Abstract
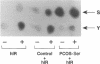
We investigated the cellular mechanisms of the unique disorder of insulin action found in the polycystic ovary syndrome (PCOS). Approximately 50% of PCOS women (PCOS-Ser) had a significant increase in insulin-independent beta-subunit [32P]phosphate incorporation (3.7-fold, P < 0.05 vs other groups) in skin fibroblast insulin receptors that was present in serine residues while insulin-induced tyrosine phosphorylation was decreased (both P < 0.05 vs other groups). PCOS skeletal muscle insulin receptors had the same abnormal phosphorylation pattern. The remaining PCOS women (PCOS-n1) had basal and insulin-stimulated receptor autophosphorylation similar to control. Phosphorylation of the artificial substrate poly GLU4:TYR1 by the PCOS-Ser insulin receptors was significantly decreased (P < 0.05) compared to control and PCOS-n1 receptors. The factor responsible for excessive serine phosphorylation appeared to be extrinsic to the receptor since no insulin receptor gene mutations were identified, immunoprecipitation before autophosphorylation corrected the phosphorylation defect and control insulin receptors mixed with lectin eluates from affected PCOS fibroblasts displayed increased serine phosphorylation. Our findings suggest that increased insulin receptor serine phosphorylation decreases its protein tyrosine kinase activity and is one mechanism for the post-binding defect in insulin action characteristic of PCOS.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accili D., Mosthaf L., Ullrich A., Taylor S. I. A mutation in the extracellular domain of the insulin receptor impairs the ability of insulin to stimulate receptor autophosphorylation. J Biol Chem. 1991 Jan 5;266(1):434–439. [PubMed] [Google Scholar]
- Ahn J., Donner D. B., Rosen O. M. Interaction of the human insulin receptor tyrosine kinase from the baculovirus expression system with protein kinase C in a cell-free system. J Biol Chem. 1993 Apr 5;268(10):7571–7576. [PubMed] [Google Scholar]
- Bak J. F., Handberg A., Beck-Nielsen H., Pedersen O. Kinetics of insulin binding and kinase activity of the partially purified insulin receptor from human skeletal muscle. Biochim Biophys Acta. 1990 May 2;1052(2):306–312. doi: 10.1016/0167-4889(90)90226-4. [DOI] [PubMed] [Google Scholar]
- Bak J. F., Møller N., Schmitz O., Saaek A., Pedersen O. In vivo insulin action and muscle glycogen synthase activity in type 2 (non-insulin-dependent) diabetes mellitus: effects of diet treatment. Diabetologia. 1992 Aug;35(8):777–784. doi: 10.1007/BF00429100. [DOI] [PubMed] [Google Scholar]
- Baltensperger K., Lewis R. E., Woon C. W., Vissavajjhala P., Ross A. H., Czech M. P. Catalysis of serine and tyrosine autophosphorylation by the human insulin receptor. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7885–7889. doi: 10.1073/pnas.89.17.7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar R. S., Gorden P., Roth J., Kahn C. R., De Meyts P. Fluctuations in the affinity and concentration of insulin receptors on circulating monocytes of obese patients: effects of starvation, refeeding, and dieting. J Clin Invest. 1976 Nov;58(5):1123–1135. doi: 10.1172/JCI108565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbetti F., Gejman P. V., Taylor S. I., Raben N., Cama A., Bonora E., Pizzo P., Moghetti P., Muggeo M., Roth J. Detection of mutations in insulin receptor gene by denaturing gradient gel electrophoresis. Diabetes. 1992 Apr;41(4):408–415. doi: 10.2337/diab.41.4.408. [DOI] [PubMed] [Google Scholar]
