Thus, most FS remain single events [2], and repeated seizures are uncommon (occurring only in ~14% of FS; [15]), although core temperatures may remain elevated in children for several hours during a febrile episode. What protects these children from having recurrent febrile seizures?
To study this question, we induced experimental FS once, twice or three times at 3–4 hour intervals to approximate the clinical situation [15]. We found that threshold temperatures for the second seizure were significantly higher compared with the first, and were even further increased for the third seizure [31]. This suggests that seizure occurrence triggers mechanisms that reduce network excitability and thus render a recurrent seizure less likely.
Our search for potential mechanisms led us to neuropeptides because: 1) Initial in vitro hippocampal slice experiments, carried out 10 to 30 minutes after experimental FS, suggested that altered inhibition through GABAA receptors was less likely to be involved within several hours after FS (Eghbal-Ahmadi et al., unpublished) and 2) In general terms, peptides induce a response of longer duration than classic neurotransmitters [47, 48]. We first considered corticotropin releasing hormone (CRH) as a potential candidate for mediating the increased threshold for a second and third experimental FS. CRH is abundantly expressed in neurons of the developing hippocampus [25]. Its release excites hippocampal neurons [1, 49] and evokes seizures in immature rodents [7, 8]. Thus, a first FS may reduce CRH levels in hippocampal synapses, leading to a decrease of the excitatory drive. However, quantitative analyses of CRH mRNA expression in the hippocampus demonstrated an increase after experimental FS, suggesting an enhanced rather than a reduced release of the endogenous peptide [45]. Down-regulation of CRH expression is therefore unlikely to provide “protection” from generating another FS.
Our next logical candidate was NPY. NPY is, like CRH, abundantly expressed in the hippocampal formation [39, 60], and, in contrast to CRH, reduces excitatory synaptic neurotransmission in the hippocampus after release, mainly through its actions on Y2-receptors [26, 27, 42, 50, 51, 58, 67, 79, 81 but see 80]. This effect has been attributed to the reduction of Ca2+ influx into presynaptic nerve terminals through several types of Ca2+ channels [26, 59, 70].
To determine whether endogenous NPY was released after a FS, and acts on its receptor(s) to reduce excitability of the hippocampal network, we used selective inhibitors of NPY receptors, Y2- (BIIE0246, 100nm/kg, 2.5 nm/μl, administered into the lateral ventricle using a semistereotaxic freehand infusion; [20, 29, 36, 37]) and Y5 (GW459633B, 30 mg/kg, injected intraperitoneally). The antagonists were infused 4 hours after a first FS at 10 (Y2) and 3 (Y5) minutes before the induction of a second experimental FS. Both antagonists abrogated the progressive increase in seizure threshold as shown in Fig. 1 [31]. These data suggest that native NPY is released after the first seizure and acts to increase threshold (i.e., reduce excitability) for a second one.
Fig. 1.
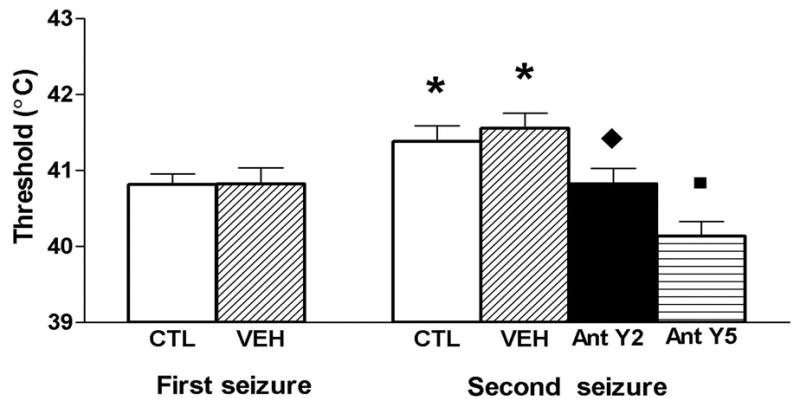
The progressive increase of threshold temperature for a second experimental febrile seizure (4 hours after the first) is abolished by selective neuropeptide Y (NPY), NPY-Y2 and NPY-Y5 receptor antagonists administered to 10 day old rats. Core threshold temperatures are significantly higher for the second seizure compared with the first for both, untreated control rats (CTL) and infused rats with vehicle (VEH). Vehicle administration does not modify the increased threshold temperature for a second febrile seizure. Asterisk denotes a significant difference from the first seizure threshold. Administration of the Y2 (BIIE0246) and Y5 (GW459633B) NPY receptor antagonists reduce the threshold temperature for the second seizure compared with animals treated with the vehicle. The diamond and square indicate significantly lower threshold compared with the threshold for the second seizure in the vehicle-infused group. Modified from Dubé C, Brunson KL, Eghbal-Ahmadi M, Gonzalez-Vega R, Baram. TZ. Endogenous neuropeptide Y prevents recurrence of experimental febrile seizures by increasing seizure threshold. J Mol Neurosci 2005;25:275–84, with permission.
