Abstract
Regulator of G-protein signaling (RGS) proteins accelerate GTP hydrolysis by heterotrimeric G-protein α subunits and thus inhibit signaling by many G protein-coupled receptors. Several RGS proteins have a multidomain architecture that adds further complexity to their roles in cell signaling in addition to their GTPase-accelerating activity. RGS12 contains a tandem repeat of Ras-binding domains but, to date, the role of this protein in Ras-mediated signal transduction has not been reported. Here, we show that RGS12 associates with the nerve growth factor (NGF) receptor tyrosine kinase TrkA, activated H-Ras, B-Raf, and MEK2 and facilitates their coordinated signaling to prolonged ERK activation. RGS12 is required for NGF-mediated neurite outgrowth of PC12 cells, but not outgrowth stimulated by basic fibroblast growth factor. siRNA-mediated knockdown of RGS12 expression also inhibits NGF-induced axonal growth in dissociated cultures of primary dorsal root ganglia neurons. These data suggest that RGS12 may play a critical, and receptor-selective, role in coordinating Ras-dependent signals that are required for promoting and/or maintaining neuronal differentiation.
Keywords: MEK, Raf, Ras, RGS12, TrkA
Introduction
Extracellular stimuli such as neurotransmitters, hormones, odorants and light are recognized by G protein-coupled receptors (GPCRs), one of the most abundant and diverse protein families in the nervous system (Davies et al, 2002b). Intercellular communication via G-protein-mediated signaling pathways in the nervous system is crucial for normal brain development and regulation of adult neural processes (Davies et al, 2002b). Thus, defining the molecular determinants that control GPCR signaling is important to understanding normal development and physiology of the nervous system and the pathophysiology of nervous system-related disorders.
One level of regulation of GPCR signaling is mediated by ‘regulators of G-protein signaling' (RGS) proteins that accelerate Gα GTP hydrolysis and inhibit signaling by many GPCRs (Ross and Wilkie, 2000). For example, mice lacking RGS9 show enhanced responses to photonic stimulation and morphine administration, suggesting that RGS9 is a potent negative regulator of both rhodopsin and opioid receptor signal transduction in vivo (Chen et al, 2000; Zachariou et al, 2003). In general, however, contributions by RGS proteins to physiological control of specific receptor signaling cascades are only now being elucidated. Multidomain RGS proteins add further complexity to the potential signaling roles played by RGS proteins in addition to their GTPase-accelerating activity (reviewed in Ross and Wilkie, 2000; Siderovski and Willard, 2005).
RGS12 is an example of a multidomain RGS protein with numerous signaling regulatory elements. In addition to a central RGS domain, RGS12 contains a PDZ (PSD-95/Discs-large/ZO-1 homology) domain, a phosphotyrosine-binding (PTB) domain, tandem Ras-binding domains (RBDs), and a GoLoco (Gαi/o-Loco) interaction motif (Ponting, 1999; Siderovski et al, 1999). The PTB domain recruits RGS12 in a phosphotyrosine-dependent manner to the SNARE-binding region of the Cav2.2 calcium channel (Richman et al, 2005), thereby determining the rate of desensitization of GABAB receptor-mediated calcium current inhibition in dorsal root ganglia (DRG) neurons (Schiff et al, 2000). Little is known about the in vivo function of other domains within mammalian RGS12. Recently, we examined RGS12 expression patterns during mouse development. Full-length RGS12 is expressed predominantly in trigeminal and DRG neurons and muscle in the E14.5 embryonic mouse (Martin-McCaffrey et al, 2005), suggesting a role for mammalian RGS12 in myo- and neurogenesis.
Loco, the Drosophila RGS12 ortholog, is essential for multiple processes in fly development, including dorsal/ventral axis formation, neuroblast asymmetric cell division, and nurse cell dumping (Pathirana et al, 2001; Yu et al, 2005). loco is expressed in lateral glial cells in the developing embryonic central nervous system and required for correct glial cell differentiation (Granderath et al, 1999). Normal glial–glial cell contacts are absent in loco-deficient flies, resulting in a loss of the blood–brain barrier and subsequent gross locomotor defects in surviving mutants. Recently, Loco, the GPCR Moody, and the Gα subunits Gαi and Gαo were found to be expressed in surface glia; these four proteins are thought to act as part of a common signaling pathway critical for blood–brain barrier formation (Bainton et al, 2005; Schwabe et al, 2005).
The requirement for Loco in glial cell development and normal locomotion suggests that mammalian RGS12 may also play a critical role in glial cell differentiation. Here, we demonstrate that RGS12 facilitates formation of a Ras/Raf/MEK/ERK multiprotein complex, suggesting a function as a scaffold for the extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) cascade. Furthermore, RGS12 interacts with the nerve growth factor (NGF) receptor TrkA, and small interfering RNA (siRNA)-mediated downregulation of endogenous RGS12 selectively inhibits NGF-induced neuritogenesis of PC12 cells and axonal growth of embryonic DRG neurons. These results suggest that RGS12 may function in promoting a differentiated phenotype by organizing a TrkA, Ras, Raf, MEK, and ERK signal transduction complex in vivo.
Results
RGS12 interacts with MAPK cascade components
Beyond two Gαi interaction domains (an RGS domain and a C-terminal GoLoco motif), RGS12 has additional protein motifs found in signaling adaptors, scaffolds, and effectors (Figure 1A): N-terminal PDZ and PTB domains and a tandem repeat of RBDs (Snow et al, 1998; Schiff et al, 2000; Kimple et al, 2001). To identify additional RGS12 interactors, yeast two-hybrid screens of mouse embryo and brain cDNA libraries were performed using the PDZ/PTB domains and an internal span including the RGS domain and tandem RBDs (Figure 1A). Two interactors from the mouse brain library were obtained with the N-terminal bait and validated as bait specific (Supplementary Figure S1): the C-terminal tail of mouse SAPAP3 (Welch et al, 2004) and the C-terminal tail of MEK2, a middle-tier protein kinase (MAP2K) within the ERK cascade (Johnson and Lapadat, 2002).
Figure 1.
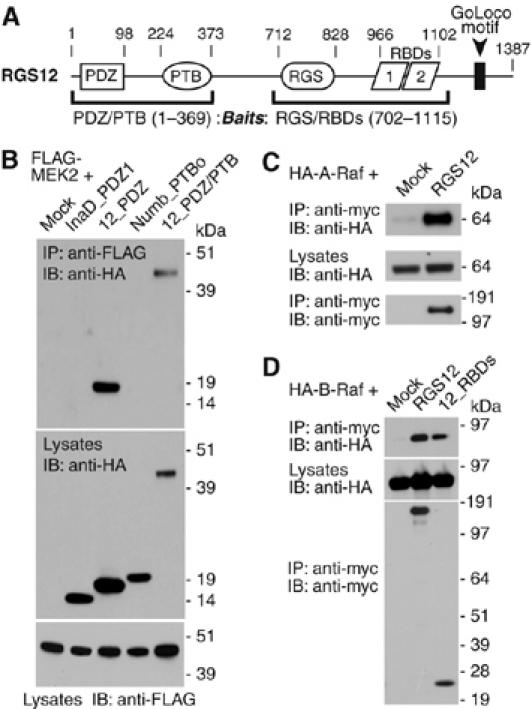
Components of the mitogen-activated protein kinase (MAPK) cascade identified as RGS12 interactors. (A) RGS12 architecture. Numbering denotes amino-acid boundaries of domains within rat RGS12. Parentheses denote baits used in yeast two-hybrid screens against mouse embryo and brain libraries. MEK2 was identified as a PDZ/PTB domain region interactor and A-Raf was identified as an interactor of the central RGS/RBD domain region (see Supplementary Figure S1). (B) HEK 293T cells were cotransfected with FLAG-MEK2 and empty vector (‘mock'), or indicated HA-tagged domain constructs. Anti-FLAG was used to immunoprecipitate (‘IP') MEK2 from resultant lysates. Co-immunoprecipitating proteins and total lysates resolved by SDS–PAGE were immunoblotted (‘IB') with anti-FLAG and anti-HA antibodies. (C) HA-A-Raf was cotransfected with empty vector or myc-RGS12 in COS-7 cells. (D) HA-B-Raf was cotransfected with empty vector, myc-RGS12, or the isolated tandem RBDs of RGS12 (‘12_RBDs') in COS-7 cells. In panels C and D, anti-myc was used to IP RGS12. Co-immunoprecipitating proteins and total lysates were then immunoblotted with anti-myc and anti-HA antibodies.
