Abstract
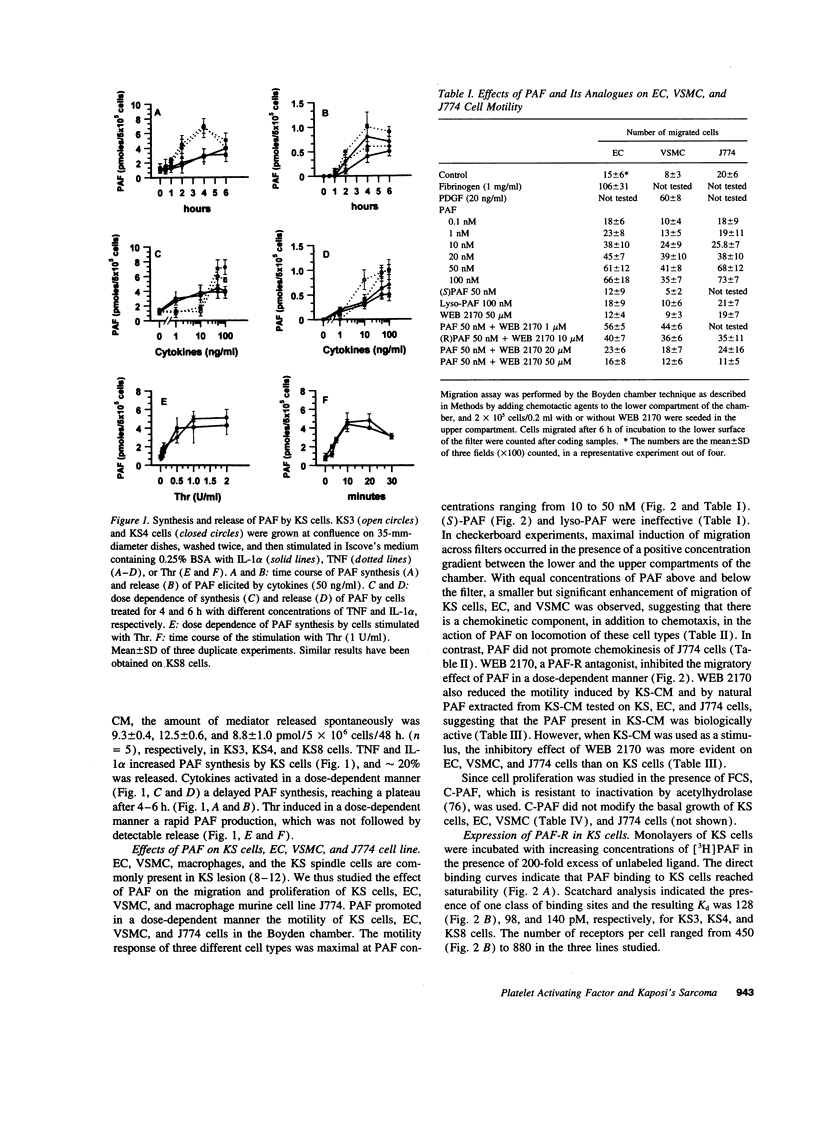
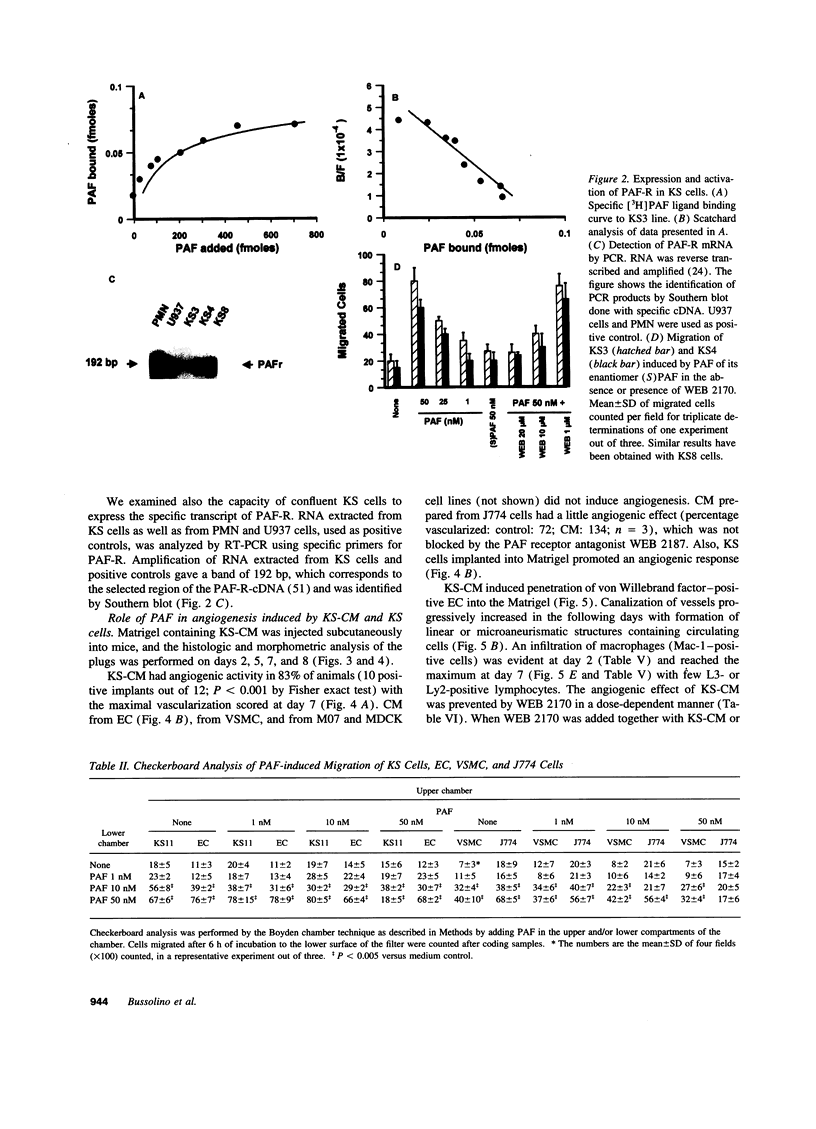
Imbalance in the network of soluble mediators may play a pivotal role in the pathogenesis of Kaposi's sarcoma (KS). In this study, we demonstrated that KS cells grown in vitro produced and in part released platelet activating factor (PAF), a powerful lipid mediator of inflammation and cell-to-cell communication. IL-1, TNF, and thrombin enhanced the synthesis of PAF. PAF receptor mRNA and specific, high affinity binding site for PAF were present in KS cells. Nanomolar concentration of PAF stimulated the chemotaxis and chemokinesis of KS cells, endothelial cells, and vascular smooth muscle cells. The migration response to PAF was inhibited by WEB 2170, a hetrazepinoic PAF receptor antagonist. Because neoangiogenesis is essential for the growth and progression of KS and since PAF can activate vascular endothelial cells, we examined the potential role of PAF as an instrumental mediator of angiogenesis associated with KS. Conditioned medium (CM) from KS cells (KS-CM) or KS cells themselves induced angiogenesis and macrophage recruitment in a murine model in which Matrigel was injected subcutaneously. These effects were inhibited by treating mice with WEB 2170. Synthetic PAF or natural PAF extracted from plasma of patients with classical KS also induced angiogenesis, which in turn was inhibited by WEB 2170. The action of PAF was amplified by expression of other angiogenic factors and chemokines: these included basic and acidic fibroblast growth factor, placental growth factor, vascular endothelial growth factor and its specific receptor flk-1, hepatocyte growth factor, KC, and macrophage inflammatory protein-2. Treatment with WEB 2170 abolished the expression of the transcripts of these molecules within Matrigel containing KS-CM. These results indicate that PAF may cooperate with other angiogenic molecules and chemokines in inducing vascular development in KS.
Full text
PDF


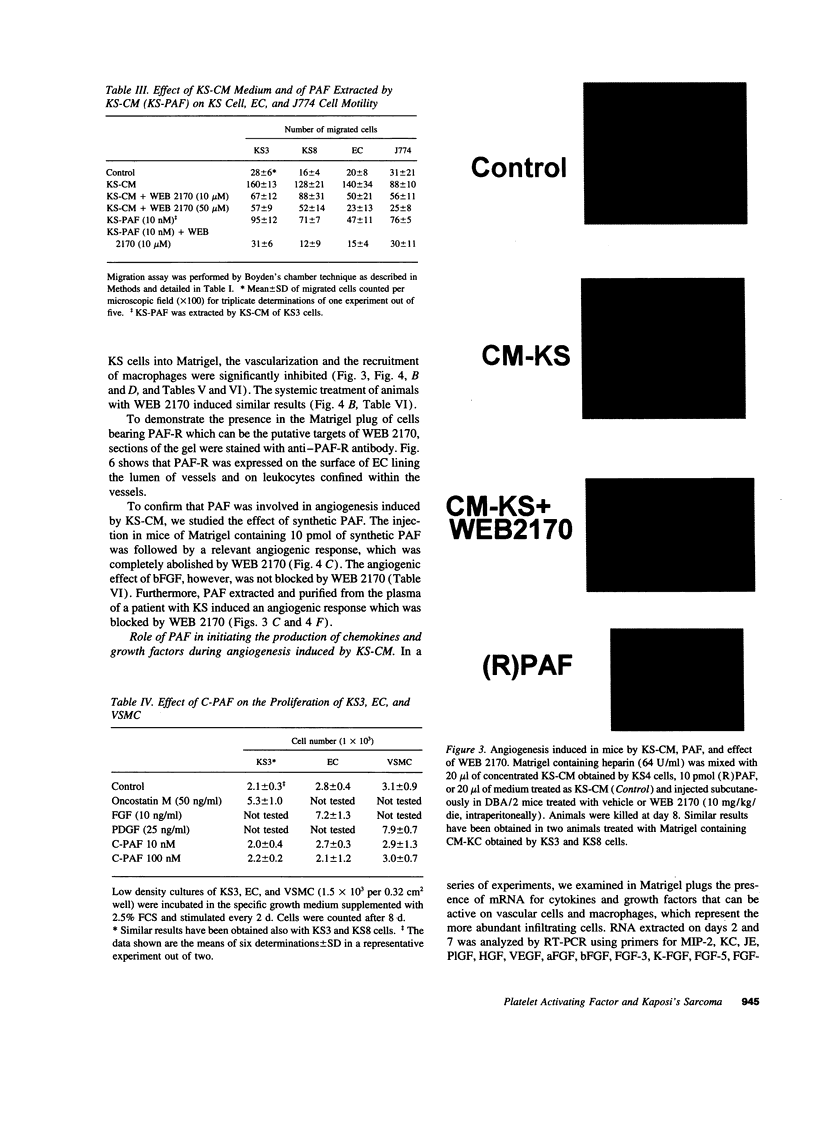

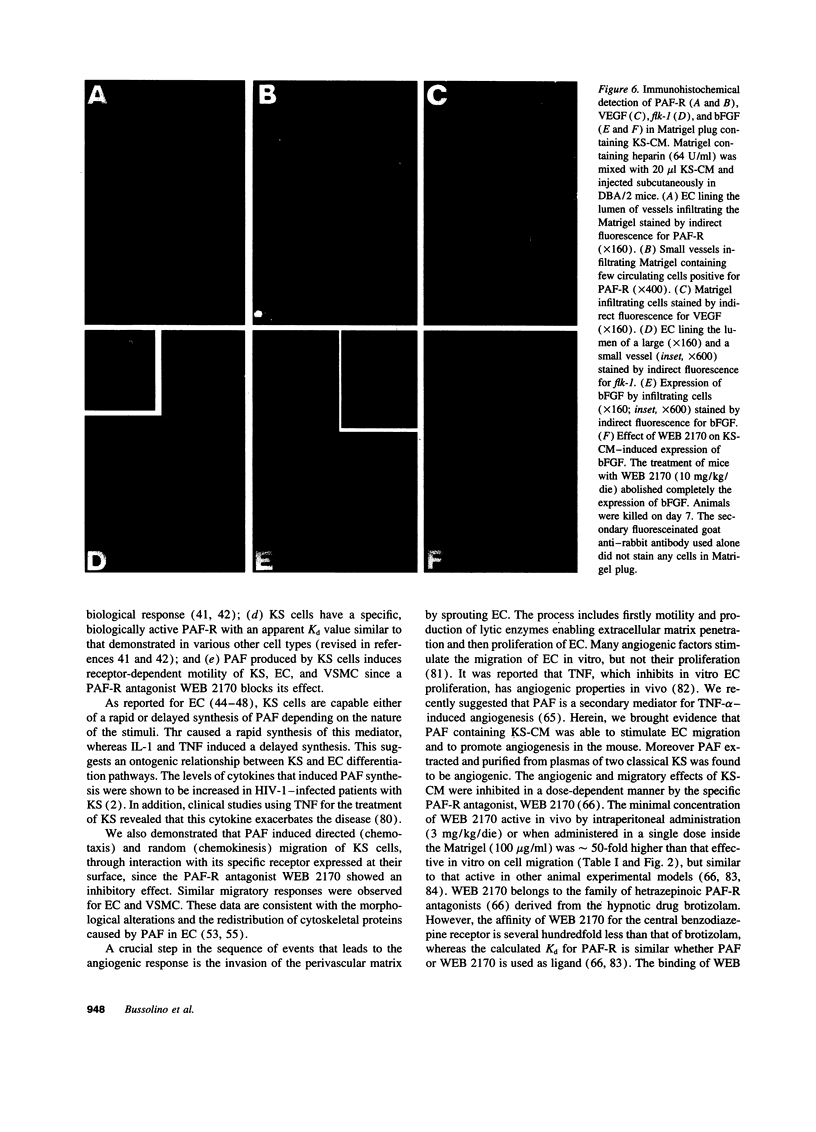
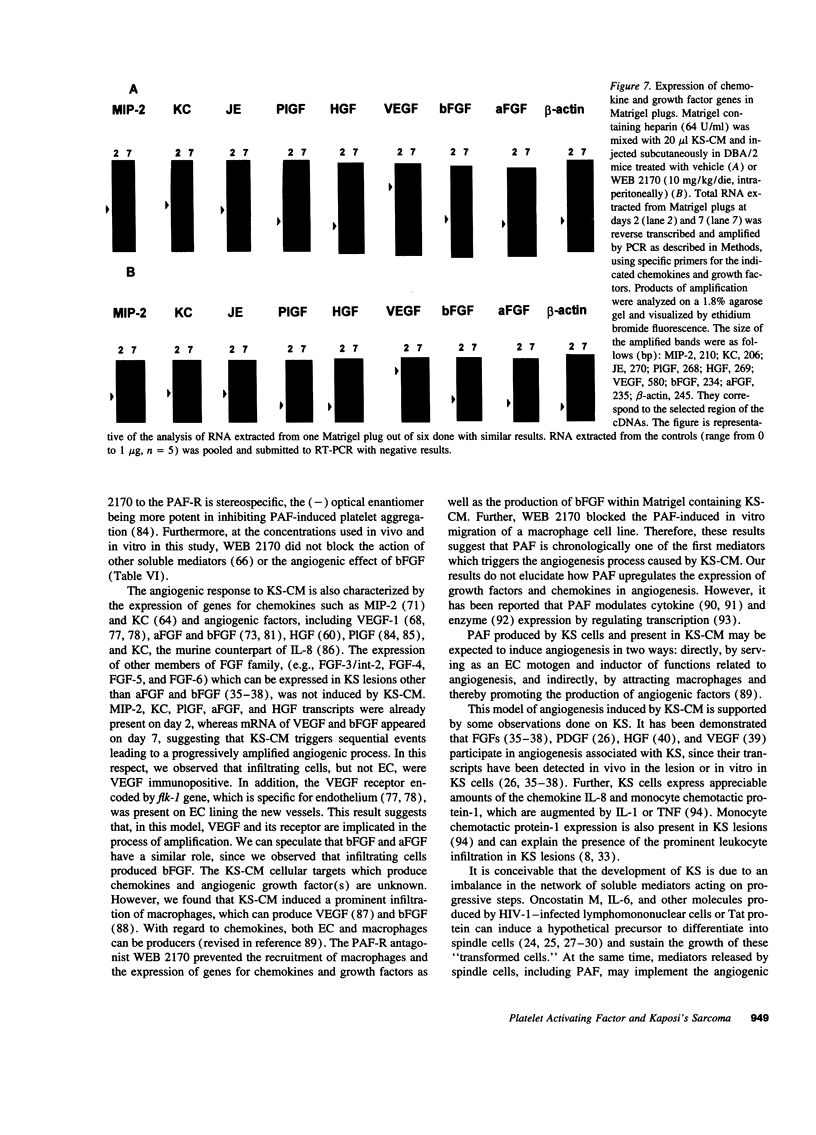



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboulafia D., Miles S. A., Saks S. R., Mitsuyasu R. T. Intravenous recombinant tumor necrosis factor in the treatment of AIDS-related Kaposi's sarcoma. J Acquir Immune Defic Syndr. 1989;2(1):54–58. [PubMed] [Google Scholar]
- Albini A., Fontanini G., Masiello L., Tacchetti C., Bigini D., Luzzi P., Noonan D. M., Stetler-Stevenson W. G. Angiogenic potential in vivo by Kaposi's sarcoma cell-free supernatants and HIV-1 tat product: inhibition of KS-like lesions by tissue inhibitor of metalloproteinase-2. AIDS. 1994 Sep;8(9):1237–1244. doi: 10.1097/00002030-199409000-00004. [DOI] [PubMed] [Google Scholar]
- Albini A., Mitchell C. D., Thompson E. W., Seeman R., Martin G. R., Wittek A. E., Quinnan G. V. Invasive activity and chemotactic response to growth factors by Kaposi's sarcoma cells. J Cell Biochem. 1988 Apr;36(4):369–376. doi: 10.1002/jcb.240360406. [DOI] [PubMed] [Google Scholar]
