Abstract
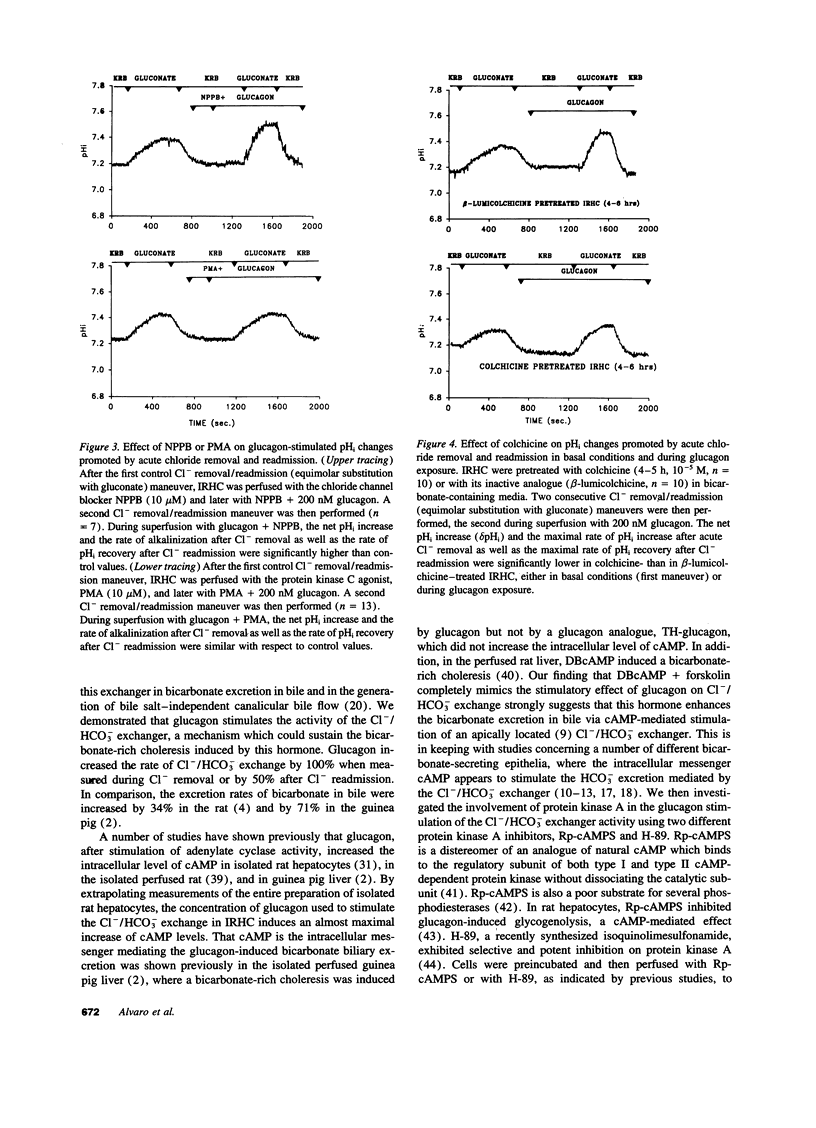
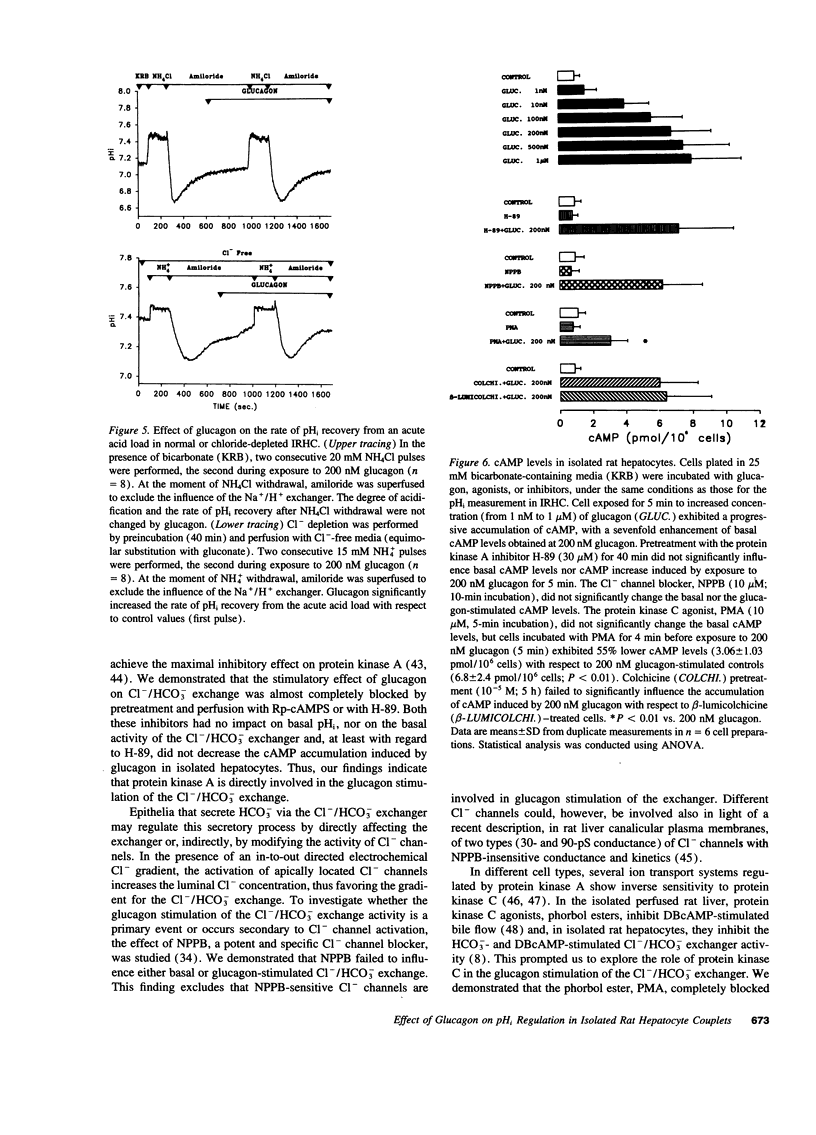
To elucidate mechanisms of glucagon-induced bicarbonate-rich choleresis, we investigated the effect of glucagon on ion transport processes involved in the regulation of intracellular pH (pHi) in isolated rat hepatocyte couplets. It was found that glucagon (200 nM), without influencing resting pHi, significantly stimulates the Cl-/HCO3- exchange activity. The effect of glucagon was associated with a sevenfold increase in cAMP levels in rat hepatocytes. The activity of the Cl-/HCO3- exchanger was also stimulated by DBcAMP + forskolin. The effect of glucagon on the Cl-/HCO3- exchange was individually blocked by two specific and selective inhibitors of protein kinase A, Rp-cAMPs (10 microM) and H-89 (30 microM), the latter having no influence on the glucagon-induced cAMP accumulation in isolated rat hepatocytes. The Cl- channel blocker, NPPB (10 microM), showed no effect on either the basal or the glucagon-stimulated Cl-/HCO3 exchange. In contrast, the protein kinase C agonist, PMA (10 microM), completely blocked the glucagon stimulation of the Cl-/HCO3- exchange; however, this effect was achieved through a significant inhibition of the glucagon-stimulated cAMP accumulation in rat hepatocytes. Colchicine pretreatment inhibited the basal as well as the glucagon-stimulated Cl-/HCO3- exchange activity. The Na+/H+ exchanger was unaffected by glucagon either at basal pHi or at acid pHi values. In contrast, glucagon, at basal pHi, stimulated the Na(+)-HCO3- symport. The main findings of this study indicate that glucagon, through the cAMP-dependent protein kinase A pathway, stimulates the activity of the Cl-/HCO3- exchanger in isolated rat hepatocyte couplets, a mechanism which could account for the in vivo induced bicarbonate-rich choleresis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvaro D., Cho W. K., Mennone A., Boyer J. L. Effect of secretion on intracellular pH regulation in isolated rat bile duct epithelial cells. J Clin Invest. 1993 Sep;92(3):1314–1325. doi: 10.1172/JCI116705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvaro D., Mennone A., Boyer J. L. Effect of ursodeoxycholic acid on intracellular pH regulation in isolated rat bile duct epithelial cells. Am J Physiol. 1993 Oct;265(4 Pt 1):G783–G791. doi: 10.1152/ajpgi.1993.265.4.G783. [DOI] [PubMed] [Google Scholar]
- Argent B. E., Arkle S., Cullen M. J., Green R. Morphological, biochemical and secretory studies on rat pancreatic ducts maintained in tissue culture. Q J Exp Physiol. 1986 Oct;71(4):633–648. doi: 10.1113/expphysiol.1986.sp003023. [DOI] [PubMed] [Google Scholar]