For neuropeptides, the increased release typically leads to enhanced synthesis [47, 48]. Indeed, increased NPY mRNA expression was detected in dentate gyrus (DG) granule cell layer of FS-experiencing rats already by 4 hours, and in DG, CA1 and CA3 hippocampal areas 24 hours after a seizure (Fig. 2), suggesting seizure-induced enhancement of endogenous NPY release [31]. Thus these findings strongly suggest that seizure-induced release of endogenous NPY is involved in endowing limbic circuits with resistance to a second experimental FS.
Fig. 2.
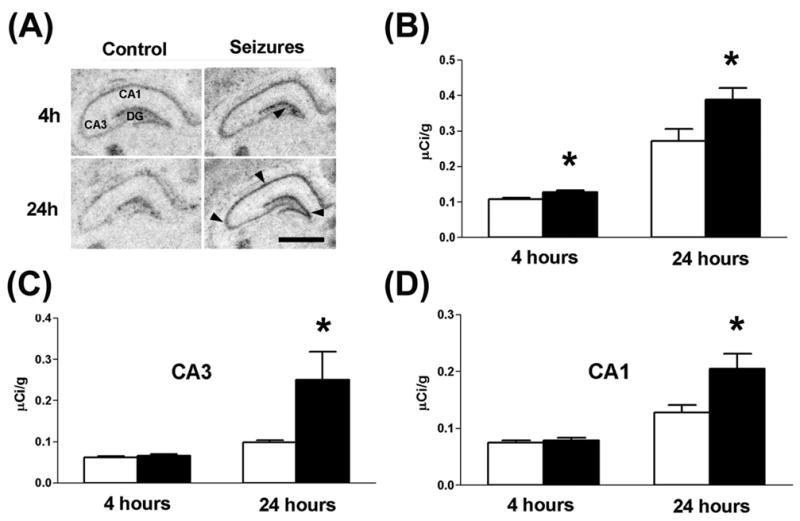
Experimental febrile seizures lead to an enhancement of NPY mRNA expression in hippocampus. In situ hybridization (ISH) histochemistry was performed on brain coronal sections (20 μm) from animals sacrificed 4 or 24 hours after experimental febrile seizure induction (n = 6 per group). (A) Example of sections illustrating the increased NPY signal after febrile seizures in the hippocampus compared with untreated rats. NPY mRNA expression is already enhanced 4 hours after seizures in the dentate gyrus (B) and 24 hours later in the dentate gyrus, CA3 (C) and CA1 (D) hippocampal areas. Reprinted with permission from Dubé C, Brunson KL, Eghbal-Ahmadi M, Gonzalez-Vega R, Baram. TZ. Endogenous neuropeptide Y prevents recurrence of experimental febrile seizures by increasing seizure threshold. J Mol Neurosci 2005;25:275–84.
4. Relevance to human studies
These findings in the FS animal model are consistent with findings in other experimental epilepsy models and with the presumed roles of NPY in human limbic epilepsy. Thus, NPY expression increases after various types of seizures, including kindling [61, 68, 77] and kainic acid-induced seizures [9, 43, 76]. NPY expression is also increased in dentate gyrus granule cells of human epileptic hippocampus [54]. In addition, NPY expression changes are accompanied by altered receptor expression both in seizure models [17, 62, 67], and in tissue from epileptic patients [40]. In all these situations, altered NPY function is assumed to increase inhibition and thus reduces hyperexcitability of the epileptic network. Our findings in an animal model of developmental seizures are novel in that they show for the first time that tight regulation of NPY expression and release is already employed early in life as a mechanism to balance network excitability, and thus to counter changes that could promote epileptogenesis. The detection of remarkably high levels of NPY relatively early in developing human brain [53], and particularly in hippocampus [5], is consistent with this hypothesized regulatory role.