- Armes J. A review of Kaposi's sarcoma. Adv Cancer Res. 1989;53:73–87. doi: 10.1016/s0065-230x(08)60279-1. [DOI] [PubMed] [Google Scholar]
- Baird A., Mormède P., Böhlen P. Immunoreactive fibroblast growth factor in cells of peritoneal exudate suggests its identity with macrophage-derived growth factor. Biochem Biophys Res Commun. 1985 Jan 16;126(1):358–364. doi: 10.1016/0006-291x(85)90614-x. [DOI] [PubMed] [Google Scholar]
- Barillari G., Buonaguro L., Fiorelli V., Hoffman J., Michaels F., Gallo R. C., Ensoli B. Effects of cytokines from activated immune cells on vascular cell growth and HIV-1 gene expression. Implications for AIDS-Kaposi's sarcoma pathogenesis. J Immunol. 1992 Dec 1;149(11):3727–3734. [PubMed] [Google Scholar]
- Bazan N. G., Fletcher B. S., Herschman H. R., Mukherjee P. K. Platelet-activating factor and retinoic acid synergistically activate the inducible prostaglandin synthase gene. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5252–5256. doi: 10.1073/pnas.91.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beral V., Bull D., Darby S., Weller I., Carne C., Beecham M., Jaffe H. Risk of Kaposi's sarcoma and sexual practices associated with faecal contact in homosexual or bisexual men with AIDS. Lancet. 1992 Mar 14;339(8794):632–635. doi: 10.1016/0140-6736(92)90793-3. [DOI] [PubMed] [Google Scholar]
- Berse B., Brown L. F., Van de Water L., Dvorak H. F., Senger D. R. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell. 1992 Feb;3(2):211–220. doi: 10.1091/mbc.3.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier G., Albrecht U., Sterrer S., Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992 Feb;114(2):521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- Brock T. A., Gimbrone M. A., Jr Platelet activating factors alters calcium homeostasis in cultured vascular endothelial cells. Am J Physiol. 1986 Jun;250(6 Pt 2):H1086–H1092. doi: 10.1152/ajpheart.1986.250.6.H1086. [DOI] [PubMed] [Google Scholar]
- Bussolino F., Camussi G., Aglietta M., Braquet P., Bosia A., Pescarmona G., Sanavio F., D'Urso N., Marchisio P. C. Human endothelial cells are target for platelet-activating factor. I. Platelet-activating factor induces changes in cytoskeleton structures. J Immunol. 1987 Oct 1;139(7):2439–2446. [PubMed] [Google Scholar]
- Bussolino F., Camussi G., Baglioni C. Synthesis and release of platelet-activating factor by human vascular endothelial cells treated with tumor necrosis factor or interleukin 1 alpha. J Biol Chem. 1988 Aug 25;263(24):11856–11861. [PubMed] [Google Scholar]
- Bussolino F., Di Renzo M. F., Ziche M., Bocchietto E., Olivero M., Naldini L., Gaudino G., Tamagnone L., Coffer A., Comoglio P. M. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992 Nov;119(3):629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F., Silvagno F., Garbarino G., Costamagna C., Sanavio F., Arese M., Soldi R., Aglietta M., Pescarmona G., Camussi G. Human endothelial cells are targets for platelet-activating factor (PAF). Activation of alpha and beta protein kinase C isozymes in endothelial cells stimulated by PAF. J Biol Chem. 1994 Jan 28;269(4):2877–2886. [PubMed] [Google Scholar]