- Arias I. M., Forgac M. The sinusoidal domain of the plasma membrane of rat hepatocytes contains an amiloride-sensitive Na+/H+ antiport. J Biol Chem. 1984 May 10;259(9):5406–5408. [PubMed] [Google Scholar]
- Benedetti A., Strazzabosco M., Corasanti J. G., Haddad P., Graf J., Boyer J. L. Cl(-)-HCO3- exchanger in isolated rat hepatocytes: role in regulation of intracellular pH. Am J Physiol. 1991 Sep;261(3 Pt 1):G512–G522. doi: 10.1152/ajpgi.1991.261.3.G512. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Strazzabosco M., Ng O. C., Boyer J. L. Regulation of activity and apical targeting of the Cl-/HCO3- exchanger in rat hepatocytes. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):792–796. doi: 10.1073/pnas.91.2.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3-. Am J Physiol. 1988 Dec;255(6 Pt 1):C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. II. Na+-dependent and -independent Cl(-)-HCO3- exchangers. Am J Physiol. 1988 Dec;255(6 Pt 1):C857–C869. doi: 10.1152/ajpcell.1988.255.6.C857. [DOI] [PubMed] [Google Scholar]
- Bruck R., Benedetti A., Strazzabosco M., Boyer J. L. Intracellular alkalinization stimulates bile flow and vesicular-mediated exocytosis in IPRL. Am J Physiol. 1993 Aug;265(2 Pt 1):G347–G353. doi: 10.1152/ajpgi.1993.265.2.G347. [DOI] [PubMed] [Google Scholar]
- Chijiwa T., Mishima A., Hagiwara M., Sano M., Hayashi K., Inoue T., Naito K., Toshioka T., Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990 Mar 25;265(9):5267–5272. [PubMed] [Google Scholar]
- Corasanti J. G., Smith N. D., Gordon E. R., Boyer J. L. Protein kinase C agonists inhibit bile secretion independently of effects on the microcirculation in the isolated perfused rat liver. Hepatology. 1989 Jul;10(1):8–13. doi: 10.1002/hep.1840100103. [DOI] [PubMed] [Google Scholar]
- Corvera S., Huerta-Bahena J., Pelton J. T., Hruby V. J., Trivedi D., García-Sáinz J. A. Metabolic effects and cyclic AMP levels produced by glucagon, (1-N alpha-Trinitrophenylhistidine,12-homoarginine)glucagon and forskolin in isolated rat hepatocytes. Biochim Biophys Acta. 1984 Aug 17;804(4):434–441. doi: 10.1016/0167-4889(84)90071-5. [DOI] [PubMed] [Google Scholar]
- Fehlmann M., Freychet P. Insulin and glucagon stimulation of (Na+-K+)-ATPase transport activity in isolated rat hepatocytes. J Biol Chem. 1981 Jul 25;256(14):7449–7453. [PubMed] [Google Scholar]
- Fitz J. G., Basavappa S., McGill J., Melhus O., Cohn J. A. Regulation of membrane chloride currents in rat bile duct epithelial cells. J Clin Invest. 1993 Jan;91(1):319–328. doi: 10.1172/JCI116188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemström G., Heylings J. R., Garner A. Gastric and duodenal HCO3- transport in vitro: effects of hormones and local transmitters. Am J Physiol. 1982 Feb;242(2):G100–G110. doi: 10.1152/ajpgi.1982.242.2.G100. [DOI] [PubMed] [Google Scholar]