- Berti L., Mosthaf L., Kroder G., Kellerer M., Tippmer S., Mushack J., Seffer E., Seedorf K., Häring H. Glucose-induced translocation of protein kinase C isoforms in rat-1 fibroblasts is paralleled by inhibition of the insulin receptor tyrosine kinase. J Biol Chem. 1994 Feb 4;269(5):3381–3386. [PubMed] [Google Scholar]
- Bollag G. E., Roth R. A., Beaudoin J., Mochly-Rosen D., Koshland D. E., Jr Protein kinase C directly phosphorylates the insulin receptor in vitro and reduces its protein-tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5822–5824. doi: 10.1073/pnas.83.16.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brillon D. J., Freidenberg G. R., Henry R. R., Olefsky J. M. Mechanism of defective insulin-receptor kinase activity in NIDDM. Evidence for two receptor populations. Diabetes. 1989 Mar;38(3):397–403. doi: 10.2337/diab.38.3.397. [DOI] [PubMed] [Google Scholar]
- Buffington C. K., Givens J. R., Kitabchi A. E. Opposing actions of dehydroepiandrosterone and testosterone on insulin sensitivity. In vivo and in vitro studies of hyperandrogenic females. Diabetes. 1991 Jun;40(6):693–700. doi: 10.2337/diab.40.6.693. [DOI] [PubMed] [Google Scholar]
- Caro J. F., Dohm L. G., Pories W. J., Sinha M. K. Cellular alterations in liver, skeletal muscle, and adipose tissue responsible for insulin resistance in obesity and type II diabetes. Diabetes Metab Rev. 1989 Dec;5(8):665–689. doi: 10.1002/dmr.5610050804. [DOI] [PubMed] [Google Scholar]
- Caro J. F., Sinha M. K., Raju S. M., Ittoop O., Pories W. J., Flickinger E. G., Meelheim D., Dohm G. L. Insulin receptor kinase in human skeletal muscle from obese subjects with and without noninsulin dependent diabetes. J Clin Invest. 1987 May;79(5):1330–1337. doi: 10.1172/JCI112958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. E., Dickens M., Tavare J. M., Roth R. A. Overexpression of protein kinase C isoenzymes alpha, beta I, gamma, and epsilon in cells overexpressing the insulin receptor. Effects on receptor phosphorylation and signaling. J Biol Chem. 1993 Mar 25;268(9):6338–6347. [PubMed] [Google Scholar]
- Ciaraldi T. P., el-Roeiy A., Madar Z., Reichart D., Olefsky J. M., Yen S. S. Cellular mechanisms of insulin resistance in polycystic ovarian syndrome. J Clin Endocrinol Metab. 1992 Aug;75(2):577–583. doi: 10.1210/jcem.75.2.1322430. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Dunaif A., Graf M., Mandeli J., Laumas V., Dobrjansky A. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab. 1987 Sep;65(3):499–507. doi: 10.1210/jcem-65-3-499. [DOI] [PubMed] [Google Scholar]
- Dunaif A., Green G., Futterweit W., Dobrjansky A. Suppression of hyperandrogenism does not improve peripheral or hepatic insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1990 Mar;70(3):699–704. doi: 10.1210/jcem-70-3-699. [DOI] [PubMed] [Google Scholar]
- Dunaif A., Green G., Phelps R. G., Lebwohl M., Futterweit W., Lewy L. Acanthosis Nigricans, insulin action, and hyperandrogenism: clinical, histological, and biochemical findings. J Clin Endocrinol Metab. 1991 Sep;73(3):590–595. doi: 10.1210/jcem-73-3-590. [DOI] [PubMed] [Google Scholar]
- Dunaif A., Segal K. R., Futterweit W., Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989 Sep;38(9):1165–1174. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- Dunaif A., Segal K. R., Shelley D. R., Green G., Dobrjansky A., Licholai T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes. 1992 Oct;41(10):1257–1266. doi: 10.2337/diab.41.10.1257. [DOI] [PubMed] [Google Scholar]
- Elkind-Hirsch K. E., Valdes C. T., Malinak L. R. Insulin resistance improves in hyperandrogenic women treated with Lupron. Fertil Steril. 1993 Oct;60(4):634–641. doi: 10.1016/s0015-0282(16)56213-x. [DOI] [PubMed] [Google Scholar]