In conclusion, a single FS leads to enhanced inhibitory processes in the normal hippocampal circuit, that protect it from ensuing hyperthermia-provoked seizures within hours of the original ictus . These processes most likely involve the actions of NPY. These findings may provide a molecular and mechanistic basis for the “single-seizure-per-febrile-episode” phenomenon occurring in most children that experience FS. In contrast, recurrence of FS during a febrile episode may be indicative of an underlying brain dysfunction that potentially involves or affects the NPY system. Importantly, improved knowledge about the role of NPY and the signaling cascade triggered by the peptide could provide targets for therapeutic intervention.
Acknowledgments
I thank M. Hinojosa for expert editorial assistance, Dr. R.A. Bender and Prof. T.Z. Baram for discussion and comments. Supported by an Epilepsy Foundation of America postdoctoral research fellowship (CD) and by NIH NS35439 (TZB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875–7. doi: 10.1126/science.6603658. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics: committee on quality improvement, subcommittee on febrile seizures. Practice parameter: long-term treatment of the child with simple febrile seizures. Pediatrics. 1999;103:1307–9. doi: 10.1542/peds.103.6.1307. [DOI] [PubMed] [Google Scholar]
- 3.Annegers JF, Hauser WA, Shirts SB, Kurland LT. Factors prognostic of unprovoked seizures after febrile convulsions. New Eng J Med. 1987;316:493–8. doi: 10.1056/NEJM198702263160901. [DOI] [PubMed] [Google Scholar]
- 4.Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–24. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai F, Sozen MA, Lukiw WJ, Argyropoulos G. Expression of AgRP, NPY, POMC and CART in human fetal and adult hippocampus. Neuropeptides. 2005;39:439–43. doi: 10.1016/j.npep.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Baram TZ. Mechanisms and outcome of febrile seizures: what have we learned from basic science approaches and what needs studying? In: Baram TZ, Shinnar S, editors. Febrile Seizures. San Diego: Academic; 2002. pp. 325–8. [Google Scholar]
- 7.Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21:471–6. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baram TZ, Hirsch E, Snead OC, III, Schultz L. Corticotropin-releasing hormone-induced seizures in infant rats originate in the amygdala. Ann Neurol. 1992;31:488–94. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellmann R, Widmann R, Olenik C, Meyer DK, Maas D, Marksteiner J, et al. Enhanced rate of expression and biosynthesis of neuropeptide Y after kainic acid-induced seizures. J Neurochem. 1991;56:525–30. doi: 10.1111/j.1471-4159.1991.tb08181.x. [DOI] [PubMed] [Google Scholar]
- 10.Bender RA, Dubé C, Gonzalez-Vega R, Mina EW, Baram TZ. Mossy fiber plasticity and enhanced hippocampal excitability, without hippocampal cell loss or altered neurogenesis, in an animal model of prolonged febrile seizures. Hippocampus. 2003;13:399–412. doi: 10.1002/hipo.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bender RA, Dubé C, Baram TZ. Febrile seizures and mechanisms of epileptogenesis: insights from an animal model. Adv Exp Med Biol. 2004;548:213–25. doi: 10.1007/978-1-4757-6376-8_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bender RA, Soleymani SV, Brewster AL, Nguyen ST, Beck H, Mathern GW, et al. Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus. J Neurosci. 2003;23:6826–36. doi: 10.1523/JNEUROSCI.23-17-06826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bendotti C, Vezzani A, Serafini R, Servadio A, Rivolta R, Samanin R. Increased preproneuropeptide Y mRNA in the rat hippocampus during the development of hippocampal kindling: comparison with the expression of preprosomatostatin mRNA. Neurosci Lett. 1991;132:175–8. doi: 10.1016/0304-3940(91)90295-5. [DOI] [PubMed] [Google Scholar]