Both SAPAP3 and MEK2 end in canonical class I PDZ-binding targets (SAPAP3: -Q-T-R-L-COOH; MEK2: -R-T-A-V-COOH), suggesting that both interactors bind the PDZ domain. The RGS12 PDZ domain is known to recognize class I PDZ-binding targets ending in Thr-x-[Leu/Val]-COOH (Snow et al, 1998). Biosensor studies indicated that the final 16 amino-acids (aa) of MEK2 were sufficient to bind directly to the RGS12 PDZ domain in vitro (Supplementary Figure S2). The interaction with MEK2 was specific for the PDZ domain of RGS12, as co-immunoprecipitation was only seen between full-length MEK2 and protein fragments containing the RGS12 PDZ domain (Figure 1B); neither the first PDZ domain of the Drosophila scaffold InaD nor the PTB domain of mouse Numb were capable of co-immunoprecipitating MEK2. RGS12 selectively interacts with MEK2, and not other MAP2K members, in cell co-immunoprecipitations (data not shown).
A separate yeast two-hybrid screen using a fragment from the RGS domain to the second RBD (Supplementary Figure S1) identified a fragment of A-Raf (aa 36–153) that spans the RBD and cysteine-rich C1 (or ‘CRD') domains N-terminal to its kinase domain (Huleihel et al, 1986). Subsequent co-immunoprecipitation studies showed that full-length A-Raf and B-Raf (Figure 1C and D), but not c-Raf-1 (Supplementary Figure S3), associate with RGS12; indeed, the interaction with B-Raf is observed upon expressing a minimal RGS12 fragment containing solely the tandem RBDs (Figure 1D). Similar to A-Raf, residues within the B-Raf N-terminus appear to mediate binding to RGS12, as B-Raf fragments spanning the first 374 aa efficiently co-immunoprecipitated with full-length RGS12 (Supplementary Figure S4).
RGS12 preferentially binds to activated H-Ras
Association of RGS12 with Raf and MEK (two tiers of the Ras-regulated ERK cascade), coupled with the presence of two RBDs within RGS12, led us to test whether RGS12 also interacts directly with Ras GTPases. RBDs are found in Ras effector proteins such as RalGDS, phosphoinositide-3′ kinase, and the Raf kinases that bind preferentially to activated Ras GTPases (Wohlgemuth et al, 2005). Upon screening several Ras family members, we found that RGS12 preferentially co-immunoprecipitates with H-Ras and not K-, M-, or R-Ras. This interaction was specific to activated H-Ras (Figure 2A and B). In some instances, we observed binding of RGS12 to activated N-Ras (Figure 2A); however, when detected, this interaction was substantially weaker than that observed with H-Ras. Association between RGS12 and active H-Ras appears to be mediated by the tandem RBD region of RGS12 (Figure 2C). Interaction with activated H-Ras is diminished by point mutation of histidine-995, within the first RBD of RGS12, to leucine (Figure 2D; H995L), a position analogous to the loss-of-function R89L mutation that abrogates c-Raf-1 binding to H-Ras (Fabian et al, 1994) (Supplementary Figure S5). However, mutation of H995 in RGS12 does not interfere with its interaction with B-Raf (Figure 2E), suggesting that the RGS12(H995L) variant is properly folded. The RGS12 paralog RGS14 is reported to interact with Rap GTPase isoforms (Traver et al, 2004; Kiel et al, 2005; Mittal and Linder, 2006); however, no interaction of RGS12 with Rap1A, Rap1B, or Rap2B was observed in cell co-immunoprecipitations (Supplementary Figure S6).
Figure 2.
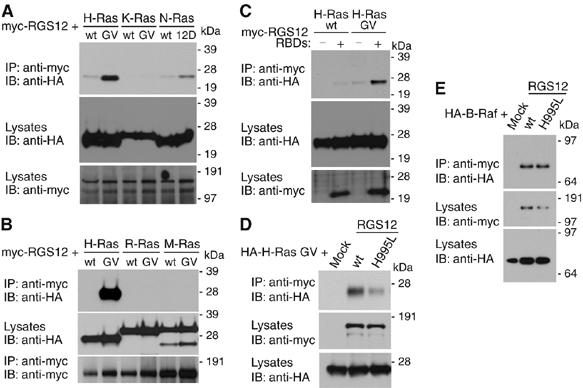
RGS12 and its isolated tandem RBDs preferentially interact with activated H-Ras. (A, B) HEK 293T cells were cotransfected with myc-RGS12 and indicated HA-Ras plasmids. Anti-myc was used to immunoprecipitate RGS12 and anti-HA used to detect associated Ras proteins. (C) Wild-type or activated (G12V) HA-H-Ras was coexpressed with empty vector or myc-tagged, isolated tandem RBD region of RGS12 in HEK 293T cells. Lysates were immunoprecipitated and immunoblotted as above. (D, E) Activated (G12V) HA-H-Ras (D) or HA-B-Raf (E) was coexpressed with empty vector, wild-type myc-RGS12 (wt), or mutant myc-RGS12 (H995L) in HEK 293T cells. Lysates were immunoprecipitated and immunoblotted as above.
RGS12 coordinates and enhances signaling to ERK activation
Interactions with activated H-Ras and the first two tiers of the Ras-regulated ERK signaling cascade suggests a role for RGS12 as a MAPK scaffold facilitating receptor signaling to ERK activation. We previously showed that RGS12 associates with the PDGFβ receptor in an agonist-independent manner (Sambi et al, 2006); thus, we measured endogenous PDGFβR signaling in CHO-K1 cells (Maudsley et al, 2000) in the presence of increasing RGS12 levels. RGS12 expression modulated PDGF-BB-stimulated activation of ERK2 phosphorylation in a concentration-dependent, biphasic manner (Figure 3A)—namely, an increase in phospho-ERK2 levels upon low RGS12 expression, transitioning to a decrease to phospho-ERK2 levels upon higher RGS12 expression. These results are reminiscent of ‘combinatorial inhibition', a hallmark of signaling scaffolds that arises because at low scaffold concentrations, signaling components are in excess and formation of a functional complex is likely to occur, but when scaffold is in excess, non-functional complexes with less than the full complement of components become more prevalent (Bray and Lay, 1997; Levchenko et al, 2000). This biphasic effect of RGS12 expression was selective for PDGFβR signaling in CHO-K1 cells, as no inhibition of epidermal growth factor (EGF)-mediated ERK2 activation was seen even at the highest RGS12 expression levels (data not shown).
Figure 3.
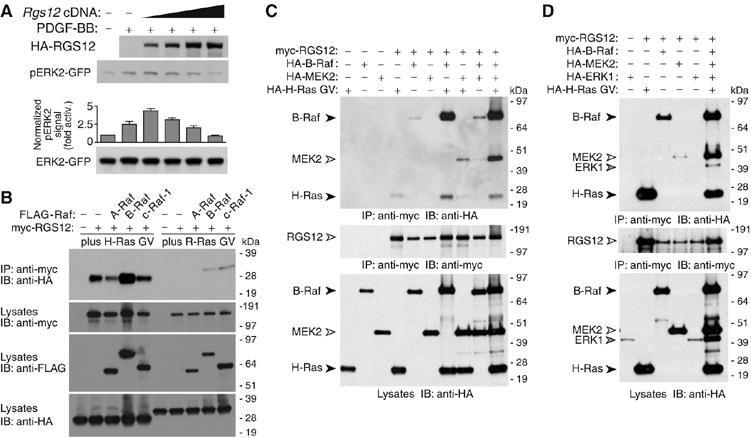
RGS12 coordinates a Ras/Raf/MEK/ERK complex and enhances signaling to ERK. (A) CHO-K1 cells with endogenous PDGFβR were transfected with ERK2–GFP and either empty vector or increasing amounts of HA-RGS12 vector, serum-starved overnight, treated with PDGF-BB (10 ng/ml) for 5 min, and resultant ERK2 activation determined by immunoblotting lysates with anti-phospho-ERK1/2. Densitometric quantitation of pERK2 and total ERK2-GFP levels (bottom subpanel) generated a normalized pERK2 signal (i.e. fold activation above absence of PDGF-BB). Error bars are standard error of the mean from three independent experiments. (B) HA-tagged, activated H-Ras (G12V) (left) or R-Ras (G38V) (right) was cotransfected with empty vector, myc-RGS12, or myc-RGS12 plus FLAG-A-Raf, B-Raf-FLAG, or c-Raf-1-FLAG vectors in HEK 293T cells. Anti-myc immunoprecipitates were immunoblotted for co-precipitated Ras proteins with anti-HA. Total lysates were immunoblotted with anti-myc, anti-FLAG, and anti-HA to confirm expression. The image shown is representative of three experiments; R-Ras interaction with RGS12 upon B-Raf or c-Raf-1 coexpression was weak and variable between experiments. (C) HEK 293T cells were transfected with myc-RGS12, HA-B-Raf, HA-MEK2, and/or HA-H-Ras G12V as indicated. Anti-myc immunoprecipitates were immunoblotted for co-precipitated HA-B-Raf, HA-MEK2, and HA-H-Ras GV using anti-HA antibody. Expression was confirmed by immunoblotting immunoprecipitated proteins with anti-myc, and total lysates with anti-HA. (D) HEK 293T cells were transfected as above but with addition of HA-ERK1 vector where indicated. Detection of expression and co-immunoprecipitation was as described above. To highlight the increase in complex formation observed when multiple Ras/MAPK components are cotransfected in panel C, we present a lighter exposure that reveals only a faint band representing HA-MEK2 co-immunoprecipitation with myc-RGS12. Panel D represents a darker exposure to highlight the co-immunoprecipitation of HA-ERK1, which in turn explains the RGS12–MEK2 interaction better than panel C.
