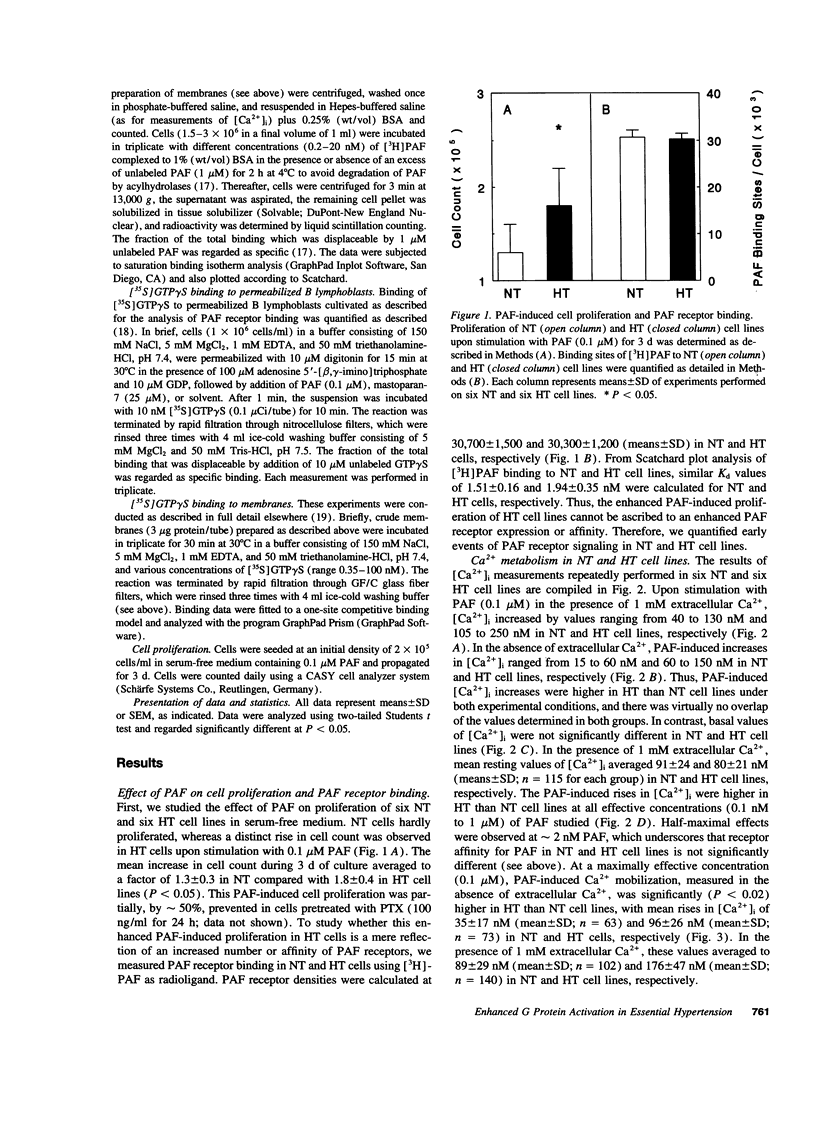
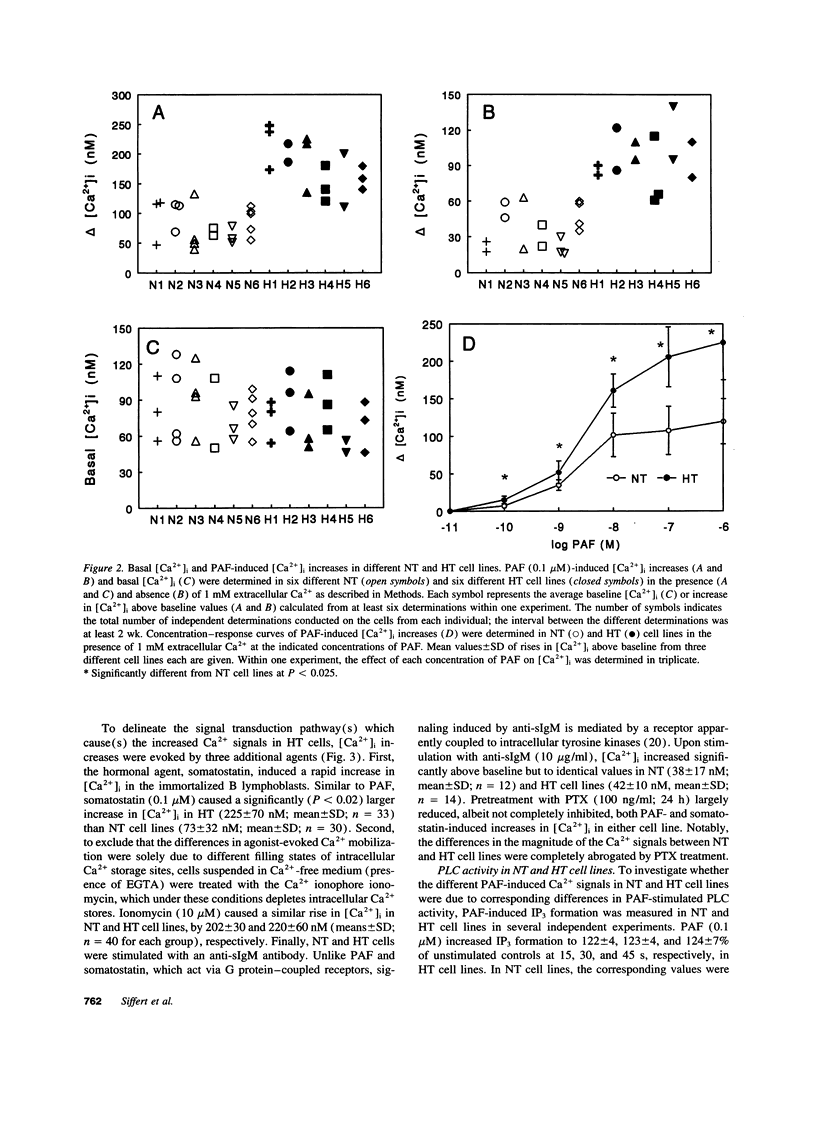
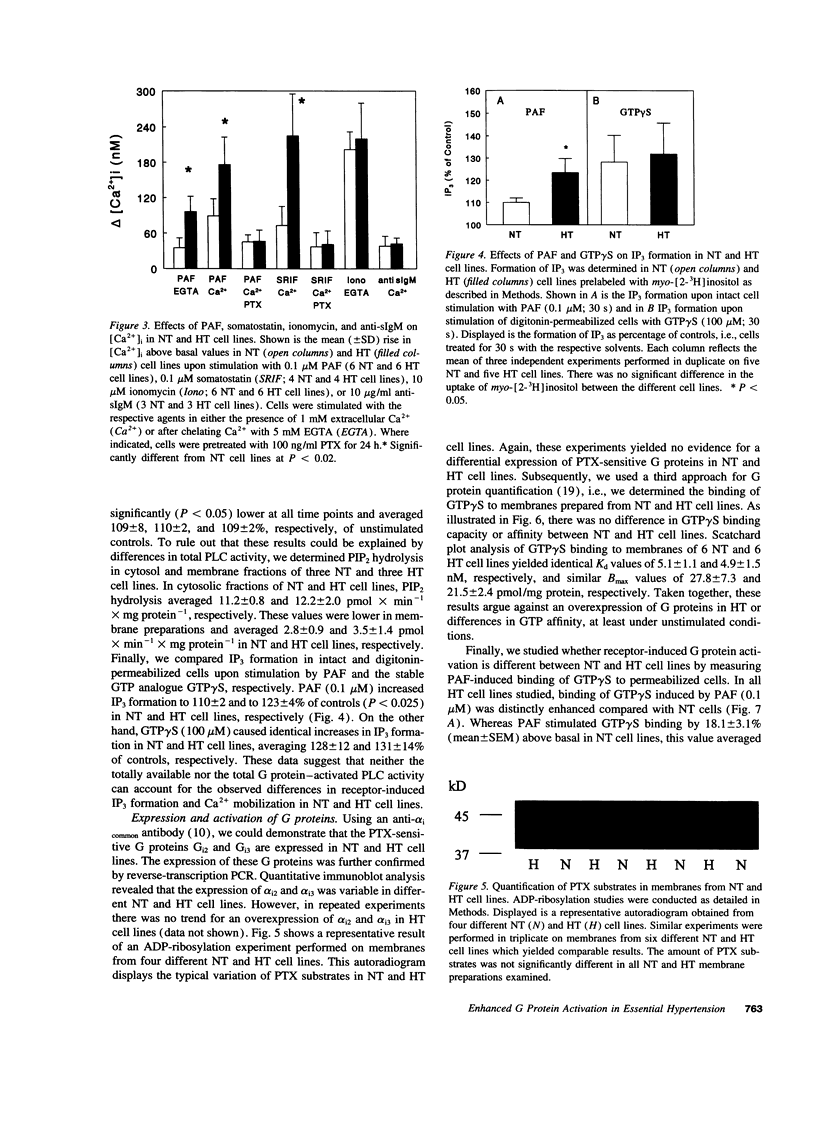
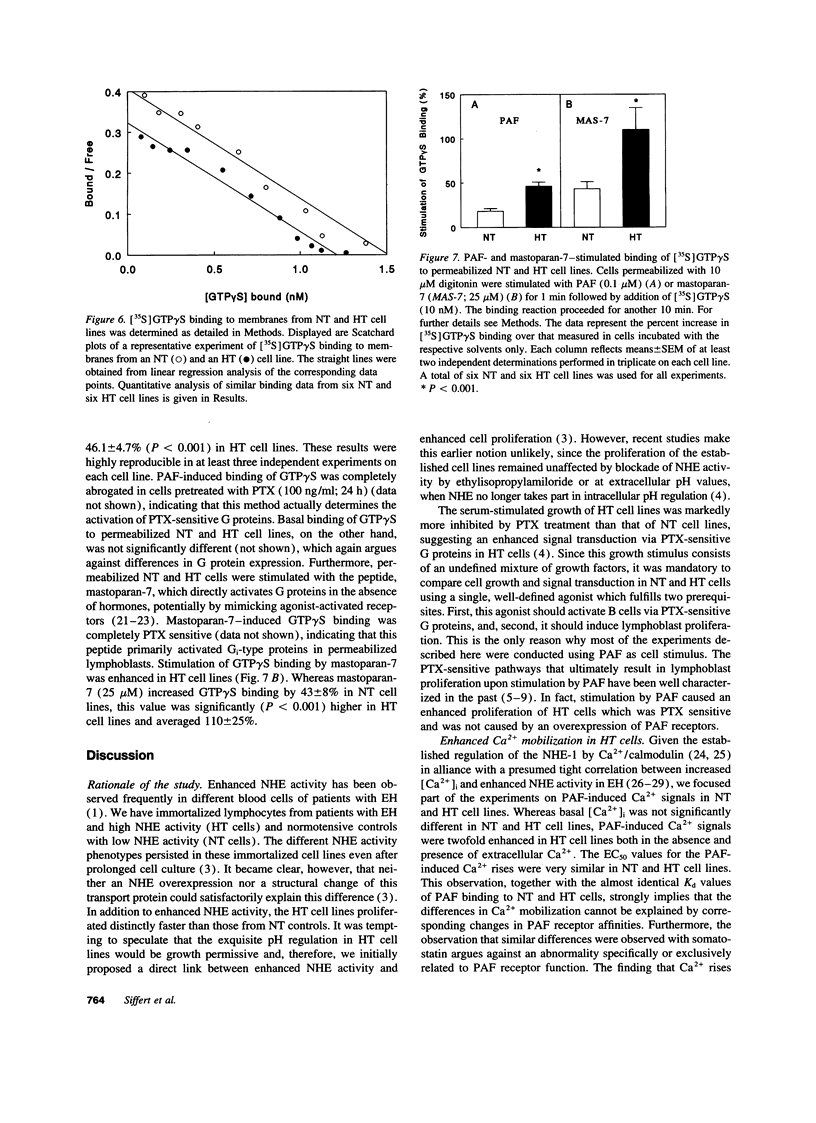
Abstract
Epstein-Barr virus-immortalized B lymphoblasts obtained from hypertensive patients with enhanced Na+/H+ exchanger activity (HT cells) proliferate distinctly faster upon serum stimulation than those from normotensive controls with low exchanger activity (NT cells) (Rosskopf, D., E. Frömter, and W. Siffert. 1993. J. Clin. Invest. 92:2553-2559). Stimulation with platelet-activating factor (PAF) as well caused an enhanced proliferation of HT cells. In analyzing possible differences in signal transduction between the immortalized NT and HT lymphoblasts, we observed that cell stimulation with PAF and somatostatin caused a twofold higher increase in [Ca2+]i in HT than in NT cell lines. This difference was completely abrogated by pertussis toxin (PTX) treatment. Furthermore, PAF-stimulated formation of inositol 1,4,5-trisphosphate (IP3) was twofold enhanced in HT cell lines. On the other hand, PAF receptor density and affinity, total cellular phospholipase C activity, expression of PTX-sensitive G proteins, and control binding of the stable GTP analogue, guanosine 5'-[gamma-thio]triphosphate (GTP gamma S), to membrane G proteins were not different in NT and HT cell lines. However, PAF- and mastoparan-stimulated binding of GTP gamma S to G proteins, which was fully PTX-sensitive, was 2.5-fold higher in HT than NT cell lines. These data suggest an enhanced receptor-mediated activation of PTX-sensitive G proteins despite unchanged receptor and G protein expression. Thus, this study not only suggests that enhanced signal transduction and cell proliferation are abnormalities in a certain group of patients with essential hypertension but also explains these findings as a result of an enhanced G protein activation in this common disorder.