- 14.Berg AT, Shinnar S, Hauser WA, Alemany M, Shapiro ED, Salomon ME, et al. A prospective study of recurrent febrile seizures. N Engl J Med. 1992;327:1122–7. doi: 10.1056/NEJM199210153271603. [DOI] [PubMed] [Google Scholar]
- 15.Berg AT, Shinnar S. Complex febrile seizures. Epilepsia. 1996;37:126–33. doi: 10.1111/j.1528-1157.1996.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 16.Berg AT, Shinnar S, Darefsky AS, Holford TR, Shapiro ED, Salomon ME, et al. Predictors of recurrent febrile seizures. A prospective cohort study. Arch Pediatr Adolesc Med. 1997;151:371–8. doi: 10.1001/archpedi.1997.02170410045006. [DOI] [PubMed] [Google Scholar]
- 17.Bregola G, Dumont Y, Fournier A, Zucchini S, Quirion R, Simonato M. Decreased levels of neuropeptide Y(5) receptor binding sites in two experimental models of epilepsy. Neuroscience. 2000;98:697–703. doi: 10.1016/s0306-4522(00)00162-7. [DOI] [PubMed] [Google Scholar]
- 18.Brewster A, Bender RA, Chen Y, Dubé C, Eghbal-Ahmadi, Baram TZ. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner. J Neurosci. 2002;22:4591–9. doi: 10.1523/JNEUROSCI.22-11-04591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brewster AL, Bernard JA, Gall CM, Baram TZ. Formation of heteromeric hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in the hippocampus is regulated by developmental seizures. Neurobiol Dis. 2005;19:200–7. doi: 10.1016/j.nbd.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci U S A. 2001;98:8856–61. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cendes F, Andermann F, Dubeau F, Gloor P, Evans A, Jones-Gotman M, et al. Early childhood prolonged febrile convulsions, atrophy and sclerosis of mesial structures, and temporal lobe epilepsy: an MRI volumetric study. Neurology. 1993;43:1083–7. doi: 10.1212/wnl.43.6.1083. [DOI] [PubMed] [Google Scholar]
- 22.Cendes F, Andermann F. Do febrile seizures promote temporal lobe epilepsy? Retrospective studies. In: Baram TZ, Shinnar S, editors. Febrile Seizures. San Diego: Academic; 2002. pp. 77–86. [Google Scholar]
- 23.Chen K, Aradi I, Thon N, Eghbal-Ahmadi M, Baram TZ, Soltesz I. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat Med. 2001;7:331–7. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen K, Baram TZ, Soltesz I. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat Med. 1999;5:888–94. doi: 10.1038/11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Bender R, Frotscher M, Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J Neurosci. 2001;21:7171–81. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colmers WF, Bleakman D. Effects of neuropeptide Y on the electrical properties of neurons. Trends Neurosci. 1994;17:373–9. doi: 10.1016/0166-2236(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 27.Colmers WF, Lukowiak K, Pittman QJ. Neuropeptide Y action in the rat hippocampal slice: site and mechanism of presynaptic inhibition. J Neurosci. 1988;8:3827–37. doi: 10.1523/JNEUROSCI.08-10-03827.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised clinical and eletroencephalographic classification of epileptic seizures. Epilepsia. 1981;33:661–6. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 29.Doods H, Gaida W, Wieland HA, Dollinger H, Schnorrenberg G, Esser F. BIIE0246: a selective and high affinity neuropeptide Y Y(2) receptor antagonist. Eur J Pharmacol. 1999;384:R3–5. doi: 10.1016/s0014-2999(99)00650-0. [DOI] [PubMed] [Google Scholar]
- 30.Dubé CM, Baram TZ. Complex Febrile Seizures- an experimental model in immature rodent. In: Pitkanen A, Schwartzkroin PA, Moshe SL, editors. Models of seizures and epilepsy. San Diego: Elsevier; 2005. pp. 333–340. [Google Scholar]