- Camussi G., Aglietta M., Malavasi F., Tetta C., Piacibello W., Sanavio F., Bussolino F. The release of platelet-activating factor from human endothelial cells in culture. J Immunol. 1983 Nov;131(5):2397–2403. [PubMed] [Google Scholar]
- Camussi G., Bussolino F., Salvidio G., Baglioni C. Tumor necrosis factor/cachectin stimulates peritoneal macrophages, polymorphonuclear neutrophils, and vascular endothelial cells to synthesize and release platelet-activating factor. J Exp Med. 1987 Nov 1;166(5):1390–1404. doi: 10.1084/jem.166.5.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Corbeil J., Evans L. A., Vasak E., Cooper D. A., Penny R. Culture and properties of cells derived from Kaposi sarcoma. J Immunol. 1991 May 1;146(9):2972–2976. [PubMed] [Google Scholar]
- Degen S. J., Stuart L. A., Han S., Jamison C. S. Characterization of the mouse cDNA and gene coding for a hepatocyte growth factor-like protein: expression during development. Biochemistry. 1991 Oct 8;30(40):9781–9791. doi: 10.1021/bi00104a030. [DOI] [PubMed] [Google Scholar]
- Delli Bovi P., Curatola A. M., Kern F. G., Greco A., Ittmann M., Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987 Aug 28;50(5):729–737. doi: 10.1016/0092-8674(87)90331-x. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Barillari G., Gallo R. C. Cytokines and growth factors in the pathogenesis of AIDS-associated Kaposi's sarcoma. Immunol Rev. 1992 Jun;127:147–155. doi: 10.1111/j.1600-065x.1992.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Barillari G., Salahuddin S. Z., Gallo R. C., Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990 May 3;345(6270):84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Nakamura S., Salahuddin S. Z., Biberfeld P., Larsson L., Beaver B., Wong-Staal F., Gallo R. C. AIDS-Kaposi's sarcoma-derived cells express cytokines with autocrine and paracrine growth effects. Science. 1989 Jan 13;243(4888):223–226. doi: 10.1126/science.2643161. [DOI] [PubMed] [Google Scholar]
- Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992 Jun 5;267(16):10931–10934. [PubMed] [Google Scholar]
- Fràter-Schröder M., Risau W., Hallmann R., Gautschi P., Böhlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill P., Rarick M., Bernstein-Singer M., Harb M., Espina B. M., Shaw V., Levine A. Treatment of advanced Kaposi's sarcoma using a combination of bleomycin and vincristine. Am J Clin Oncol. 1990 Aug;13(4):315–319. doi: 10.1097/00000421-199008000-00010. [DOI] [PubMed] [Google Scholar]
- Gorski J., Rollini P., Long E., Mach B. Molecular organization of the HLA-SB region of the human major histocompatibility complex and evidence for two SB beta-chain genes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3934–3938. doi: 10.1073/pnas.81.13.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutterman J. U. Cytokine therapeutics: lessons from interferon alpha. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1198–1205. doi: 10.1073/pnas.91.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A. C., Kahn L. B. Myogenic cells in Kaposi's sarcoma: an ultrastructural study. J Pathol. 1978 Mar;124(3):157–160. doi: 10.1002/path.1711240305. [DOI] [PubMed] [Google Scholar]