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv A. Cytosolic Ca2+, Na+/H+ antiport, protein kinase C trio in essential hypertension. Am J Hypertens. 1994 Feb;7(2):205–212. doi: 10.1093/ajh/7.2.205. [DOI] [PubMed] [Google Scholar]
- Aviv A., Livne A. The Na+/H+ antiport, cytosolic free Ca2+, and essential hypertension: a hypothesis. Am J Hypertens. 1988 Oct;1(4 Pt 1):410–413. doi: 10.1093/ajh/1.4.410. [DOI] [PubMed] [Google Scholar]
- Aviv A. The link between cytosolic Ca2+ and the Na+-H+ antiport: a unifying factor for essential hypertension. J Hypertens. 1988 Sep;6(9):685–691. doi: 10.1097/00004872-198809000-00001. [DOI] [PubMed] [Google Scholar]
- Aviv A. The roles of cell Ca2+, protein kinase C and the Na(+)-H+ antiport in the development of hypertension and insulin resistance. J Am Soc Nephrol. 1992 Nov;3(5):1049–1063. doi: 10.1681/ASN.V351049. [DOI] [PubMed] [Google Scholar]
- Bertrand B., Wakabayashi S., Ikeda T., Pouysségur J., Shigekawa M. The Na+/H+ exchanger isoform 1 (NHE1) is a novel member of the calmodulin-binding proteins. Identification and characterization of calmodulin-binding sites. J Biol Chem. 1994 May 6;269(18):13703–13709. [PubMed] [Google Scholar]
- Birnbaumer L. G proteins in signal transduction. Annu Rev Pharmacol Toxicol. 1990;30:675–705. doi: 10.1146/annurev.pa.30.040190.003331. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for beta gamma dimers as well as alpha subunits. Cell. 1992 Dec 24;71(7):1069–1072. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Canessa M., Morgan K., Goldszer R., Moore T. J., Spalvins A. Kinetic abnormalities of the red blood cell sodium-proton exchange in hypertensive patients. Hypertension. 1991 Mar;17(3):340–348. doi: 10.1161/01.hyp.17.3.340. [DOI] [PubMed] [Google Scholar]