- 31.Dubé C, Brunson KL, Eghbal-Ahmadi M, Gonzalez-Vega R, Baram TZ. Endogenous neuropeptide Y prevents recurrence of experimental febrile seizures by increasing seizure threshold. J Mol Neurosci. 2005;25:275–84. doi: 10.1385/JMN:25:3:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dubé C, Chen K, Eghbal-Ahmadi M, Brunson K, Soltesz I, Baram TZ. Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long-term. Ann Neurol. 2000;47:336–44. [PMC free article] [PubMed] [Google Scholar]
- 33.Dubé C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911–22. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubé C, Vezzani A, Behrens M, Bartfai T, Baram TZ. Interleukin-1beta contributes to the generation of experimental febrile seizures. Ann Neurol. 2005;57:152–5. doi: 10.1002/ana.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubé C, Yu H, Nalcioglu O, Baram TZ. Serial MRI after prolonged experimental febrile seizures: altered T2 signal does not signify neuronal death. Ann Neurol. 2004;56:709–14. doi: 10.1002/ana.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumont Y, Cadieux A, Doods H, Pheng LH, Abounader R, Hamel E, et al. BIIE0246, a potent and highly selective non-peptide neuropeptide Y Y(2) receptor antagonist. Br J Pharmacol. 2000;129:1075–88. doi: 10.1038/sj.bjp.0703162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Bahh B, Cao JQ, Beck-Sickinger AG, Colmers WF. Blockade of neuropeptide Y(2) receptors and suppression of NPY's anti-epileptic actions in the rat hippocampal slice by BIIE0246. Br J Pharmacol. 2002;136:502–9. doi: 10.1038/sj.bjp.0704751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–80. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- 39.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 40.Furtinger S, Pirker S, Czech T, Baumgartner C, Ransmayr G, Sperk G. Plasticity of Y1 and Y2 receptors and neuropeptide Y fibers in patients with temporal lobe epilepsy. J Neurosci. 2001;21:5804–12. doi: 10.1523/JNEUROSCI.21-15-05804.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gatti S, Vezzani A, Bartfai T. Mechanisms of fever and febrile seizures: putative role of interleukin-1 system. In: Baram TZ, Shinnar S, editors. Febrile Seizures. San Diego: Academic; 2002. pp. 169–188. [Google Scholar]
- 42.Greber S, Schwarzer C, Sperk G. Neuropeptide Y inhibits potassium-stimulated glutamate release through Y2 receptors in rat hippocampal slices in vitro. Br J Pharmacol. 1994;113:737–40. doi: 10.1111/j.1476-5381.1994.tb17055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gruber B, Greber S, Rupp E, Sperk G. Differential NPY mRNA expression in granule cells and interneurons of the rat dentate gyrus after kainic acid injection. Hippocampus. 1994;4:474–82. doi: 10.1002/hipo.450040409. [DOI] [PubMed] [Google Scholar]
- 44.Hamati-Haddad A, Abou-Khalil B. Epilepsy diagnosis and localization in patients with antecedent childhood febrile convulsions. Neurology. 1998;50:917–22. doi: 10.1212/wnl.50.4.917. [DOI] [PubMed] [Google Scholar]
- 45.Hatalski CG, Brunson KL, Tantayanubutr B, Chen Y, Baram TZ. Neuronal activity and stress differentially regulate hippocampal and hypothalamic corticotropin-releasing hormone expression in the immature rat. Neuroscience. 2000;101:571–80. doi: 10.1016/s0306-4522(00)00386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994;35:S1–S6. doi: 10.1111/j.1528-1157.1994.tb05932.x. [DOI] [PubMed] [Google Scholar]
- 47.Hokfelt T. Neuropeptides in perspective: the last ten years. Neuron. 1991;7:867–79. doi: 10.1016/0896-6273(91)90333-u. [DOI] [PubMed] [Google Scholar]
