Abstract
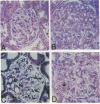
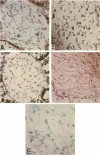
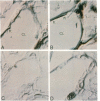
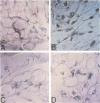
The development of progressive glomerulosclerosis in the renal ablation model has been ascribed to a number of humoral and hemodynamic events, including the peptide growth factor, transforming growth factor-beta 1 (TGF-beta 1). An important role has also been attributed to angiotensin II (AII), which, in addition to its hemodynamic effects, can stimulate transcription of TGF-beta 1. We postulated that increased glomerular production of AII, resulting from enhanced intrinsic angiotensinogen expression, stimulates local TGF-beta 1 synthesis, activating glomerular matrix protein synthesis, and leads to sclerosis. Using in situ reverse transcription, the glomerular cell sites of alpha-1 (IV) collagen, fibronectin, laminin B1, angiotensinogen, and TGF-beta 1 mRNA synthesis were determined at sequential periods following renal ablation. The early hypertrophic phase was associated with global, but transient, increases in the mRNA for alpha-1 (IV) collagen. No changes were noted for fibronectin, TGF-beta 1, and angiotensinogen mRNAs. At 24 d after ablation, at which time sclerosis is not evident, endothelial cells, particularly in the dilated capillaries at the vascular pole, expressed angiotensinogen and TGF-beta 1 mRNAs, as well as fibronectin and laminin B1 RNA transcripts. By 74 d after ablation angiotensinogen and TGF-beta 1 mRNAs were widely distributed among endothelial and mesangial cells, and were particularly prominent in regions of evolving sclerosis. These same regions were also notable for enhanced expression of matrix protein mRNAs, particularly fibronectin. All receptor blockade inhibited angiotensinogen, TGF-beta 1, fibronectin, and laminin B1 mRNA expression by the endothelium. We conclude that, as a result of