- Forker E. L. Mechanisms of hepatic bile formation. Annu Rev Physiol. 1977;39:323–347. doi: 10.1146/annurev.ph.39.030177.001543. [DOI] [PubMed] [Google Scholar]
- Gautam A., Ng O. C., Boyer J. L. Isolated rat hepatocyte couplets in short-term culture: structural characteristics and plasma membrane reorganization. Hepatology. 1987 Mar-Apr;7(2):216–223. doi: 10.1002/hep.1840070203. [DOI] [PubMed] [Google Scholar]
- Gleeson D., Smith N. D., Boyer J. L. Bicarbonate-dependent and -independent intracellular pH regulatory mechanisms in rat hepatocytes. Evidence for Na+-HCO3- cotransport. J Clin Invest. 1989 Jul;84(1):312–321. doi: 10.1172/JCI114156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J., Henderson R. M., Krumpholz B., Boyer J. L. Cell membrane and transepithelial voltages and resistances in isolated rat hepatocyte couplets. J Membr Biol. 1987;95(3):241–254. doi: 10.1007/BF01869486. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Greenwell J. R., Argent B. E. Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J Membr Biol. 1988 Oct;105(2):131–142. doi: 10.1007/BF02009166. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Greenwell J. R., Argent B. E. Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J Membr Biol. 1988 Oct;105(2):131–142. doi: 10.1007/BF02009166. [DOI] [PubMed] [Google Scholar]
- Hayakawa T., Bruck R., Ng O. C., Boyer J. L. DBcAMP stimulates vesicle transport and HRP excretion in isolated perfused rat liver. Am J Physiol. 1990 Nov;259(5 Pt 1):G727–G735. doi: 10.1152/ajpgi.1990.259.5.G727. [DOI] [PubMed] [Google Scholar]
- Heyworth C. M., Wilson S. P., Gawler D. J., Houslay M. D. The phorbol ester TPA prevents the expression of both glucagon desensitisation and the glucagon-mediated block of insulin stimulation of the peripheral plasma membrane cyclic AMP phosphodiesterase in rat hepatocytes. FEBS Lett. 1985 Aug 5;187(2):196–200. doi: 10.1016/0014-5793(85)81241-2. [DOI] [PubMed] [Google Scholar]
- Jarvest R. L., Lowe G., Baraniak J., Stec W. J. A stereochemical investigation of the hydrolysis of cyclic AMP and the (Sp)-and (Rp)-diastereoisomers of adenosine cyclic 3':5'-phosphorothioate by bovine heart and baker's-yeast cyclic AMP phosphodiesterases. Biochem J. 1982 May 1;203(2):461–470. doi: 10.1042/bj2030461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedis A., Dumont M., Duval M., Erlinger S. Influence of glucagon on canalicular bile production in the dog. Biomedicine. 1974 Apr 10;21(4):176–181. [PubMed] [Google Scholar]