- Carty D. J. Pertussis toxin-catalyzed ADP-ribosylation of G proteins. Methods Enzymol. 1994;237:63–70. doi: 10.1016/s0076-6879(94)37053-2. [DOI] [PubMed] [Google Scholar]
- Cushley W., Harnett M. M. Cellular signalling mechanisms in B lymphocytes. Biochem J. 1993 Jun 1;292(Pt 2):313–332. doi: 10.1042/bj2920313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. A., Mazer B., Sawami H., Mills G. B., Terada N., Lucas J. J., Gelfand E. W. Platelet-activating factor triggers the phosphorylation and activation of MAP-2 kinase and S6 peptide kinase activity in human B cell lines. J Immunol. 1993 Aug 15;151(4):1802–1810. [PubMed] [Google Scholar]
- Higashijima T., Burnier J., Ross E. M. Regulation of Gi and Go by mastoparan, related amphiphilic peptides, and hydrophobic amines. Mechanism and structural determinants of activity. J Biol Chem. 1990 Aug 25;265(24):14176–14186. [PubMed] [Google Scholar]
- Higashijima T., Uzu S., Nakajima T., Ross E. M. Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins). J Biol Chem. 1988 May 15;263(14):6491–6494. [PubMed] [Google Scholar]
- Kovár J., Franek F. Serum-free medium for hybridoma and parental myeloma cell cultivation. Methods Enzymol. 1986;121:277–292. doi: 10.1016/0076-6879(86)21025-3. [DOI] [PubMed] [Google Scholar]
- Kuruvilla A., Putcha G., Poulos E., Shearer W. T. Tyrosine phosphorylation of phospholipase C concomitant with its activation by platelet-activating factor in a human B cell line. J Immunol. 1993 Jul 15;151(2):637–648. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laugwitz K. L., Offermanns S., Spicher K., Schultz G. mu and delta opioid receptors differentially couple to G protein subtypes in membranes of human neuroblastoma SH-SY5Y cells. Neuron. 1993 Feb;10(2):233–242. doi: 10.1016/0896-6273(93)90314-h. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Kaziro Y., Asano S., Ogata E. Analysis of the expression of seven G protein alpha subunit genes in hematopoietic cells. Am J Med Sci. 1993 Aug;306(2):89–93. doi: 10.1097/00000441-199308000-00004. [DOI] [PubMed] [Google Scholar]