- 48.Hokfelt T, Broberger C, Xu ZQ, Sergeyev V, Ubink R, Diez M. Neuropeptides- an overview. Neuropharmacology. 2000;39:1337–56. doi: 10.1016/s0028-3908(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 49.Hollrigel GS, Chen K, Baram TZ, Soltesz I. The pro-convulsant actions of corticotropin-releasing hormone in the hippocampus of infant rats. Neuroscience. 1998;84:71–9. doi: 10.1016/s0306-4522(97)00499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klapstein GJ, Colmers WF. On the sites of presynaptic inhibition by neuropeptide Y in rat hippocampus in vitro. Hippocampus. 1993;3:103–11. doi: 10.1002/hipo.450030111. [DOI] [PubMed] [Google Scholar]
- 51.Klapstein GJ, Colmers WF. Neuropeptide Y suppresses epileptiform activity in rat hippocampus in vitro. J Neurophysiol. 1997;78:1651–61. doi: 10.1152/jn.1997.78.3.1651. [DOI] [PubMed] [Google Scholar]
- 52.Lewis DV. Febrile convulsions and mesial temporal sclerosis. Curr Opin Neurol. 1999;12:197. doi: 10.1097/00019052-199904000-00011. [DOI] [PubMed] [Google Scholar]
- 53.Lotstra F, Schiffmann SN, Vanderhaeghen JJ. Neuropeptide Y-containing neurons in the human infant hippocampus. Brain Res. 1989;478:211–26. doi: 10.1016/0006-8993(89)91501-1. [DOI] [PubMed] [Google Scholar]
- 54.Mathern GW, Babb TL, Pretorius JK, Leite JP. Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci. 1995;15:3990–4004. doi: 10.1523/JNEUROSCI.15-05-03990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelson KB, Ellenberg JH. Predictors of epilepsy in children who have experienced febrile seizures. N Engl J Med. 1976;295:1029–33. doi: 10.1056/NEJM197611042951901. [DOI] [PubMed] [Google Scholar]
- 56.Nelson KB, Ellenberg JH. Prognosis in children with febrile seizures. Pediatrics. 1978;61:720–7. [PubMed] [Google Scholar]
- 57.Nelson KB, Ellenberg JH. Febrile seizures. New York: Raven Press; 1981. [Google Scholar]
- 58.Patrylo PR, van den Pol AN, Spencer DD, Williamson A. NPY inhibits glutamatergic excitation in the epileptic human dentate gyrus. J Neurophysiol. 1999;82:478–83. doi: 10.1152/jn.1999.82.1.478. [DOI] [PubMed] [Google Scholar]
- 59.Qian J, Colmers WF, Saggau P. Inhibition of synaptic transmission by neuropeptide Y in rat hippocampal area CA1: modulation of presynaptic Ca2+ entry. J Neurosci. 1997;17:8169–77. doi: 10.1523/JNEUROSCI.17-21-08169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Redrobe JP, Dumont Y, St-Pierre JA, Quirion R. Multiple receptors for neuropeptide Y in the hippocampus: putative roles in seizures and cognition. Brain Res. 1999;848:153–66. doi: 10.1016/s0006-8993(99)02119-8. [DOI] [PubMed] [Google Scholar]
- 61.Rizzi M, Monno A, Samanin R, Sperk G, Vezzani A. Electrical kindling of the hippocampus is associated with functional activation of neuropeptide Y-containing neurons. Eur J Neurosci. 1993;5:1534–8. doi: 10.1111/j.1460-9568.1993.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 62.Roder C, Schwarzer C, Vezzani A, Gobbi M, Mennini T, Sperk G. Autoradiographic analysis of neuropeptide Y receptor binding sites in the rat hippocampus after kainic acid-induced limbic seizures. Neuroscience. 1996;70:47–55. doi: 10.1016/0306-4522(95)00332-d. [DOI] [PubMed] [Google Scholar]
- 63.Rothwell NJ, Luheshi GN. Pharmacology of interleukin-1 actions in the brain. Adv Pharmacol. 1994;25:1–20. doi: 10.1016/s1054-3589(08)60428-7. [DOI] [PubMed] [Google Scholar]
- 64.Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–25. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- 65.Sadamatsu M, Kanai H, Masui A, Serikawa T, Yamada J, Sasa M, et al. Altered brain contents of neuropeptides in spontaneously epileptic rats (SER) and tremor rats with absence seizures. Life Sci. 1995;57:523–31. doi: 10.1016/0024-3205(95)00302-m. [DOI] [PubMed] [Google Scholar]