To investigate the interaction of RGS12 with multiple Ras/MAPK signaling components, we coexpressed RGS12 and activated Ras GTPases with Raf kinase isoforms A-Raf, B-Raf, or c-Raf-1, and examined the ability of RGS12 to bind to Ras. RGS12 does not interact with activated R-Ras (Figure 2A); however, activated H-Ras and R-Ras both interact with all three Raf isoforms (reviewed in Wittinghofer and Herrmann, 1995). Activated R-Ras did not co-immunoprecipitate with RGS12 in the absence or presence of any of the three Raf kinases (Figure 3B). In contrast, the amount of H-Ras bound to RGS12 increased upon concomitant expression of B-Raf, but not A-Raf or c-Raf-1 (Figure 3B), suggesting that these binary interactions (H-Ras/RGS12, RGS12/B-Raf, and H-Ras/B-Raf) are mutually supportive and/or a trimeric complex of all three proteins exists.
In addition to H-Ras and B-Raf, RGS12 interacts with MEK2 (Figure 1); thus, we examined whether a complex of all four proteins might assemble in cells. Activated H-Ras (G12V mutant), B-Raf, and MEK2 were each detected in RGS12 immunoprecipitates upon coexpression (Figure 3C and D). Simultaneous expression of MEK2 with either activated H-Ras or B-Raf had no effect on the amount of H-Ras or B-Raf that co-immunoprecipitated with RGS12 (Figure 3C). In contrast, coexpression of activated H-Ras enhanced the amount of both B-Raf and MEK2 associating with RGS12 (Figure 3C). Amounts of co-immunoprecipitating H-Ras, B-Raf, and MEK2 were additionally enhanced when all four proteins were simultaneously coexpressed in HEK 293T cells (Figure 3C and D). These results suggest that the binding of activated H-Ras to RGS12 facilitates the assembly of a multiprotein complex on RGS12 that functionally links H-Ras with the first two tiers of the MAPK cascade (B-Raf and MEK2).
Several MAPK scaffold proteins (e.g. KSR, MP1, β-arrestin-2; reviewed in Kolch, 2005) also form macromolecular complexes with the third-tier kinase ERK. As RGS12 interacts with activated H-Ras, B-Raf, and MEK2, we reasoned that RGS12 may also assemble a MAPK complex containing the third-tier kinase. An interaction between RGS12 and ERK1 was not observed when only those two proteins were coexpressed in HEK 293T cells (Figure 3D); however, when activated H-Ras, B-Raf, and MEK2 were also coexpressed, ERK1 co-immunoprecipitated with RGS12 (Figure 3D). These findings further support the hypothesis that RGS12 acts as a scaffold to assemble multiple components of the Ras-activated MAPK cascade.
Loss of RGS12 inhibits NGF-mediated and MAPK cascade-dependent neuritogenesis
To define the role of RGS12 as a cellular MAPK scaffold, we identified cell lines that express RGS12 endogenously. Rabbit antisera against both the N-terminus and RGS domain of RGS12 confirmed the expression of full-length RGS12 in PC12 rat pheochromocytoma cells (Supplementary Figure S7). RGS12 co-immunoprecipitated with both endogenous H-Ras and B-Raf in PC12 cells (Figure 4A), but not with Rap1, suggesting that a complex of RGS12 with specific MAPK pathway members exists between endogenous components in PC12 cells.
Figure 4.
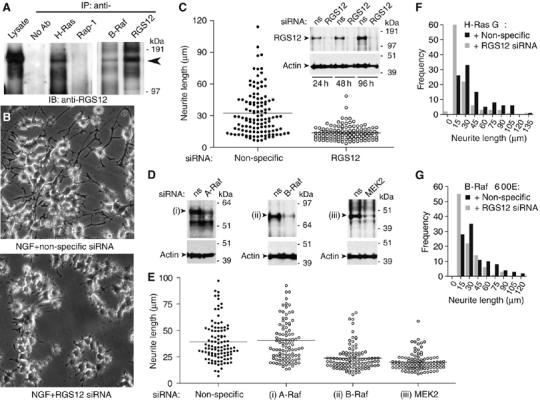
RGS12 is critical for NGF-mediated and MAPK-dependent neurite outgrowth in PC12 cells. (A) PC12 lysates were immunoprecipitated with beads alone (no Ab), or anti-H-Ras, anti-Rap-1, anti-B-Raf, or anti-RGS12 antibodies. Anti-RGS12 was used to detect associated endogenous RGS12. (B) PC12 cells were transfected with non-specific (top) or RGS12 siRNA (bottom) for 24 h, and treated with 100 ng/ml NGF for an additional 48 h. Images were obtained as described in Materials and methods. (C) PC12 cells were transfected, treated with NGF, and imaged as in panel B. Neurite lengths were measured for >100 cells per condition in three independent experiments as described in Materials and methods. The average for each condition is represented by a black line. Median neurite length in non-specific versus RGS12 siRNA-transfected cultures were significantly different (P<0.0001; Mann–Whitney test used given non-Gaussian distributions). Inset: PC12 cells were transfected with non-specific (ns) or RGS12 siRNA, and lysed 24, 48, and 96 h post-transfection for immunoblotting for RGS12 and actin. (D) PC12 cells were transfected with non-specific or (i) A-Raf-, (ii) B-Raf-, or (iii) MEK2-directed siRNA, and lysed 72 h post-transfection for immunoblotting for expression of actin and relevant protein target. (E) PC12 cells were transfected with gene-specific siRNAs as in panel D and resultant neurite length statistics were quantitated as in panel C. Neurite length was not statistically different between non-specific and A-Raf-directed siRNA treatment (subpanel (i); P>0.05, Mann–Whitney test), but was significantly reduced upon B-Raf and MEK2 knockdown (P<0.0001 for both subpanels (ii) and (iii); Mann–Whitney test versus non-specific siRNA). (F, G) PC12 neuritogenesis stimulated by transfection of activated (G12V) H-Ras (mean±s.e.m.: 42.1±2.8 μm) and activated (V600E) B-Raf (mean±s.e.m.: 40.6±2.6 μm) is blunted by RGS12 knockdown (mean±s.e.m.: 27.6±2.2 μm (F) and 27.3±1.8 μm (G)). One hundred independent measurements of neurite length are made for each treatment.
NGF stimulation of the TrkA receptor causes terminal differentiation, growth inhibition, and neurite formation in PC12 cells (Altin et al, 1991; Ng and Shooter, 1993). NGF induces rapid and sustained activation of both Ras and ERK, and inhibition of either Ras or ERK blocks neurite induction (Cowley et al, 1994). Thus, NGF-induced neurite formation is mediated by Ras activation of the ERK MAPK cascade. To address a possible role for RGS12 in NGF-induced neurite formation, we employed siRNA to suppress endogenous RGS12 expression. A pool of four duplexes efficiently reduced RGS12 expression (Supplementary Figure S8); upon separation, each of the four siRNA oligonucleotides also reduced expression of RGS12 (Figure 4C inset and Supplementary Figure S8). RNAi suppression of RGS12 expression impaired NGF-mediated neurite formation (Figure 4B and Supplementary Figure S9), reducing the average length of NGF-promoted neurites over 48 h compared with cells transfected with non-specific siRNA (Figure 4C). These results suggest that RGS12 positively regulates NGF signaling during PC12 cell neuritogenesis.