- Mazer B. D., Sawami H., Tordai A., Gelfand E. W. Platelet-activating factor-mediated transmembrane signaling in human B lymphocytes is regulated through a pertussis- and cholera toxin-sensitive pathway. J Clin Invest. 1992 Sep;90(3):759–765. doi: 10.1172/JCI115948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazer B., Domenico J., Sawami H., Gelfand E. W. Platelet-activating factor induces an increase in intracellular calcium and expression of regulatory genes in human B lymphoblastoid cells. J Immunol. 1991 Mar 15;146(6):1914–1920. [PubMed] [Google Scholar]
- McLellan A. R., Milligan G., Houslay M. D., Connell J. M. G-proteins in essential hypertension: a study of human platelet plasma membranes. J Hypertens. 1993 May;11(5):543–549. doi: 10.1097/00004872-199305000-00010. [DOI] [PubMed] [Google Scholar]
- Milligan G. Specificity and functional applications of antipeptide antisera which identify G-protein alpha subunits. Methods Enzymol. 1994;237:268–283. doi: 10.1016/s0076-6879(94)37068-0. [DOI] [PubMed] [Google Scholar]
- Rosskopf D., Düsing R., Siffert W. Membrane sodium-proton exchange and primary hypertension. Hypertension. 1993 May;21(5):607–617. doi: 10.1161/01.hyp.21.5.607. [DOI] [PubMed] [Google Scholar]
- Rosskopf D., Frömter E., Siffert W. Hypertensive sodium-proton exchanger phenotype persists in immortalized lymphoblasts from essential hypertensive patients. A cell culture model for human hypertension. J Clin Invest. 1993 Nov;92(5):2553–2559. doi: 10.1172/JCI116865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosskopf D., Schröder K. J., Siffert W. Role of sodium-hydrogen exchange in the proliferation of immortalised lymphoblasts from patients with essential hypertension and normotensive subjects. Cardiovasc Res. 1995 Feb;29(2):254–259. [PubMed] [Google Scholar]