- Haverkos H. W., Drotman D. P. Prevalence of Kaposi's sarcoma among patients with AIDS. N Engl J Med. 1985 Jun 6;312(23):1518–1518. [PubMed] [Google Scholar]
- Heuer H. O., Casals-Stenzel J., Muacevic G., Weber K. H. Pharmacologic activity of bepafant (WEB 2170), a new and selective hetrazepinoic antagonist of platelet activating factor. J Pharmacol Exp Ther. 1990 Dec;255(3):962–968. [PubMed] [Google Scholar]
- Honda Z., Nakamura M., Miki I., Minami M., Watanabe T., Seyama Y., Okado H., Toh H., Ito K., Miyamoto T. Cloning by functional expression of platelet-activating factor receptor from guinea-pig lung. Nature. 1991 Jan 24;349(6307):342–346. doi: 10.1038/349342a0. [DOI] [PubMed] [Google Scholar]
- Huang Y. Q., Li J. J., Moscatelli D., Basilico C., Nicolaides A., Zhang W. G., Poiesz B. J., Friedman-Kien A. E. Expression of int-2 oncogene in Kaposi's sarcoma lesions. J Clin Invest. 1993 Mar;91(3):1191–1197. doi: 10.1172/JCI116279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. Q., Li J. J., Rush M. G., Poiesz B. J., Nicolaides A., Jacobson M., Zhang W. G., Coutavas E., Abbott M. A., Friedman-Kien A. E. HPV-16-related DNA sequences in Kaposi's sarcoma. Lancet. 1992 Feb 29;339(8792):515–518. doi: 10.1016/0140-6736(92)90338-4. [DOI] [PubMed] [Google Scholar]
- Hébert J. M., Basilico C., Goldfarb M., Haub O., Martin G. R. Isolation of cDNAs encoding four mouse FGF family members and characterization of their expression patterns during embryogenesis. Dev Biol. 1990 Apr;138(2):454–463. doi: 10.1016/0012-1606(90)90211-z. [DOI] [PubMed] [Google Scholar]
- Ikegami K., Meade C. J., Heuer H. O., Birke F. Hetrazepine PAF antagonists. J Lipid Mediat. 1992 Jun-Jul;5(2):177–182. [PubMed] [Google Scholar]
- Kaaya E. E., Parravicini C., Sundelin B., Mgaya E., Kitinya J., Lema L., Luande J., Biberfeld P. Spindle cell ploidy and proliferation in endemic and epidemic African Kaposi's sarcoma. Eur J Cancer. 1992;28A(11):1890–1894. doi: 10.1016/0959-8049(92)90030-6. [DOI] [PubMed] [Google Scholar]
- Kahn J. O., Northfelt D. W., Miles S. A. AIDS-associated Kaposi's sarcoma. AIDS Clin Rev. 1992:261–280. [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Kunkel S. L., Harlow L. A., DiPietro L. A., Elner V. M., Elner S. G., Strieter R. M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992 Dec 11;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Kuijpers T. W., Hakkert B. C., Hart M. H., Roos D. Neutrophil migration across monolayers of cytokine-prestimulated endothelial cells: a role for platelet-activating factor and IL-8. J Cell Biol. 1992 May;117(3):565–572. doi: 10.1083/jcb.117.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacasse C., Rola-Pleszczynski M. Immune regulation by platelet-activating factor: II. Mediation of suppression by cytokine-stimulated endothelial cells in vitro. J Leukoc Biol. 1991 Mar;49(3):245–252. doi: 10.1002/jlb.49.3.245. [DOI] [PubMed] [Google Scholar]