To establish whether the identified interaction partners of RGS12 are also essential for NGF-mediated neurite outgrowth, we employed siRNA to suppress endogenous A-Raf, B-Raf, and MEK2 levels (Figure 4D). Knockdown of B-Raf and MEK2 resulted in reduced PC12 neuritogenesis upon NGF treatment, similar to that of RGS12 knockdown (Figure 4E); in contrast, knockdown of A-Raf did not alter average neurite length promoted by NGF treatment. These results support the hypothesis that RGS12 and its MAPK-cascade-binding partners B-Raf and MEK2 participate in NGF-mediated neuritogenesis and suggest that the scaffolding function of RGS12 may be important in this process.
To establish further the role of RGS12 in MAPK cascade-dependent neuritogenesis, we examined the effect of RGS12 knockdown on PC12 neurite outgrowth stimulated by activated mutants of H-Ras (G12V; Taparowsky et al, 1982) and B-Raf (V600E; Davies et al, 2002a). Knockdown of RGS12 impaired both H-Ras- and B-Raf-stimulated neurite formation (Figure 4F and G), suggesting that RGS12 is an effector and scaffold for activated H-Ras and B-Raf in PC12 cells.
Prolonged ERK activation by NGF is reduced upon RGS12 depletion
Prolonged ERK activation promotes PC12 cell differentiation, whereas transient ERK activation promotes PC12 growth (Heasley and Johnson, 1992; Traverse et al, 1992; Nguyen et al, 1993). EGF acting through the EGF receptor induces transient activation of ERK, resulting in proliferation (Huff et al, 1981; Marshall, 1995). In contrast, NGF acting through the TrkA receptor induces both transient and prolonged phosphorylation of ERK, with the prolonged activation required for differentiation (Qui and Green, 1992; Traverse et al, 1992). We thus examined the effect of RGS12 knockdown on ERK activation kinetics in PC12 cells stimulated with NGF and EGF. Knockdown of RGS12 did not change the levels of transient ERK activation (5–10 min) by NGF (Figure 5A) or EGF (data not shown). However, the duration of ERK activation upon NGF treatment was shortened by RGS12 knockdown (Figure 5A), suggesting a mechanistic explanation for the impairment in NGF-mediated neuritogenesis seen upon RGS12 depletion (Figure 4). Knockdown of B-Raf and MEK2 levels, but not A-Raf levels, also leads to a reduction in sustained ERK activation (e.g. Figure 5B; 120 min post-NGF treatment).
Figure 5.
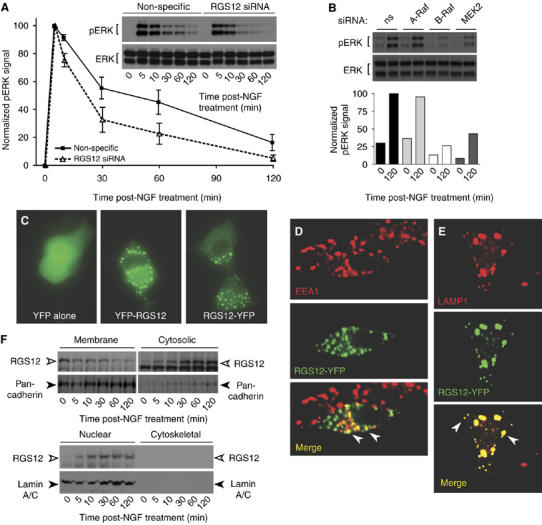
Prolonged ERK activation by NGF is reduced upon RGS12 depletion, expressed RGS12 is punctate and co-incident with endosomal markers, and translocation of endogenous RGS12 from membranes is regulated by NGF. (A) PC12 cells were transfected with non-specific or RGS12 siRNA and stimulated with NGF. ERK activation (pERK) and total ERK expression was determined by immunoblots (inset) with anti-phospho-ERK1/2 or anti-ERK1/2 at indicated times after NGF treatment. Scion Image was used for densitometric quantitation of pERK1/2 levels and normalization to maximum signal (non-specific siRNA at 5 min). Error bars represent standard error of the mean from three independent experiments. (B) Prolonged ERK activation is also reduced by siRNA-mediated knockdown of B-Raf and MEK2 levels, but not A-Raf levels, in PC12 cells. Densitometric quantitation of pERK1/2 levels was normalized to maximum signal (non-specific (ns) siRNA at 120 min). (C) PC12 cells were transfected with pEYFP-N1 empty vector, YFP-RGS12, or RGS12-YFP vectors for 24 h, and treated with 100 ng/ml NGF for an additional 48 h before epifluorescence imaging. (D, E) PC12 cells were transfected with RGS12-YFP (green) for 24 h, treated with 100 ng/ml NGF for an additional 48 h, fixed, permeabilized, and stained with early endosomal marker EEA1 (D, red) or late endosomal marker LAMP1 (E, red) before confocal microscopy. Colocalization of RGS12-YFP and endosomal markers is shown in yellow. White arrows indicate colocalization of RGS12-YFP with EEA1 or LAMP1 on individual puncta. (F) PC12 cells were stimulated with NGF at indicated time points, then fractionated into membrane, cytosolic, nuclear, and cytoskeletal fractions as described in Materials and methods. Proteins resolved by SDS-PAGE were immunoblotted with anti-RGS12, or antibodies against nuclear envelope marker lamin A/C or plasma membrane marker anti-pan-cadherin.
Subcellular localization of MAPK scaffolds, and the proteins that they coordinate, is critical for their function (Teis et al, 2002). We previously established that epitope-tagged RGS12 localizes to intracellular puncta in primary guinea-pig airway smooth muscle cells and regulates PDGFβR trafficking and PDGF-mediated activation of the MAPK cascade (Sambi et al, 2006). Similarly, in PC12 cells, a YFP fusion of RGS12 localizes to punctate structures, in comparison with YFP alone (Figure 5C). To determine whether these puncta were endocytic vesicles, we stained with early endosomal and late endosomal markers, EEA1 and LAMP1, respectively (Fukuda, 1991; Christoforidis et al, 1999). RGS12-YFP showed colocalization with EEA1 (Figure 5D) as well as with LAMP1 (Figure 5E), suggesting that overexpressed RGS12 is endosomal.
To explore the expression pattern of endogenous RGS12, we used subcellular fractionation following NGF stimulation of PC12 cells. Detection of RGS12 first occurs in the crude membrane fraction (Figure 5F; 0 min), but RGS12 subsequently is enriched in both cytosolic and nuclear fractions after NGF stimulation, with a concomitant decrease in the membrane fraction (Figure 5F). We observed little change in the localization or protein levels of nuclear and membrane markers (lamin A/C and pan-cadherin, respectively; McKeon et al, 1986; Takeichi et al, 1988), suggesting that the movement of endogenous RGS12 from membrane to cytosolic and nuclear fractions is specific.
RGS12 forms a multiprotein complex containing TrkA, H-Ras, B-Raf, and MEK2
MAPK pathway scaffolds have been identified that promote formation of stable complexes containing receptors (Morrison and Davis, 2003; Kolch, 2005). For example, β-arrestin-2 forms a complex with the angiotensin II type 1a receptor and component kinases of the ERK MAPK cascade (Luttrell et al, 2001). RGS12 interacts with activated H-Ras, B-Raf, and MEK2 (Figures 1, 2, and 3), and appears to regulate, and redistribute, upon NGF signaling in PC12 cells (Figures 4 and 5). Thus, we hypothesized that RGS12 may also associate with the primary NGF receptor in PC12 cells: TrkA (Chao and Hempstead, 1995). Therefore, we transiently expressed full-length RGS12 and TrkA in HEK 293T cells and assayed for protein complex formation (Figure 6). RGS12 co-immunoprecipitated with the TrkA receptor but not with fibroblast growth factor receptor isoforms FGFR1 (Figure 6A) or FGFR2 (data not shown). Coexpression of TrkA, but not FGFR1, redistributed YFP-RGS12 out of endosomes (Figure 6B and C), suggesting that RGS12 subcellular localization is regulated specifically by TrkA. This subcellular redistribution of YFP-RGS12 appears to be dependent on TrkA kinase activity, given that kinase-inactive and kinase-domain-deleted mutants of TrkA were unable to elicit redistribution; furthermore, treatment with the TrkA inhibitor K-252a (Berg et al, 1992) blocked RGS12 redistribution by wild-type TrkA coexpression (Supplementary Figure S10). A multiprotein complex containing RGS12, TrkA, activated H-Ras, B-Raf, and MEK2 was detected by co-immunoprecipitation (Figure 6D). These findings suggest that RGS12 associates with TrkA and potentially tethers the Ras–Raf–MEK signaling module to this receptor.