- Rosskopf D., Siffert G., Osswald U., Witte K., Düsing R., Akkerman J. W., Siffert W. Platelet Na(+)-H+ exchanger activity in normotensive and hypertensive subjects: effect of enalapril therapy upon antiport activity. J Hypertens. 1992 Aug;10(8):839–847. [PubMed] [Google Scholar]
- Schulam P. G., Kuruvilla A., Putcha G., Mangus L., Franklin-Johnson J., Shearer W. T. Platelet-activating factor induces phospholipid turnover, calcium flux, arachidonic acid liberation, eicosanoid generation, and oncogene expression in a human B cell line. J Immunol. 1991 Mar 1;146(5):1642–1648. [PubMed] [Google Scholar]
- Valone F. H. Binding of platelet-activating factor 1-O-alkyl-2-acetyl-sn-glycero-3-phosphorylcholine to intact platelets and platelet membranes. Methods Enzymol. 1992;215:224–228. doi: 10.1016/0076-6879(92)15066-l. [DOI] [PubMed] [Google Scholar]
- Wakabayashi S., Bertrand B., Ikeda T., Pouysségur J., Shigekawa M. Mutation of calmodulin-binding site renders the Na+/H+ exchanger (NHE1) highly H(+)-sensitive and Ca2+ regulation-defective. J Biol Chem. 1994 May 6;269(18):13710–13715. [PubMed] [Google Scholar]
- Weiland T., Jakobs K. H. Measurement of receptor-stimulated guanosine 5'-O-(gamma-thio)triphosphate binding by G proteins. Methods Enzymol. 1994;237:3–13. doi: 10.1016/s0076-6879(94)37048-6. [DOI] [PubMed] [Google Scholar]
- Wieland T., Liedel K., Kaldenberg-Stasch S., Meyer zu Heringdorf D., Schmidt M., Jakobs K. H. Analysis of receptor-G protein interactions in permeabilized cells. Naunyn Schmiedebergs Arch Pharmacol. 1995 Apr;351(4):329–336. doi: 10.1007/BF00169072. [DOI] [PubMed] [Google Scholar]