- Foley J. E. Mechanisms of impaired insulin action in isolated adipocytes from obese and diabetic subjects. Diabetes Metab Rev. 1988 Aug;4(5):487–505. doi: 10.1002/dmr.5610040506. [DOI] [PubMed] [Google Scholar]
- Freidenberg G. R., Henry R. R., Klein H. H., Reichart D. R., Olefsky J. M. Decreased kinase activity of insulin receptors from adipocytes of non-insulin-dependent diabetic subjects. J Clin Invest. 1987 Jan;79(1):240–250. doi: 10.1172/JCI112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidenberg G. R., Reichart D., Olefsky J. M., Henry R. R. Reversibility of defective adipocyte insulin receptor kinase activity in non-insulin-dependent diabetes mellitus. Effect of weight loss. J Clin Invest. 1988 Oct;82(4):1398–1406. doi: 10.1172/JCI113744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Jen P., Freychet P. Insulin receptors in human circulating cells and fibroblasts. Proc Natl Acad Sci U S A. 1972 Mar;69(3):747–751. doi: 10.1073/pnas.69.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorescu F., Flier J. S., Kahn C. R. Defect in insulin receptor phosphorylation in erythrocytes and fibroblasts associated with severe insulin resistance. J Biol Chem. 1984 Dec 25;259(24):15003–15006. [PubMed] [Google Scholar]
- Guo H., Damuni Z. Autophosphorylation-activated protein kinase phosphorylates and inactivates protein phosphatase 2A. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2500–2504. doi: 10.1073/pnas.90.6.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. I., Hadden W. C., Knowler W. C., Bennett P. H. Prevalence of diabetes and impaired glucose tolerance and plasma glucose levels in U.S. population aged 20-74 yr. Diabetes. 1987 Apr;36(4):523–534. doi: 10.2337/diab.36.4.523. [DOI] [PubMed] [Google Scholar]
- Holmäng A., Svedberg J., Jennische E., Björntorp P. Effects of testosterone on muscle insulin sensitivity and morphology in female rats. Am J Physiol. 1990 Oct;259(4 Pt 1):E555–E560. doi: 10.1152/ajpendo.1990.259.4.E555. [DOI] [PubMed] [Google Scholar]
- Kahn C. R. Banting Lecture. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes. 1994 Aug;43(8):1066–1084. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- Karasik A., Rothenberg P. L., Yamada K., White M. F., Kahn C. R. Increased protein kinase C activity is linked to reduced insulin receptor autophosphorylation in liver of starved rats. J Biol Chem. 1990 Jun 25;265(18):10226–10231. [PubMed] [Google Scholar]
- Kohanski R. A., Frost S. C., Lane M. D. Insulin-dependent phosphorylation of the insulin receptor-protein kinase and activation of glucose transport in 3T3-L1 adipocytes. J Biol Chem. 1986 Sep 15;261(26):12272–12281. [PubMed] [Google Scholar]
- Kohanski R. A., Lane M. D. Homogeneous functional insulin receptor from 3T3-L1 adipocytes. Purification using N alpha B1-(biotinyl-epsilon-aminocaproyl)insulin and avidin-sepharose. J Biol Chem. 1985 Apr 25;260(8):5014–5025. [PubMed] [Google Scholar]
- Lewis R. E., Cao L., Perregaux D., Czech M. P. Threonine 1336 of the human insulin receptor is a major target for phosphorylation by protein kinase C. Biochemistry. 1990 Feb 20;29(7):1807–1813. doi: 10.1021/bi00459a020. [DOI] [PubMed] [Google Scholar]
- Lima F. B., Thies R. S., Garvey W. T. Glucose and insulin regulate insulin sensitivity in primary cultured adipocytes without affecting insulin receptor kinase activity. Endocrinology. 1991 May;128(5):2415–2426. doi: 10.1210/endo-128-5-2415. [DOI] [PubMed] [Google Scholar]