Figure 6.
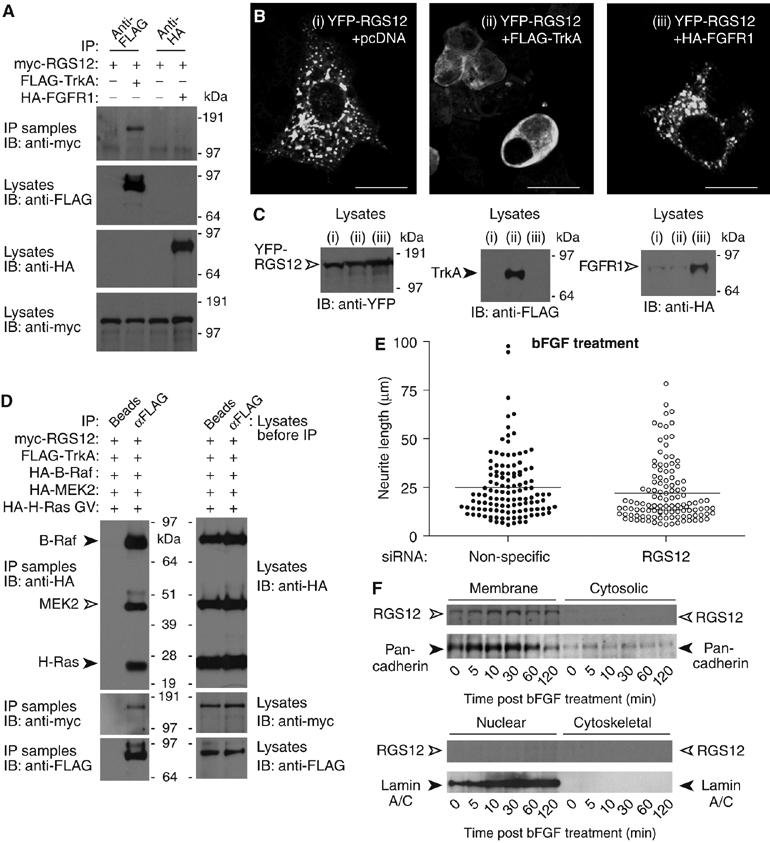
RGS12 forms a multiprotein complex containing TrkA and shows selectivity for TrkA signaling versus FGFR signaling. (A) HEK 293T cells were cotransfected with myc-RGS12, and either empty vector, FLAG-TrkA, or HA-FGFR1 plasmids. Anti-FLAG or anti-HA antibody was used to immunoprecipitate FLAG-TrkA or HA-FGFR1, respectively. Anti-myc was used to detect associated myc-RGS12 protein. (B) HEK 293T cells were cotransfected with YFP-RGS12 and either (i) pcDNA3.1 empty vector, (ii) FLAG-TrkA, or (iii) HA-FGFR1 vectors and imaged by confocal microscopy; scale bar represents 20 μm. (C) HEK 293T cells were transfected as described in panel B. At 72 h post-transfection, cells were lysed and immunoblotted to verify expression of YFP-RGS12, FLAG-TrkA, and HA-FGFR1. (D) HEK 293T cells were transfected with myc-RGS12, FLAG-TrkA, HA-B-Raf, HA-MEK2, and HA-H-Ras G12V plasmids. Lysates were immunoprecipitated with beads alone or anti-FLAG antibody. Anti-HA and anti-myc were used to detect associated myc-RGS12, HA-B-Raf, HA-MEK2, and HA-H-Ras G12V. (E) PC12 cells were transfected with non-specific or RGS12 siRNA for 24 h and treated with 100 ng/ml bFGF for an additional 48 h. Differences in neurite length were quantitated as described in Figure 4C. Median neurite lengths of non-specific siRNA-transfected cells were not significantly different from those of RGS12 siRNA-transfected cells (P>0.03; Mann–Whitney test). (F) PC12 cells were stimulated with bFGF for the indicated durations. Cells were then fractionated into membrane, cytosolic, nuclear, and cytoskeletal fractions as in Figure 5F. Proteins resolved by SDS–PAGE were immunoblotted with anti-RGS12, or antibodies against nuclear envelope marker lamin A/C or plasma membrane marker anti-pan-cadherin.
RGS12 receptor selectivity and role in axonal growth of primary neurons
As an interaction between RGS12 and FGFRs was not detected, we investigated whether RGS12 might selectively regulate NGF-mediated but not bFGF-mediated signaling in PC12 cells. bFGF can reproduce the entire spectrum of PC12 cell responses known to be elicited by NGF, including neurite outgrowth (Rydel and Greene, 1987). Whereas RGS12 knockdown attenuated NGF-promoted neurite outgrowth (Figure 4), no significant change in neurite outgrowth was seen with bFGF (Figure 6E). Consistent with this result, no change in RGS12 subcellular localization was observed upon stimulation with bFGF for up to 120 min (Figure 6F), in contrast to the movement seen upon NGF stimulation (Figure 5F). These data strongly suggest that RGS12 is critical for NGF-mediated, but not bFGF-mediated, neurite outgrowth and this may be due, at least in part, to a specific interaction between RGS12 and the NGF receptor TrkA.
With our findings that RGS12 (i) forms a complex with multiple proteins involved in the NGF-stimulated MAPK signaling pathway, (ii) is critical for MAPK cascade-promoted neuritogenesis in PC12 cells, and (iii) is expressed in trigeminal and DRG neurons in the E14.5 embryonic mouse (Martin-McCaffrey et al, 2005), we surmised that RGS12 may regulate a MAPK-mediated process in primary developing neurons. The Ras/Raf/ERK cascade is known to mediate axon elongation in NGF-containing embryonic DRG cultures (Liu and Snider, 2001; Markus et al, 2002; see also Atwal et al, 2000). Expression of Ras and Raf dominant inhibitory constructs strongly reduces NGF-induced axon growth from dissociated cultures of embryonic DRG neurons, and overexpression of activated H-Ras, Raf-1, or ERK leads to axonal growth comparable to that elicited by NGF treatment (Markus et al, 2002). Here, we found that siRNA knockdown of mouse RGS12 (Figure 7A) leads to a significant reduction in NGF-stimulated axonal growth in E14 DRG cultures from both wild–type and Bax-null mice (Figure 7B–D). Experiments were performed with Bax-null as well as wild-type mice to exclude any effects of RGS12 inhibition on neuronal survival.
Figure 7.
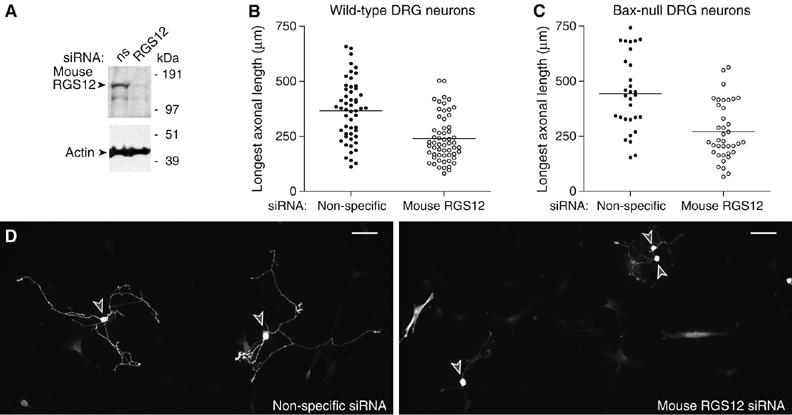
RGS12 is critical for NGF-mediated axonal growth in primary mouse DRG neurons. (A) N1E-115 mouse neuroblastoma cells were transfected with non-specific or mouse RGS12-directed siRNA and lysed 72 h post-transection for immunoblotting of RGS12 and actin. (B) Wild-type mouse embryo (E14) DRG neurons were transfected with non-specific or mouse RGS12 siRNA (along with YFP tracer), cultured for 2 days with NGF (50 ng/ml), replated, and stimulated with NGF for 1 day. Axons of YFP-positive cells were traced in camera lucida and quantitated using IPlab. RGS12 knockdown resulted in a significant decrease in longest axonal length (P<0.0001; Mann–Whitney test). (C) Bax-deficient mouse E14 DRG neurons were transfected as in panel B, cultured without NGF for 2 days, and stimulated with NGF (50 ng/ml) for 36 h. Axonal length of YFP-positive cells was quantitated as in panel B. RGS12 knockdown resulted in a significant decrease in longest axonal length (P<0.0001; Mann–Whitney test). (D) Photomicrographs of representative wild-type DRG neurons transfected with non-specific (left) or mouse RGS12 (right) siRNA. Cell bodies are denoted with gray arrowheads; scale bar represents 100 μm.