- Krapf R., Alpern R. J. Cell pH and transepithelial H/HCO3 transport in the renal proximal tubule. J Membr Biol. 1993 Jan;131(1):1–10. doi: 10.1007/BF02258529. [DOI] [PubMed] [Google Scholar]
- Lenzen R., Hruby V. J., Tavoloni N. mechanism of glucagon choleresis in guinea pigs. Am J Physiol. 1990 Nov;259(5 Pt 1):G736–G744. doi: 10.1152/ajpgi.1990.259.5.G736. [DOI] [PubMed] [Google Scholar]
- Martínez-Ansó E., Castillo J. E., Díez J., Medina J. F., Prieto J. Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology. 1994 Jun;19(6):1400–1406. [PubMed] [Google Scholar]
- Meier P. J., Knickelbein R., Moseley R. H., Dobbins J. W., Boyer J. L. Evidence for carrier-mediated chloride/bicarbonate exchange in canalicular rat liver plasma membrane vesicles. J Clin Invest. 1985 Apr;75(4):1256–1263. doi: 10.1172/JCI111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson M. H., Boyer J. L. Mechanisms and regulation of bile secretion. Hepatology. 1991 Sep;14(3):551–566. [PubMed] [Google Scholar]
- Novak I., Greger R. Properties of the luminal membrane of isolated perfused rat pancreatic ducts. Effect of cyclic AMP and blockers of chloride transport. Pflugers Arch. 1988 May;411(5):546–553. doi: 10.1007/BF00582376. [DOI] [PubMed] [Google Scholar]
- Renner E. L., Lake J. R., Scharschmidt B. F., Zimmerli B., Meier P. J. Rat hepatocytes exhibit basolateral Na+/HCO3- cotransport. J Clin Invest. 1989 Apr;83(4):1225–1235. doi: 10.1172/JCI114005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss L. Ion transport across gallbladder epithelium. Physiol Rev. 1989 Apr;69(2):503–545. doi: 10.1152/physrev.1989.69.2.503. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Rothermel J. D., Jastorff B., Botelho L. H. Inhibition of glucagon-induced glycogenolysis in isolated rat hepatocytes by the Rp diastereomer of adenosine cyclic 3',5'-phosphorothioate. J Biol Chem. 1984 Jul 10;259(13):8151–8155. [PubMed] [Google Scholar]
- Rothermel J. D., Parker Botelho L. H. A mechanistic and kinetic analysis of the interactions of the diastereoisomers of adenosine 3',5'-(cyclic)phosphorothioate with purified cyclic AMP-dependent protein kinase. Biochem J. 1988 May 1;251(3):757–762. doi: 10.1042/bj2510757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster V. L. Cyclic adenosine monophosphate-stimulated bicarbonate secretion in rabbit cortical collecting tubules. J Clin Invest. 1985 Jun;75(6):2056–2064. doi: 10.1172/JCI111925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schworer C. M., Mortimore G. E. Glucagon-induced autophagy and proteolysis in rat liver: mediation by selective deprivation of intracellular amino acids. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3169–3173. doi: 10.1073/pnas.76.7.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Sellinger M., Weinman S. A., Henderson R. M., Zweifach A., Boyer J. L., Graf J. Anion channels in rat liver canalicular plasma membranes reconstituted into planar lipid bilayers. Am J Physiol. 1992 Jun;262(6 Pt 1):G1027–G1032. doi: 10.1152/ajpgi.1992.262.6.G1027. [DOI] [PubMed] [Google Scholar]