- Longo N., Langley S. D., Griffin L. D., Elsas L. J. Activation of glucose transport by a natural mutation in the human insulin receptor. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):60–64. doi: 10.1073/pnas.90.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo N., Shuster R. C., Griffin L. D., Elsas L. J. Insulin-receptor autophosphorylation and kinase activity are constitutively increased in fibroblasts cultured from a patient with heritable insulin-resistance. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1229–1234. doi: 10.1016/0006-291x(90)90655-7. [DOI] [PubMed] [Google Scholar]
- Maddux B. A., Sbraccia P., Kumakura S., Sasson S., Youngren J., Fisher A., Spencer S., Grupe A., Henzel W., Stewart T. A. Membrane glycoprotein PC-1 and insulin resistance in non-insulin-dependent diabetes mellitus. Nature. 1995 Feb 2;373(6513):448–451. doi: 10.1038/373448a0. [DOI] [PubMed] [Google Scholar]
- Maddux B. A., Sbraccia P., Reaven G. M., Moller D. E., Goldfine I. D. Inhibitors of insulin receptor tyrosine kinase in fibroblasts from diverse patients with impaired insulin action: evidence for a novel mechanism of postreceptor insulin resistance. J Clin Endocrinol Metab. 1993 Jul;77(1):73–79. doi: 10.1210/jcem.77.1.7686917. [DOI] [PubMed] [Google Scholar]
- New M. I., Lorenzen F., Lerner A. J., Kohn B., Oberfield S. E., Pollack M. S., Dupont B., Stoner E., Levy D. J., Pang S. Genotyping steroid 21-hydroxylase deficiency: hormonal reference data. J Clin Endocrinol Metab. 1983 Aug;57(2):320–326. doi: 10.1210/jcem-57-2-320. [DOI] [PubMed] [Google Scholar]
- Oda Y., Kuo M. D., Huang S. S., Huang J. S. The plasma cell membrane glycoprotein, PC-1, is a threonine-specific protein kinase stimulated by acidic fibroblast growth factor. J Biol Chem. 1991 Sep 5;266(25):16791–16795. [PubMed] [Google Scholar]
- Petruzziello A., Formisano P., Miele C., Di Finizio B., Riccardi G., Ferrara A., Beguinot L., Beguinot F. Defective insulin action in fibroblasts from noninsulin-dependent diabetes mellitus patients with Gln1152 insulin receptor mutation. J Clin Endocrinol Metab. 1993 Aug;77(2):409–412. doi: 10.1210/jcem.77.2.8393885. [DOI] [PubMed] [Google Scholar]
- Pillay T. S., Whittaker J., Lammers R., Ullrich A., Siddle K. Multisite serine phosphorylation of the insulin and IGF-I receptors in transfected cells. FEBS Lett. 1991 Aug 19;288(1-2):206–211. doi: 10.1016/0014-5793(91)81035-7. [DOI] [PubMed] [Google Scholar]
- Polson D. W., Adams J., Wadsworth J., Franks S. Polycystic ovaries--a common finding in normal women. Lancet. 1988 Apr 16;1(8590):870–872. doi: 10.1016/s0140-6736(88)91612-1. [DOI] [PubMed] [Google Scholar]
- Prigent S. A., Stanley K. K., Siddle K. Identification of epitopes on the human insulin receptor reacting with rabbit polyclonal antisera and mouse monoclonal antibodies. J Biol Chem. 1990 Jun 15;265(17):9970–9977. [PubMed] [Google Scholar]
- Rapuano M., Rosen O. M. Phosphorylation of the insulin receptor by a casein kinase I-like enzyme. J Biol Chem. 1991 Jul 15;266(20):12902–12907. [PubMed] [Google Scholar]
- Rosenbaum D., Haber R. S., Dunaif A. Insulin resistance in polycystic ovary syndrome: decreased expression of GLUT-4 glucose transporters in adipocytes. Am J Physiol. 1993 Feb;264(2 Pt 1):E197–E202. doi: 10.1152/ajpendo.1993.264.2.E197. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. G., Dollar L. A. Characterization of the somatomedin-C/insulin-like growth factor I (SM-C/IGF-I) receptor on cultured human fibroblast monolayers: regulation of receptor concentrations by SM-C/IGF-I and insulin. J Clin Endocrinol Metab. 1982 Sep;55(3):434–440. doi: 10.1210/jcem-55-3-434. [DOI] [PubMed] [Google Scholar]