Discussion
The Raf–MEK–ERK cascade is often depicted as a linear signaling pathway traversing cytoplasm to connect receptor activation at the membrane with transcriptional effects in the nucleus. However, regulation of this pathway is complex—this kinase cascade lies downstream of various receptors and is involved in many biological processes (Johnson and Lapadat, 2002). Specificity in receptor-promoted signaling to ERK activation and spatio-temporal regulation of these signals are achieved by scaffolds. Several MAPK scaffolds have now been identified, with Saccharomyces cerevisiae Ste5 serving as the archetype, coordinating the kinases Ste11 (MAP3K), Ste7 (MAP2K), and Fus3 (MAPK) for efficient, pheromone-dependent activation of the MAPK cascade in haploid yeast (Gustin et al, 1998; Elion, 2001). Another well-characterized ERK scaffold, kinase suppressor of Ras (KSR), binds c-Raf-1, MEK1/2, ERK1/2, and other proteins (Kolch, 2005), and is thought to be critical to Ras function, given that KSR1-deficient fibroblasts are impaired in both Ras-mediated ERK activation and cell transformation (Kortum and Lewis, 2004). Our data suggest that RGS12, analogous to these established functions of Ste5 and KSR, sustains NGF-mediated ERK activation in PC12 cells and primary neurons by forming a signaling complex containing TrkA, H-Ras, B-Raf, MEK2, and ERK proteins.
MAPK scaffolds not only tether proteins together, but also specify their subcellular localization and guide their ultimate output. In tethering Ste11, Ste7, and Fus3 through independent binding sites (Choi et al, 1994), Ste5 recruits these kinases to the plasma membrane—the site at which upstream proteins participate in pheromone-dependent activation of the MAPK cascade (Pryciak and Huntress, 1998). In this way, Ste5 not only increases MAPK signaling efficiency, but also generates specificity by insulating the pheromone response from other parallel pathways (nutritional cues and osmotic stress), which use some or all of these same signaling components (Elion, 2001). The ubiquity of these signaling components is even more dramatic in mammalian cells, as underscored by the fact that over 160 substrates for the final-tier kinases ERK1/2 have now been described. The majority of these ERK substrates are nuclear; however, numerous others exist in the cytosol and at other organelles, including endosomes (Roux and Blenis, 2004; Yoon and Seger, 2006). Scaffold-mediated regulation of the utilization of particular MAPK substrates contributes to the distinct, and at times opposing, cellular events regulated by the ubiquitous MAPK cascade. In this context, our results provide several lines of evidence that RGS12 is a selective scaffold regulating prolonged ERK activation downstream of particular inputs and components: for example, NGF versus bFGF, activated H-Ras versus related GTPases, and B-Raf versus c-Raf-1.
In contrast to its robust association with activated H-Ras, RGS12 did not bind appreciably to other Ras isoforms. As H-, N-, and K-Ras are highly homologous, it is likely that their unique hypervariable linker domain sequences and differential post-translational modifications, known to sequester these Ras family members to distinct cellular locales (Ashery et al, 2006), may dictate, in part, the functional differences seen among Ras isoforms for RGS12 association. Ras signaling from internal membrane compartments is well documented, with one well-described mechanism involving endocytosis (Jiang and Sorkin, 2002), whereby EGF promotes internalization of active H-Ras, EGFR, and Ras effectors into endosomes. Similarly, the scaffold protein MP1, which binds to MEK1 and enhances ERK1 activation, localizes EGF-promoted Ras/MAPK signaling to late endosomes (Teis et al, 2002); MP1 is dispensable for transient EGFR activation of ERK at the plasma membrane, but essential for delayed activation of ERK on late endosomes. The enhancement of MAPK activation by MP1 is strictly dependent on the endosomal localization of the complex, as the artificial mislocalization of MP1 to the plasma membrane abolishes its positive effect. Thus, compartmentalizing Ras at endosomes appears to provide a means for trafficking and promoting MAPK signaling. These results, and our findings that RGS12 localizes to endocytic vesicles and binds multiple MAPK cascade components in addition to activated H-Ras, suggest that RGS12 may be a novel endosomal Ras effector scaffold.
In PC12 cells, transient ERK activation promotes cell proliferation, whereas prolonged ERK activation promotes neuronal differentiation (Heasley and Johnson, 1992; Traverse et al, 1992; Nguyen et al, 1993; Marshall, 1995). Furthermore, endosomal localization of MAPK signaling components appears to be critical for these differences, suggesting that endosomes may serve as the sites that generate persistent, rather than transient, signaling through Ras. Here, we show that RGS12 localizes to endosomes and its depletion attenuates prolonged ERK activation and neuronal differentiation. Proteins involved in NGF-mediated neurite outgrowth are known to localize to endosomes, and this localization is critical for their participation in neuronal differentiation of PC12 cells (Zhang et al, 2000; Howe et al, 2001). Isolated clathrin-coated vesicles from NGF-treated PC12 cells were found to contain NGF bound to TrkA together with activated signaling proteins of the Ras/MAPK pathway (Howe et al, 2001). Additionally, neuronal differentiation is stimulated by catalytically active Trk receptors specifically trafficked to endocytic vesicles; if NGF internalization in PC12 cells is inhibited by dominant-negative dynamin, neurite outgrowth is blunted (Zhang et al, 2000). These results suggest that RGS12, by binding TrkA and components of the Ras/MAPK cascade, might serve to coordinate this endosomal MAPK signaling required for prolonged ERK activation and neuritogenesis.
The most compelling context for this new data regarding RGS12 as a putative endosomal scaffold for TrkA-activated MAPK signaling is in the known response of primary neurons to trophic factors. NGF signals through TrkA to promote differentiation, survival, and maintenance of primary neurons (Casaccia-Bonnefil et al, 1999; Sofroniew et al, 2001). For neurotrophins to induce these pleiotropic effects, the signal must be communicated from axon terminals to cell bodies. One hypothesis suggests that ‘signaling endosomes' containing NGF-bound TrkA are formed at the axon terminal and traffic in a retrograde manner to the cell body, where they initiate local signal transduction cascades (Howe and Mobley, 2004); such retrograde transport and signaling of neurotrophins like NGF is thought to be critical to the development and maintenance of the nervous system (Sofroniew et al, 2001). NGF-containing vesicles transported retrogradely contain active components of the ERK pathway in vivo (Johanson et al, 1995). Thus, it is possible that RGS12 represents a key component of the NGF-derived signaling endosome.
In addition to their originally described roles as Gα GAPs and negative regulators of GPCR signaling, RGS proteins increasingly appear as scaffolds that integrate signaling from diverse receptors and steer pathway output to divergent cytoplasmic signaling networks (Siderovski and Willard, 2005). Our observation that RGS12 preferentially binds to activated H-Ras is in line with yeast two-hybrid data that Loco (the fly ortholog) interacts with Drosophila Ras1 (Formstecher et al, 2005), suggesting that RGS12 might constitute an evolutionarily conserved molecular link that integrates receptor tyrosine kinase signaling with GPCR signaling in both vertebrates and invertebrates. Interestingly, Loco localization in surface glia is punctate throughout the cytosol (Schwabe et al, 2005), similar to RGS12 in PC12 cells, HEK 293T cells, primary airway smooth muscle cells, and primary DRG neurons (this report, Sambi et al, 2006, and unpublished results). Although the mechanism by which RGS12 localizes to endosomes is unknown, it is possible that the Gαi-binding C-terminal GoLoco motif plays a role; we have previously reported that a loss-of-function mutation in the GoLoco motif mislocalizes RGS12 to the nucleus (Sambi et al, 2006). Thus, the multiple functional domains within RGS12 may cooperate to define the spatial and temporal nature of an RGS12-coordinated signaling output initiated from receptor tyrosine kinases and both monomeric and heterotrimeric G-protein subunits. Our observation that reducing RGS12 expression is able to blunt the actions of oncogenic alleles of H-Ras and B-Raf highlights the importance of future investigations into RGS12 functions in transformation-promoting MAPK signal transduction beyond that of NGF-mediated axonal growth.