- Le Gouvello S., Vivier E., Debre P., Thomas Y., Colard O. CD2 triggering stimulates the formation of platelet-activating factor-acether from alkyl-arachidonoyl-glycerophosphocholine in a human CD4+ T lymphocyte clone. J Immunol. 1992 Aug 15;149(4):1289–1293. [PubMed] [Google Scholar]
- Li J. J., Huang Y. Q., Moscatelli D., Nicolaides A., Zhang W. C., Friedman-Kien A. E. Expression of fibroblast growth factors and their receptors in acquired immunodeficiency syndrome-associated Kaposi sarcoma tissue and derived cells. Cancer. 1993 Oct 1;72(7):2253–2259. doi: 10.1002/1097-0142(19931001)72:7<2253::aid-cncr2820720732>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Lorant D. E., Patel K. D., McIntyre T. M., McEver R. P., Prescott S. M., Zimmerman G. A. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: a juxtacrine system for adhesion and activation of neutrophils. J Cell Biol. 1991 Oct;115(1):223–234. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglione D., Guerriero V., Viglietto G., Delli-Bovi P., Persico M. G. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Bottazzi B., Colotta F., Sozzani S., Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992 Jul;13(7):265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- McManus L. M., Woodard D. S., Deavers S. I., Pinckard R. N. PAF molecular heterogeneity: pathobiological implications. Lab Invest. 1993 Dec;69(6):639–650. [PubMed] [Google Scholar]
- McNutt N. S., Fletcher V., Conant M. A. Early lesions of Kaposi's sarcoma in homosexual men. An ultrastructural comparison with other vascular proliferations in skin. Am J Pathol. 1983 Apr;111(1):62–77. [PMC free article] [PubMed] [Google Scholar]
- Miles S. A., Martínez-Maza O., Rezai A., Magpantay L., Kishimoto T., Nakamura S., Radka S. F., Linsley P. S. Oncostatin M as a potent mitogen for AIDS-Kaposi's sarcoma-derived cells. Science. 1992 Mar 13;255(5050):1432–1434. doi: 10.1126/science.1542793. [DOI] [PubMed] [Google Scholar]
- Miles S. A., Rezai A. R., Salazar-González J. F., Vander Meyden M., Stevens R. H., Logan D. M., Mitsuyasu R. T., Taga T., Hirano T., Kishimoto T. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4068–4072. doi: 10.1073/pnas.87.11.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millauer B., Wizigmann-Voos S., Schnürch H., Martinez R., Møller N. P., Risau W., Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993 Mar 26;72(6):835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Montrucchio G., Bergerone S., Bussolino F., Alloatti G., Silvestro L., Lupia E., Cravetto A., Di Leo M., Emanuelli G., Camussi G. Streptokinase induces intravascular release of platelet-activating factor in patients with acute myocardial infarction and stimulates its synthesis by cultured human endothelial cells. Circulation. 1993 Oct;88(4 Pt 1):1476–1483. doi: 10.1161/01.cir.88.4.1476. [DOI] [PubMed] [Google Scholar]