- 66.Santoro B, Baram TZ. The multiple personalities of h-channels. Trends Neurosci. 2003;26:550–4. doi: 10.1016/j.tins.2003.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwarzer C, Kofler N, Sperk G. Up-regulation of neuropeptide Y-Y2 receptors in an animal model of temporal lobe epilepsy. Mol Pharmacol. 1998;53:6–13. doi: 10.1124/mol.53.1.6. [DOI] [PubMed] [Google Scholar]
- 68.Schwarzer C, Williamson JM, Lothman EW, Vezzani A, Sperk G. Somatostatin, neuropeptide Y, neurokinin B and cholecystokinin immunoreactivity in two chronic models of temporal lobe epilepsy. Neuroscience. 1995;69:831–45. doi: 10.1016/0306-4522(95)00268-n. [DOI] [PubMed] [Google Scholar]
- 69.Shinnar S. Do febrile seizures lead to temporal lobe epilepsy? Prospective and epidemiological studies. In: Baram TZ, Shinnar S, editors. Febrile Seizures. San Diego: Academic; 2002. pp. 87–101. [Google Scholar]
- 70.Silva AP, Carvalho AP, Carvalho CM, Malva JO. Functional interaction between neuropeptide Y receptors and modulation of calcium channels in the rat hippocampus. Neuropharmacology. 2003;44:282–92. doi: 10.1016/s0028-3908(02)00382-9. [DOI] [PubMed] [Google Scholar]
- 71.Stafstrom CE. The incidence and prevalence of febrile seizures. In: Baram TZ, Shinnar S, editors. Febrile Seizures. San Diego: Academic; 2002. pp. 1–25. [Google Scholar]
- 72.Theodore WH, Bhatia S, Hatta J, Fazilat S, DeCarli C, Bookheimer SY, et al. Hippocampal atrophy, epilepsy duration, and febrile seizures in patients with partial seizures. Neurology. 1999;52:132–6. doi: 10.1212/wnl.52.1.132. [DOI] [PubMed] [Google Scholar]
- 73.Toth Z, Yan XX, Haftoglou S, Ribak CE, Baram TZ. Seizure-induced neuronal injury: vulnerability to febrile seizures in an immature rat model. J Neurosci. 1998;18:4285–94. doi: 10.1523/JNEUROSCI.18-11-04285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tu B, Timofeeva O, Jiao Y, Nadler JV. Spontaneous release of neuropeptide Y tonically inhibits recurrent mossy fiber synaptic transmission in epileptic brain. J Neurosci. 2005;25:1718–29. doi: 10.1523/JNEUROSCI.4835-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vezzani A, Civenni G, Rizzi M, Monno A, Messali S, Samanin R. Enhanced neuropeptide Y release in the hippocampus is associated with chronic seizure susceptibility in kainic acid treated rats. Brain Res. 1994;660:138–43. doi: 10.1016/0006-8993(94)90847-8. [DOI] [PubMed] [Google Scholar]
- 76.Vezzani A, Monhemius R, Tutka P, Milani R, Samanin R. Functional activation of somatostatin- and neuropeptide Y-containing neurons in the entorhinal cortex of chronically epileptic rats. Neuroscience. 1996;75:551–7. doi: 10.1016/0306-4522(96)00261-8. [DOI] [PubMed] [Google Scholar]
- 77.Vezzani A, Schwarzer C, Lothman EW, Williamson J, Sperk G. Functional changes in somatostatin and neuropeptide Y containing neurons in the rat hippocampus in chronic models of limbic seizures. Epilepsy Res. 1996;26:267–79. doi: 10.1016/s0920-1211(96)00059-9. [DOI] [PubMed] [Google Scholar]
- 78.Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- 79.Woldbye DP. Antiepileptic effects of NPY on pentylenetetrazole seizures. Regul Pept. 1998;75–76:279–282. doi: 10.1016/s0167-0115(98)00079-2. [DOI] [PubMed] [Google Scholar]
- 80.Woldbye DP, Larsen PJ, Mikkelsen JD, Klemp K, Madsen TM, Bolwig TG. Powerful inhibition of kainic acid seizures by neuropeptide Y via Y5-like receptors. Nat Med. 1997;3:761–4. doi: 10.1038/nm0797-761. [DOI] [PubMed] [Google Scholar]
- 81.Woldbye DP, Madsen TM, Larsen PJ, Mikkelsen JD, Bolwig TG. Neuropeptide Y inhibits hippocampal seizures and wet dog shakes. Brain Res. 1996;737:162–8. doi: 10.1016/0006-8993(96)00730-5. [DOI] [PubMed] [Google Scholar]