- Roth R. A., Beaudoin J. Phosphorylation of purified insulin receptor by cAMP kinase. Diabetes. 1987 Jan;36(1):123–126. doi: 10.2337/diab.36.1.123. [DOI] [PubMed] [Google Scholar]
- Sbraccia P., Goodman P. A., Maddux B. A., Wong K. Y., Chen Y. D., Reaven G. M., Goldfine I. D. Production of inhibitor of insulin-receptor tyrosine kinase in fibroblasts from patient with insulin resistance and NIDDM. Diabetes. 1991 Feb;40(2):295–299. doi: 10.2337/diab.40.2.295. [DOI] [PubMed] [Google Scholar]
- Soos M. A., Siddle K. Immunological relationships between receptors for insulin and insulin-like growth factor I. Evidence for structural heterogeneity of insulin-like growth factor I receptors involving hybrids with insulin receptors. Biochem J. 1989 Oct 15;263(2):553–563. doi: 10.1042/bj2630553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soos M. A., Whittaker J., Lammers R., Ullrich A., Siddle K. Receptors for insulin and insulin-like growth factor-I can form hybrid dimers. Characterisation of hybrid receptors in transfected cells. Biochem J. 1990 Sep 1;270(2):383–390. doi: 10.1042/bj2700383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbara L. R., Tang Z., Cama A., Xia J., Schenker E., Kohanski R. A., Poretsky L., Koller E., Taylor S. I., Dunaif A. Absence of insulin receptor gene mutations in three insulin-resistant women with the polycystic ovary syndrome. Metabolism. 1994 Dec;43(12):1568–1574. doi: 10.1016/0026-0495(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Takayama S., White M. F., Kahn C. R. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J Biol Chem. 1988 Mar 5;263(7):3440–3447. [PubMed] [Google Scholar]
- Tavaré J. M., O'Brien R. M., Siddle K., Denton R. M. Analysis of insulin-receptor phosphorylation sites in intact cells by two-dimensional phosphopeptide mapping. Biochem J. 1988 Aug 1;253(3):783–788. doi: 10.1042/bj2530783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. I., Cama A., Accili D., Barbetti F., Quon M. J., de la Luz Sierra M., Suzuki Y., Koller E., Levy-Toledano R., Wertheimer E. Mutations in the insulin receptor gene. Endocr Rev. 1992 Aug;13(3):566–595. doi: 10.1210/edrv-13-3-566. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A. M., Sutcliffe I. C., Johnson A. B., Taylor R. Abnormal activation of glycogen synthesis in fibroblasts from NIDDM subjects. Evidence for an abnormality specific to glucose metabolism. Diabetes. 1993 Apr;42(4):583–589. doi: 10.2337/diab.42.4.583. [DOI] [PubMed] [Google Scholar]
- White M. F., Takayama S., Kahn C. R. Differences in the sites of phosphorylation of the insulin receptor in vivo and in vitro. J Biol Chem. 1985 Aug 5;260(16):9470–9478. [PubMed] [Google Scholar]
- Whittaker J., Okamoto A. K., Thys R., Bell G. I., Steiner D. F., Hofmann C. A. High-level expression of human insulin receptor cDNA in mouse NIH 3T3 cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5237–5241. doi: 10.1073/pnas.84.15.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Goncalves E., Kahn C. R., Shoelson S. E. Substitution of the insulin receptor transmembrane domain with the c-neu/erbB2 transmembrane domain constitutively activates the insulin receptor kinase in vitro. J Biol Chem. 1992 Jun 25;267(18):12452–12461. [PubMed] [Google Scholar]
- Zick Y., Grunberger G., Podskalny J. M., Moncada V., Taylor S. I., Gorden P., Roth J. Insulin stimulates phosphorylation of serine residues in soluble insulin receptors. Biochem Biophys Res Commun. 1983 Nov 15;116(3):1129–1135. doi: 10.1016/s0006-291x(83)80260-5. [DOI] [PubMed] [Google Scholar]