- Montrucchio G., Lupia E., Battaglia E., Passerini G., Bussolino F., Emanuelli G., Camussi G. Tumor necrosis factor alpha-induced angiogenesis depends on in situ platelet-activating factor biosynthesis. J Exp Med. 1994 Jul 1;180(1):377–382. doi: 10.1084/jem.180.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Casey G., Brookes S., Dixon M., Peters G., Dickson C. Sequence, topography and protein coding potential of mouse int-2: a putative oncogene activated by mouse mammary tumour virus. EMBO J. 1986 May;5(5):919–924. doi: 10.1002/j.1460-2075.1986.tb04304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller E., Dagenais P., Alami N., Rola-Pleszczynski M. Identification and functional characterization of platelet-activating factor receptors in human leukocyte populations using polyclonal anti-peptide antibody. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5818–5822. doi: 10.1073/pnas.90.12.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu Y. M., Rosen E. M., Zitnick R., Goldberg I., Park M., Naujokas M., Polverini P. J., Nickoloff B. J. Role of scatter factor in the pathogenesis of AIDS-related Kaposi sarcoma. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5281–5285. doi: 10.1073/pnas.91.12.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair B. C., DeVico A. L., Nakamura S., Copeland T. D., Chen Y., Patel A., O'Neil T., Oroszlan S., Gallo R. C., Sarngadharan M. G. Identification of a major growth factor for AIDS-Kaposi's sarcoma cells as oncostatin M. Science. 1992 Mar 13;255(5050):1430–1432. doi: 10.1126/science.1542792. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Sakurada S., Salahuddin S. Z., Osada Y., Tanaka N. G., Sakamoto N., Sekiguchi M., Gallo R. C. Inhibition of development of Kaposi's sarcoma-related lesions by a bacterial cell wall complex. Science. 1992 Mar 13;255(5050):1437–1440. doi: 10.1126/science.1371891. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Salahuddin S. Z., Biberfeld P., Ensoli B., Markham P. D., Wong-Staal F., Gallo R. C. Kaposi's sarcoma cells: long-term culture with growth factor from retrovirus-infected CD4+ T cells. Science. 1988 Oct 21;242(4877):426–430. doi: 10.1126/science.3262925. [DOI] [PubMed] [Google Scholar]
- Passaniti A., Taylor R. M., Pili R., Guo Y., Long P. V., Haney J. A., Pauly R. R., Grant D. S., Martin G. R. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992 Oct;67(4):519–528. [PubMed] [Google Scholar]
- Prescott S. M., Zimmerman G. A., McIntyre T. M. Platelet-activating factor. J Biol Chem. 1990 Oct 15;265(29):17381–17384. [PubMed] [Google Scholar]
- Quinn T. P., Peters K. G., De Vries C., Ferrara N., Williams L. T. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola-Pleszczynski M., Stankova J. Differentiation-dependent modulation of TNF production by PAF in human HL-60 myeloid leukemia cells. J Leukoc Biol. 1992 Jun;51(6):609–616. doi: 10.1002/jlb.51.6.609. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Morrison E. D., Stiles C. D. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3738–3742. doi: 10.1073/pnas.85.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutgers J. L., Wieczorek R., Bonetti F., Kaplan K. L., Posnett D. N., Friedman-Kien A. E., Knowles D. M., 2nd The expression of endothelial cell surface antigens by AIDS-associated Kaposi's sarcoma. Evidence for a vascular endothelial cell origin. Am J Pathol. 1986 Mar;122(3):493–499. [PMC free article] [PubMed] [Google Scholar]