Materials and methods
Biological reagents and cellular assays
A list of plasmids, antibodies, agonists, and other biological materials used, as well as routine procedures for cell culturing, transient transfection of plasmid DNA, cell imaging by confocal microscopy, harvesting of cell lysates, subcellular fractionation, immunoprecipitation, and immunoblotting are described in the Supplementary data.
siRNA transfection
siRNAs against rat A-Raf, B-Raf, MEK2, and RGS12 (the latter including SMARTpool and constituent individual duplexes) were from Dharmacon (Lafayette, CO). Non-specific siRNA (5′-CUACGUCCAGGAGCGCACC-3′) was used as a negative control (Gullapalli et al, 2006). PC12 cells were plated the day before transfection at 130 000 or 260 000 per well in 12-well culture dishes coated with poly-D-lysine (BD Biocoat, Franklin Lakes, NJ) and transfected with 150 nM siRNA using Lipofectamine 2000 (L2K; Invitrogen) according to the manufacturer's protocol. N1E-115 cells were plated the day before transfection at 50 000 cells per well in a 12-well culture dish and transfected using L2K with 150 nM mouse RGS12 SMARTpool siRNA (Dharmacon).
PC12 differentiation
PC12 cells were transfected with non-specific or RGS12 siRNA for 24 h and treated with 100 ng/ml NGF or bFGF (in 1% serum) for an additional 48 h. Brightfield images were obtained using an Olympus IX70 with a Q-Fire CCD camera. To quantitate differences in neurite length, >100 cells were counted per condition in three independent experiments using the ImageJ Measure feature (http://rsb.info.nih.gov/ij/); length was converted into micrometer and analyzed for statistical significance using Prism 4.0 (GraphPad, San Diego, CA).
Transfection and imaging of primary neurons
Isolation, culture, transfection, and imaging of wild-type and Bax-null mouse embryonic DRG (embryonic day 14) were performed as described (Kim et al, 2006), with the following modifications: DRG neuron cultures were coelectroporated with siRNA and YFP tracer DNA (pCS2-Venus; 3 μg) and subsequently stained with GFP antibody (A11122; Invitrogen) to highlight axon morphology. Quantitative determinations of axon length were based on analyses of >100 transfected neurons each in the non-specific and RGS12 siRNA-transfected cultures.
Supplementary Material
Supplementary data
Acknowledgments
We thank K-L Guan, D Morrison, J Frost, D Anderson, F Lee, CJ Der, A Cox, L Quilliam, and CJ McGlade for constructs, J Trejo, P Reddig, N Sciaky, and N Johnson for microscopy guidance, and S Chasse, AJ Kimple, and CI Behe for help with cloning, and especially thank E Maher at the University of Wisconsin Biotechnology Center for the yeast two-hybrid screens. This work was supported by NIH R01 GM062338 (to DPS) and R01 NS031768 (to WDS). MDW was supported by a PhRMA Foundation predoctoral fellowship. SDC was supported by NIH training grant T32 GM007040.
References
- Altin JG, Wetts R, Bradshaw RA (1991) Microinjection of a p21ras antibody into PC12 cells inhibits neurite outgrowth induced by nerve growth factor and basic fibroblast growth factor. Growth Factors 4: 145–155 [DOI] [PubMed] [Google Scholar]
- Ashery U, Yizhar O, Rotblat B, Kloog Y (2006) Nonconventional trafficking of Ras associated with Ras signal organization. Traffic 7: 119–126 [DOI] [PubMed] [Google Scholar]
- Atwal JK, Massie B, Miller FD, Kaplan DR (2000) The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron 27: 265–277 [DOI] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, Heberlein U (2005) Moody encodes two GPCRs that regulate cocaine behaviors and blood–brain barrier permeability in Drosophila. Cell 123: 145–156 [DOI] [PubMed] [Google Scholar]
- Berg MM, Sternberg DW, Parada LF, Chao MV (1992) K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J Biol Chem 267: 13–16 [PubMed] [Google Scholar]
- Bray D, Lay S (1997) Computer-based analysis of the binding steps in protein complex formation. Proc Natl Acad Sci USA 94: 13493–13498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Gu C, Chao MV (1999) Neurotrophins in cell survival/death decisions. Adv Exp Med Biol 468: 275–282 [DOI] [PubMed] [Google Scholar]
- Chao MV, Hempstead BL (1995) p75 and Trk: a two-receptor system. Trends Neurosci 18: 321–326 [PubMed] [Google Scholar]
- Chen CK, Burns ME, He W, Wensel TG, Baylor DA, Simon MI (2000) Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9-1. Nature 403: 557–560 [DOI] [PubMed] [Google Scholar]
- Choi KY, Satterberg B, Lyons DM, Elion EA (1994) Ste5 tethers multiple protein kinases in the MAP kinase cascade required for mating in S. cerevisiae. Cell 78: 499–512 [DOI] [PubMed] [Google Scholar]
- Christoforidis S, McBride HM, Burgoyne RD, Zerial M (1999) The Rab5 effector EEA1 is a core component of endosome docking. Nature 397: 621–625 [DOI] [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ (1994) Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell 77: 841–852 [DOI] [PubMed] [Google Scholar]
- Davies H et al. (2002a) Mutations of the BRAF gene in human cancer. Nature 417: 949–954 [DOI] [PubMed] [Google Scholar]
- Davies M, Collingridge GL, Hunt SP (2002b) Understanding G Protein-Coupled Receptors and their Role in the CNS. Oxford, UK: Oxford University Press [Google Scholar]
- Elion EA (2001) The Ste5p scaffold. J Cell Sci 114: 3967–3978 [DOI] [PubMed] [Google Scholar]
- Fabian JR, Vojtek AB, Cooper JA, Morrison DK (1994) A single amino acid change in Raf-1 inhibits Ras binding and alters Raf-1 function. Proc Natl Acad Sci USA 91: 5982–5986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formstecher E et al. (2005) Protein interaction mapping: a Drosophila case study. Genome Res 15: 376–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M (1991) Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem 266: 21327–21330 [PubMed] [Google Scholar]
- Granderath S, Stollewerk A, Greig S, Goodman CS, O'Kane CJ, Klambt C (1999) loco encodes an RGS protein required for Drosophila glial differentiation. Development 126: 1781–1791 [DOI] [PubMed] [Google Scholar]
- Gullapalli A, Wolfe BL, Griffin CT, Magnuson T, Trejo J (2006) An essential role for SNX1 in lysosomal sorting of protease-activated receptor-1: evidence for retromer-, Hrs-, and Tsg101-independent functions of sorting nexins. Mol Biol Cell 17: 1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin MC, Albertyn J, Alexander M, Davenport K (1998) MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev 62: 1264–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasley LE, Johnson GL (1992) The beta-PDGF receptor induces neuronal differentiation of PC12 cells. Mol Biol Cell 3: 545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CL, Mobley WC (2004) Signaling endosome hypothesis: a cellular mechanism for long distance communication. J Neurobiol 58: 207–216 [DOI] [PubMed] [Google Scholar]
- Howe CL, Valletta JS, Rusnak AS, Mobley WC (2001) NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras–MAPK pathway. Neuron 32: 801–814 [DOI] [PubMed] [Google Scholar]
- Huff K, End D, Guroff G (1981) Nerve growth factor-induced alteration in the response of PC12 pheochromocytoma cells to epidermal growth factor. J Cell Biol 88: 189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huleihel M, Goldsborough M, Cleveland J, Gunnell M, Bonner T, Rapp UR (1986) Characterization of murine A-raf, a new oncogene related to the v-raf oncogene. Mol Cell Biol 6: 2655–2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Sorkin A (2002) Coordinated traffic of Grb2 and Ras during epidermal growth factor receptor endocytosis visualized in living cells. Mol Biol Cell 13: 1522–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson SO, Crouch MF, Hendry IA (1995) Retrograde axonal transport of signal transduction proteins in rat sciatic nerve. Brain Res 690: 55–63 [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R (2002) Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298: 1911–1912 [DOI] [PubMed] [Google Scholar]
- Kiel C, Wohlgemuth S, Rousseau F, Schymkowitz J, Ferkinghoff-Borg J, Wittinghofer F, Serrano L (2005) Recognizing and defining true Ras binding domains II: in silico prediction based on homology modelling and energy calculations. J Mol Biol 348: 759–775 [DOI] [PubMed] [Google Scholar]