- Soleimani M., Lesoine G. A., Bergman J. A., McKinney T. D. A pH modifier site regulates activity of the Na+:HCO3- cotransporter in basolateral membranes of kidney proximal tubules. J Clin Invest. 1991 Oct;88(4):1135–1140. doi: 10.1172/JCI115413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson D. L., Beauwens R., Palmisano J., Mitchell P. P., Steinmetz P. R. A double-membrane model for urinary bicarbonate secretion. Am J Physiol. 1985 Oct;249(4 Pt 2):F546–F552. doi: 10.1152/ajprenal.1985.249.4.F546. [DOI] [PubMed] [Google Scholar]
- Strazzabosco M., Sakisaka S., Hayakawa T., Boyer J. L. Effect of UDCA on intracellular and biliary pH in isolated rat hepatocyte couplets and perfused livers. Am J Physiol. 1991 Jan;260(1 Pt 1):G58–G69. doi: 10.1152/ajpgi.1991.260.1.G58. [DOI] [PubMed] [Google Scholar]
- Stuenkel E. L., Machen T. E., Williams J. A. pH regulatory mechanisms in rat pancreatic ductal cells. Am J Physiol. 1988 Jun;254(6 Pt 1):G925–G930. doi: 10.1152/ajpgi.1988.254.6.G925. [DOI] [PubMed] [Google Scholar]
- Sundaram U., Knickelbein R. G., Dobbins J. W. pH regulation in ileum: Na(+)-H+ and Cl(-)-HCO3- exchange in isolated crypt and villus cells. Am J Physiol. 1991 Mar;260(3 Pt 1):G440–G449. doi: 10.1152/ajpgi.1991.260.3.G440. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Thomsen O. O., Larsen J. A. The effect of glucagon, dibutyrylic cyclic AMP and insulin on bile production in the intact rat and the perfused rat liver. Acta Physiol Scand. 1981 Jan;111(1):23–30. doi: 10.1111/j.1748-1716.1981.tb06700.x. [DOI] [PubMed] [Google Scholar]
- Wangemann P., Wittner M., Di Stefano A., Englert H. C., Lang H. J., Schlatter E., Greger R. Cl(-)-channel blockers in the thick ascending limb of the loop of Henle. Structure activity relationship. Pflugers Arch. 1986;407 (Suppl 2):S128–S141. doi: 10.1007/BF00584942. [DOI] [PubMed] [Google Scholar]
- Weintraub W. H., Machen T. E. pH regulation in hepatoma cells: roles for Na-H exchange, Cl-HCO3 exchange, and Na-HCO3 cotransport. Am J Physiol. 1989 Sep;257(3 Pt 1):G317–G327. doi: 10.1152/ajpgi.1989.257.3.G317. [DOI] [PubMed] [Google Scholar]
- Wenzl E., Machen T. E. Intracellular pH dependence of buffer capacity and anion exchange in the parietal cell. Am J Physiol. 1989 Nov;257(5 Pt 1):G741–G747. doi: 10.1152/ajpgi.1989.257.5.G741. [DOI] [PubMed] [Google Scholar]