- Safai B., Johnson K. G., Myskowski P. L., Koziner B., Yang S. Y., Cunningham-Rundles S., Godbold J. H., Dupont B. The natural history of Kaposi's sarcoma in the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Nov;103(5):744–750. doi: 10.7326/0003-4819-103-5-744. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Nakamura S., Biberfeld P., Kaplan M. H., Markham P. D., Larsson L., Gallo R. C. Angiogenic properties of Kaposi's sarcoma-derived cells after long-term culture in vitro. Science. 1988 Oct 21;242(4877):430–433. doi: 10.1126/science.2459779. [DOI] [PubMed] [Google Scholar]
- Schwertschlag U. S., Whorton A. R. Platelet-activating factor-induced homologous and heterologous desensitization in cultured vascular smooth muscle cells. J Biol Chem. 1988 Sep 25;263(27):13791–13796. [PubMed] [Google Scholar]
- Sciacca F. L., Stürzl M., Bussolino F., Sironi M., Brandstetter H., Zietz C., Zhou D., Matteucci C., Peri G., Sozzani S. Expression of adhesion molecules, platelet-activating factor, and chemokines by Kaposi's sarcoma cells. J Immunol. 1994 Nov 15;153(10):4816–4825. [PubMed] [Google Scholar]
- Seeds E. A., Coyle A. J., Page C. P. The effect of the selective PAF antagonist WEB 2170 on PAF and antigen induced airway hyperresponsiveness and eosinophil infiltration. J Lipid Mediat. 1991 Jul-Aug;4(1):111–121. [PubMed] [Google Scholar]
- Smith C. S., Parker L., Shearer W. T. Cytokine regulation by platelet-activating factor in a human B cell line. J Immunol. 1994 Nov 1;153(9):3997–4005. [PubMed] [Google Scholar]
- Smith C. S., Shearer W. T. Activation of NF-kappa B and immunoglobulin expression in response to platelet-activating factor in a human B cell line. Cell Immunol. 1994 May;155(2):292–303. doi: 10.1006/cimm.1994.1123. [DOI] [PubMed] [Google Scholar]
- Stürzl M., Roth W. K., Brockmeyer N. H., Zietz C., Speiser B., Hofschneider P. H. Expression of platelet-derived growth factor and its receptor in AIDS-related Kaposi sarcoma in vivo suggests paracrine and autocrine mechanisms of tumor maintenance. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7046–7050. doi: 10.1073/pnas.89.15.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappero J. W., Conant M. A., Wolfe S. F., Berger T. G. Kaposi's sarcoma. Epidemiology, pathogenesis, histology, clinical spectrum, staging criteria and therapy. J Am Acad Dermatol. 1993 Mar;28(3):371–395. doi: 10.1016/0190-9622(93)70057-z. [DOI] [PubMed] [Google Scholar]
- Taylor J. F., Smith P. G., Bull D., Pike M. C. Kaposi's sarcoma in Uganda: geographic and ethnic distribution. Br J Cancer. 1972 Dec;26(6):483–497. doi: 10.1038/bjc.1972.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekamp-Olson P., Gallegos C., Bauer D., McClain J., Sherry B., Fabre M., van Deventer S., Cerami A. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J Exp Med. 1990 Sep 1;172(3):911–919. doi: 10.1084/jem.172.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessner T. G., O'Flaherty J. T., Wykle R. L. Stimulation of platelet-activating factor synthesis by a nonmetabolizable bioactive analog of platelet-activating factor and influence of arachidonic acid metabolites. J Biol Chem. 1989 Mar 25;264(9):4794–4799. [PubMed] [Google Scholar]