- Kim WY, Zhou FQ, Zhou J, Yokota Y, Wang YM, Yoshimura T, Kaibuchi K, Woodgett JR, Anton ES, Snider WD (2006) Essential roles for GSK-3s and GSK-3-primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron 52: 981–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimple RJ, De Vries L, Tronchere H, Behe CI, Morris RA, Gist Farquhar M, Siderovski DP (2001) RGS12 and RGS14 GoLoco motifs are G alpha(i) interaction sites with guanine nucleotide dissociation inhibitor activity. J Biol Chem 276: 29275–29281 [DOI] [PubMed] [Google Scholar]
- Kolch W (2005) Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol 6: 827–837 [DOI] [PubMed] [Google Scholar]
- Kortum RL, Lewis RE (2004) The molecular scaffold KSR1 regulates the proliferative and oncogenic potential of cells. Mol Cell Biol 24: 4407–4416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchenko A, Bruck J, Sternberg PW (2000) Scaffold proteins may biphasically affect the levels of mitogen-activated protein kinase signaling and reduce its threshold properties. Proc Natl Acad Sci USA 97: 5818–5823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RY, Snider WD (2001) Different signaling pathways mediate regenerative versus developmental sensory axon growth. J Neurosci 21: RC164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ (2001) Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci USA 98: 2449–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus A, Zhong J, Snider WD (2002) Raf and akt mediate distinct aspects of sensory axon growth. Neuron 35: 65–76 [DOI] [PubMed] [Google Scholar]
- Marshall CJ (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80: 179–185 [DOI] [PubMed] [Google Scholar]
- Martin-McCaffrey L, Hains MD, Pritchard GA, Pajak A, Dagnino L, Siderovski DP, D'Souza SJ (2005) Differential expression of regulator of G-protein signaling R12 subfamily members during mouse development. Dev Dyn 234: 438–444 [DOI] [PubMed] [Google Scholar]
- Maudsley S, Zamah AM, Rahman N, Blitzer JT, Luttrell LM, Lefkowitz RJ, Hall RA (2000) Platelet-derived growth factor receptor association with Na(+)/H(+) exchanger regulatory factor potentiates receptor activity. Mol Cell Biol 20: 8352–8363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon FD, Kirschner MW, Caput D (1986) Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature 319: 463–468 [DOI] [PubMed] [Google Scholar]
- Mittal V, Linder ME (2006) Biochemical characterization of RGS14: RGS14 activity towards G-protein alpha subunits is independent of its binding to Rap2A. Biochem J 394: 309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison DK, Davis RJ (2003) Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol 19: 91–118 [DOI] [PubMed] [Google Scholar]
- Ng NF, Shooter EM (1993) Activation of p21ras by nerve growth factor in embryonic sensory neurons and PC12 cells. J Biol Chem 268: 25329–25333 [PubMed] [Google Scholar]
- Nguyen TT, Scimeca JC, Filloux C, Peraldi P, Carpentier JL, Van Obberghen E (1993) Co-regulation of the mitogen-activated protein kinase, extracellular signal-regulated kinase 1, and the 90-kDa ribosomal S6 kinase in PC12 cells. Distinct effects of the neurotrophic factor, nerve growth factor, and the mitogenic factor, epidermal growth factor. J Biol Chem 268: 9803–9810 [PubMed] [Google Scholar]
- Pathirana S, Zhao D, Bownes M (2001) The Drosophila RGS protein Loco is required for dorsal/ventral axis formation of the egg and embryo, and nurse cell dumping. Mech Dev 109: 137–150 [DOI] [PubMed] [Google Scholar]
- Ponting CP (1999) Raf-like Ras/Rap-binding domains in RGS12- and still-life-like signalling proteins. J Mol Med 77: 695–698 [DOI] [PubMed] [Google Scholar]
- Pryciak PM, Huntress FA (1998) Membrane recruitment of the kinase cascade scaffold protein Ste5 by the Gbetagamma complex underlies activation of the yeast pheromone response pathway. Genes Dev 12: 2684–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui MS, Green SH (1992) PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity. Neuron 9: 705–717 [DOI] [PubMed] [Google Scholar]
- Richman RW, Strock J, Hains MD, Cabanilla NJ, Lau KK, Siderovski DP, Diverse-Pierluissi M (2005) RGS12 interacts with the SNARE-binding region of the Cav2.2 calcium channel. J Biol Chem 280: 1521–1528 [DOI] [PubMed] [Google Scholar]
- Ross EM, Wilkie TM (2000) GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem 69: 795–827 [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J (2004) ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68: 320–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydel RE, Greene LA (1987) Acidic and basic fibroblast growth factors promote stable neurite outgrowth and neuronal differentiation in cultures of PC12 cells. J Neurosci 7: 3639–3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambi BS, Hains MD, Waters CM, Connell MC, Willard FS, Kimple AJ, Pyne S, Siderovski DP, Pyne NJ (2006) The effect of RGS12 on PDGFbeta receptor signalling to p42/p44 mitogen activated protein kinase in mammalian cells. Cell Signal 18: 971–981 [DOI] [PubMed] [Google Scholar]
- Schiff ML, Siderovski DP, Jordan JD, Brothers G, Snow B, De Vries L, Ortiz DF, Diverse-Pierluissi M (2000) Tyrosine-kinase-dependent recruitment of RGS12 to the N-type calcium channel. Nature 408: 723–727 [DOI] [PubMed] [Google Scholar]
- Schwabe T, Bainton RJ, Fetter RD, Heberlein U, Gaul U (2005) GPCR signaling is required for blood–brain barrier formation in Drosophila. Cell 123: 133–144 [DOI] [PubMed] [Google Scholar]
- Siderovski DP, Diverse-Pierluissi M, De Vries L (1999) The GoLoco motif: a Galphai/o binding motif and potential guanine-nucleotide exchange factor. Trends Biochem Sci 24: 340–341 [DOI] [PubMed] [Google Scholar]
- Siderovski DP, Willard FS (2005) The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int J Biol Sci 1: 51–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow BE, Hall RA, Krumins AM, Brothers GM, Bouchard D, Brothers CA, Chung S, Mangion J, Gilman AG, Lefkowitz RJ, Siderovski DP (1998) GTPase activating specificity of RGS12 and binding specificity of an alternatively spliced PDZ (PSD-95/Dlg/ZO-1) domain. J Biol Chem 273: 17749–17755 [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Howe CL, Mobley WC (2001) Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 24: 1217–1281 [DOI] [PubMed] [Google Scholar]
- Takeichi M, Hatta K, Nose A, Nagafuchi A (1988) Identification of a gene family of cadherin cell adhesion molecules. Cell Differ Dev 25 (Suppl): 91–94 [DOI] [PubMed] [Google Scholar]
- Taparowsky E, Suard Y, Fasano O, Shimizu K, Goldfarb M, Wigler M (1982) Activation of the T24 bladder carcinoma transforming gene is linked to a single amino acid change. Nature 300: 762–765 [DOI] [PubMed] [Google Scholar]
- Teis D, Wunderlich W, Huber LA (2002) Localization of the MP1–MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev Cell 3: 803–814 [DOI] [PubMed] [Google Scholar]
- Traver S, Splingard A, Gaudriault G, De Gunzburg J (2004) The RGS (regulator of G-protein signalling) and GoLoco domains of RGS14 co-operate to regulate Gi-mediated signalling. Biochem J 379: 627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse S, Gomez N, Paterson H, Marshall C, Cohen P (1992) Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J 288 (Part 2): 351–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JM, Wang D, Feng G (2004) Differential mRNA expression and protein localization of the SAP90/PSD-95-associated proteins (SAPAPs) in the nervous system of the mouse. J Comp Neurol 472: 24–39 [DOI] [PubMed] [Google Scholar]
- Wittinghofer A, Herrmann C (1995) Ras-effector interactions, the problem of specificity. FEBS Lett 369: 52–56 [DOI] [PubMed] [Google Scholar]
- Wohlgemuth S, Kiel C, Kramer A, Serrano L, Wittinghofer F, Herrmann C (2005) Recognizing and defining true Ras binding domains I: biochemical analysis. J Mol Biol 348: 741–758 [DOI] [PubMed] [Google Scholar]
- Yoon S, Seger R (2006) The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 24: 21–44 [DOI] [PubMed] [Google Scholar]
- Yu F, Wang H, Qian H, Kaushik R, Bownes M, Yang X, Chia W (2005) Locomotion defects, together with Pins, regulates heterotrimeric G-protein signaling during Drosophila neuroblast asymmetric divisions. Genes Dev 19: 1341–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Georgescu D, Sanchez N, Rahman Z, DiLeone R, Berton O, Neve RL, Sim-Selley LJ, Selley DE, Gold SJ, Nestler EJ (2003) Essential role for RGS9 in opiate action. Proc Natl Acad Sci USA 100: 13656–13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Moheban DB, Conway BR, Bhattacharyya A, Segal RA (2000) Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J Neurosci 20: 5671–5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