- Thompson E. W., Nakamura S., Shima T. B., Melchiori A., Martin G. R., Salahuddin S. Z., Gallo R. C., Albini A. Supernatants of acquired immunodeficiency syndrome-related Kaposi's sarcoma cells induce endothelial cell chemotaxis and invasiveness. Cancer Res. 1991 May 15;51(10):2670–2676. [PubMed] [Google Scholar]
- Vogel J., Hinrichs S. H., Reynolds R. K., Luciw P. A., Jay G. The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature. 1988 Oct 13;335(6191):606–611. doi: 10.1038/335606a0. [DOI] [PubMed] [Google Scholar]
- Weich H. A., Salahuddin S. Z., Gill P., Nakamura S., Gallo R. C., Folkmann J. AIDS-associated Kaposi's sarcoma-derived cells in long-term culture express and synthesize smooth muscle alpha-actin. Am J Pathol. 1991 Dec;139(6):1251–1258. [PMC free article] [PubMed] [Google Scholar]
- Weindel K., Marmé D., Weich H. A. AIDS-associated Kaposi's sarcoma cells in culture express vascular endothelial growth factor. Biochem Biophys Res Commun. 1992 Mar 31;183(3):1167–1174. doi: 10.1016/s0006-291x(05)80313-4. [DOI] [PubMed] [Google Scholar]
- Werner S., Hofschneider P. H., Roth W. K. Cells derived from sporadic and AIDS-related Kaposi's sarcoma reveal identical cytochemical and molecular properties in vitro. Int J Cancer. 1989 Jun 15;43(6):1137–1144. doi: 10.1002/ijc.2910430629. [DOI] [PubMed] [Google Scholar]
- Wick M. R. Kaposi's sarcoma unrelated to the acquired immunodeficiency syndrome. Curr Opin Oncol. 1991 Apr;3(2):377–383. doi: 10.1097/00001622-199104000-00021. [DOI] [PubMed] [Google Scholar]
- Wittek A. E., Mitchell C. D., Armstrong G. R., Albini A., Martin G. R., Seemann R., Levenbook I. S., Wierenga D. E., Ridge J., Dunlap R. C. Propagation and properties of Kaposi's sarcoma-derived cell lines obtained from patients with AIDS: similarity of cultured cells to smooth muscle cells. AIDS. 1991 Dec;5(12):1485–1493. doi: 10.1097/00002030-199112000-00011. [DOI] [PubMed] [Google Scholar]
- Xerri L., Hassoun J., Planche J., Guigou V., Grob J. J., Parc P., Birnbaum D., deLapeyriere O. Fibroblast growth factor gene expression in AIDS-Kaposi's sarcoma detected by in situ hybridization. Am J Pathol. 1991 Jan;138(1):9–15. [PMC free article] [PubMed] [Google Scholar]
- Ye R. D., Prossnitz E. R., Zou A. H., Cochrane C. G. Characterization of a human cDNA that encodes a functional receptor for platelet activating factor. Biochem Biophys Res Commun. 1991 Oct 15;180(1):105–111. doi: 10.1016/s0006-291x(05)81261-6. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lapeyriere O., Rosnet O., Benharroch D., Raybaud F., Marchetto S., Planche J., Galland F., Mattei M. G., Copeland N. G., Jenkins N. A. Structure, chromosome mapping and expression of the murine Fgf-6 gene. Oncogene. 1990 Jun;5(6):823–831. [PubMed] [Google Scholar]