hemodynamic changes, injured or activated endothelium synthesizes angiotensinogen, triggering a cascade of TGF-beta 1 and matrix protein gene expression with resultant development of the segmental glomerular sclerotic lesion.
Full text
PDF




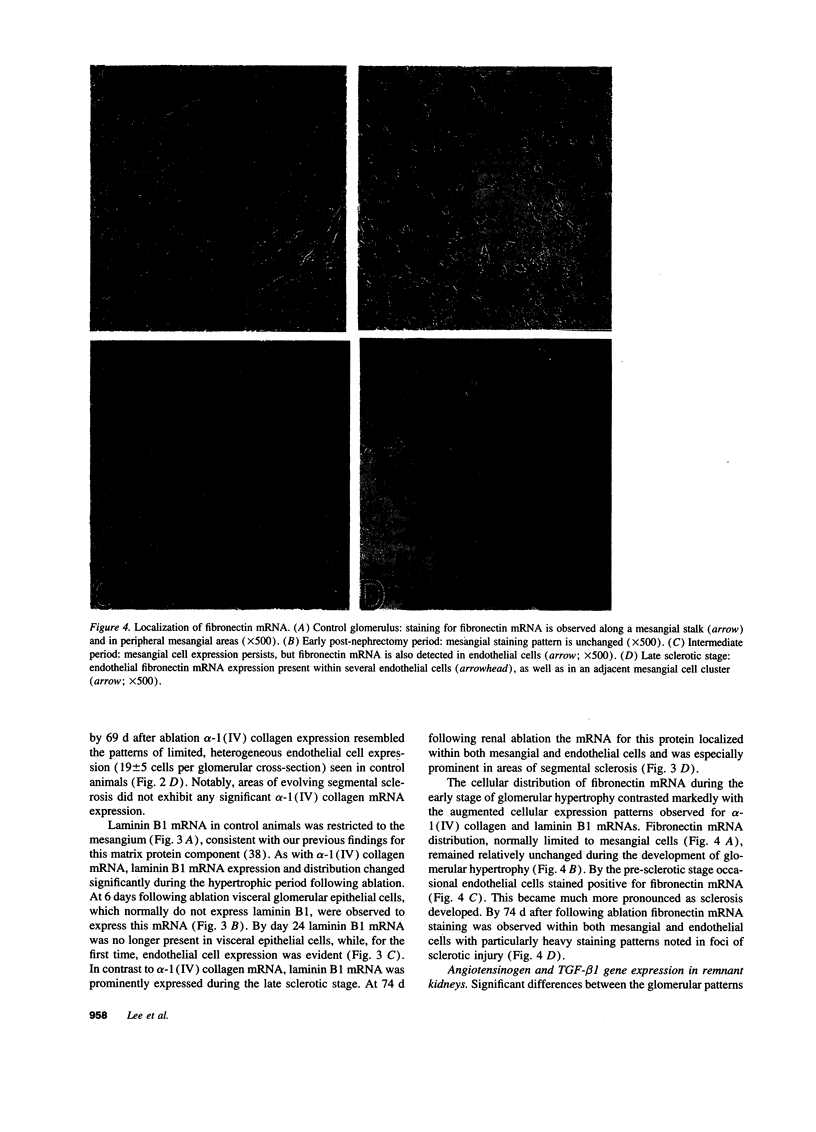

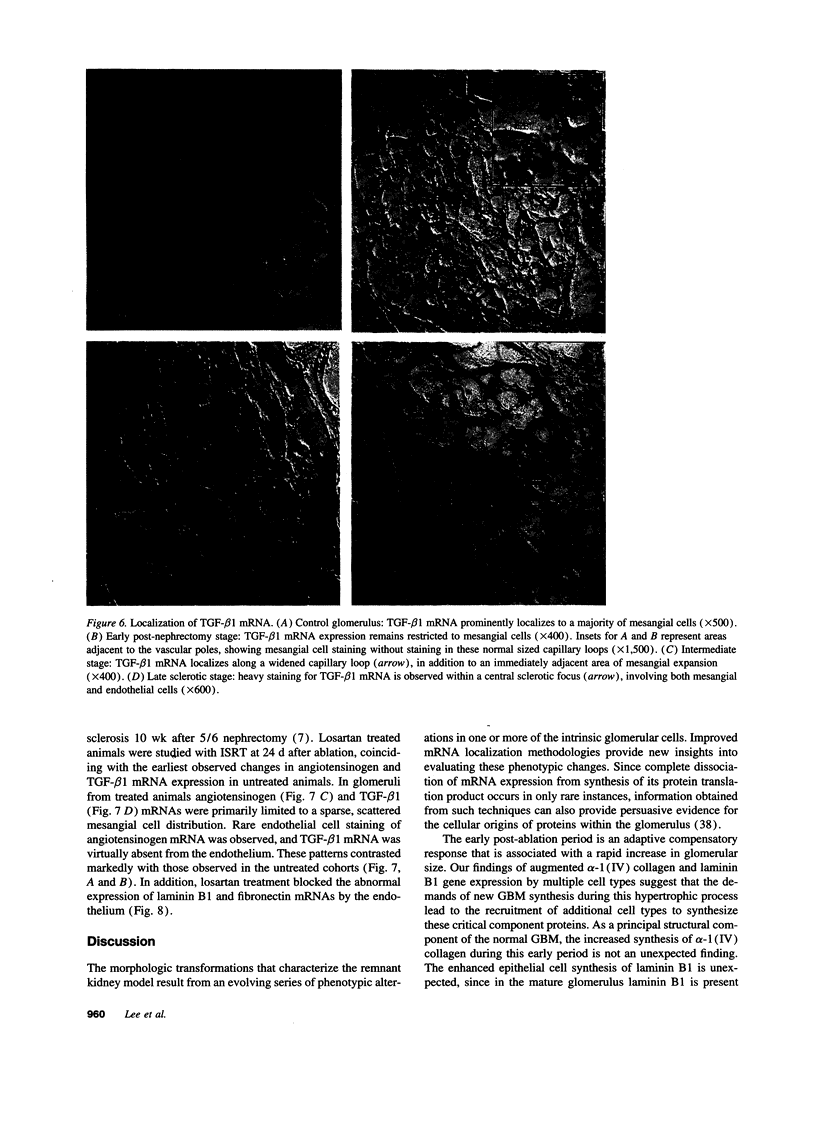
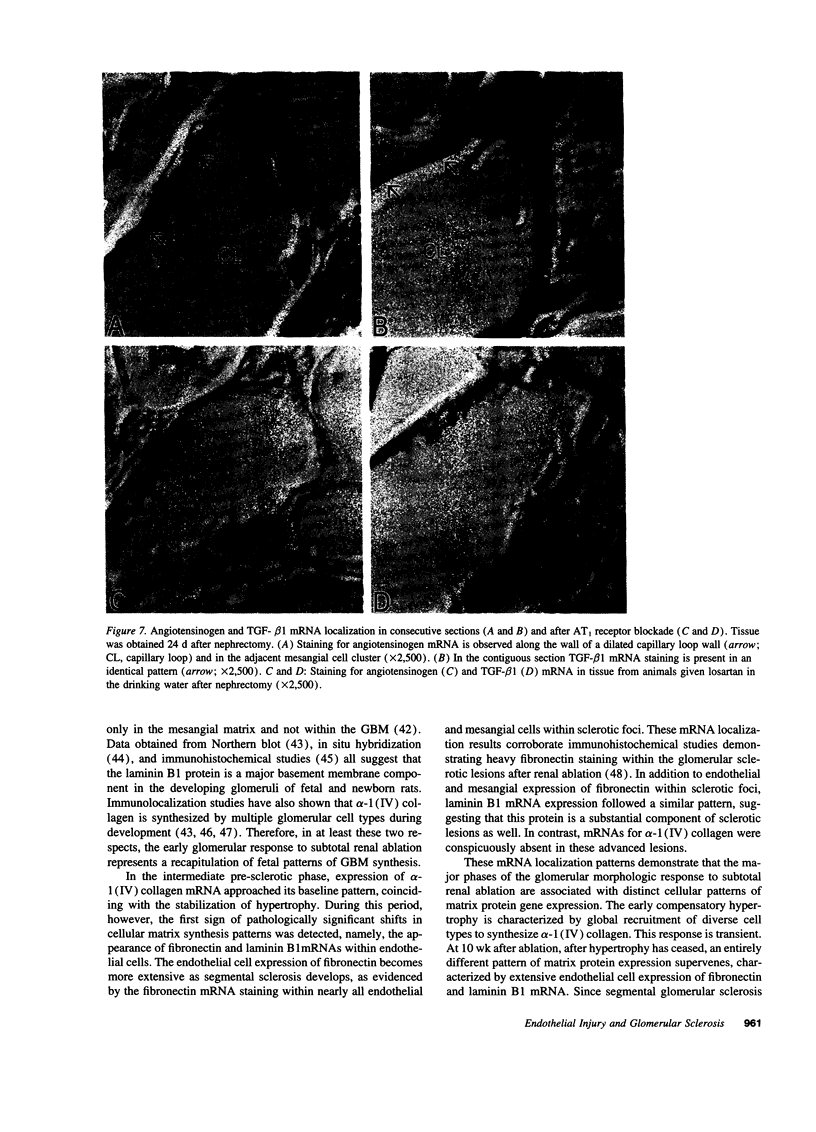
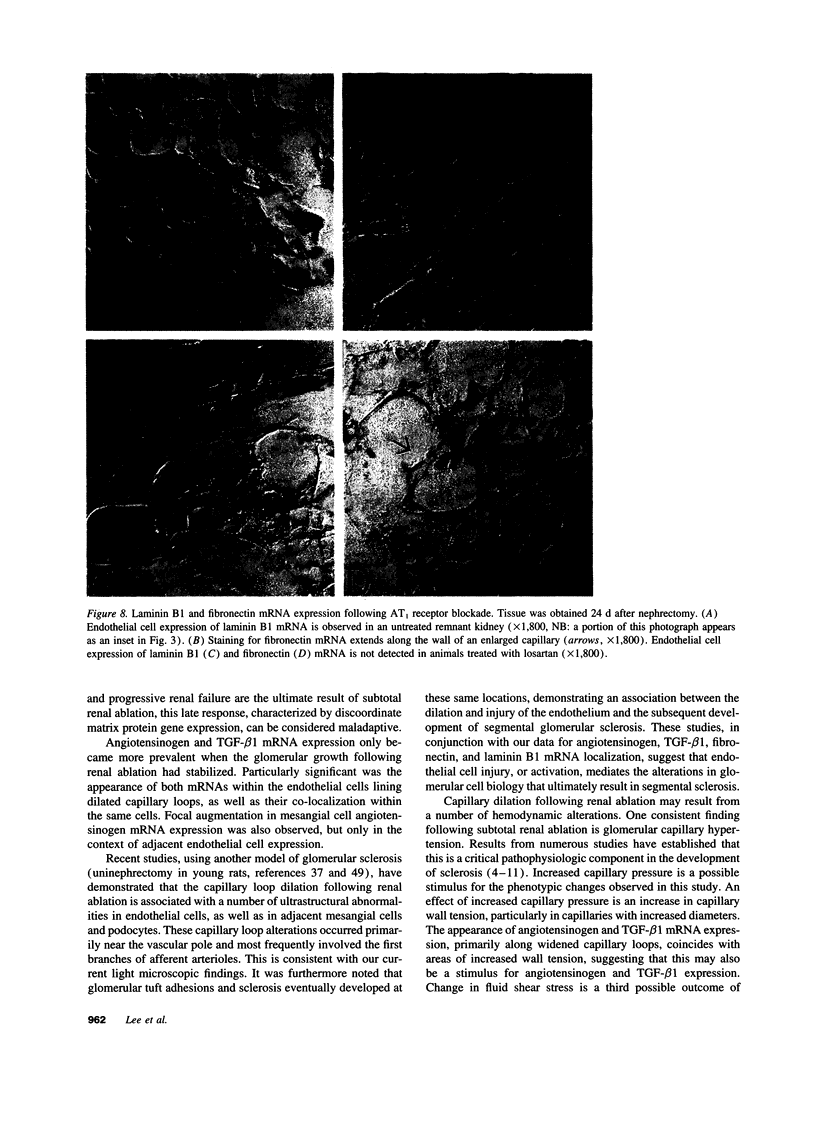


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R. Origin of the glomerular basement membrane visualized after in vivo labeling of laminin in newborn rat kidneys. J Cell Biol. 1985 Jun;100(6):1988–2000. doi: 10.1083/jcb.100.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson D. R., St John P. L. Laminin distribution in developing glomerular basement membranes. Kidney Int. 1993 Jan;43(1):73–78. doi: 10.1038/ki.1993.13. [DOI] [PubMed] [Google Scholar]
- Adler S., Chen X., Eng B. Control of rat glomerular epithelial cell growth in vitro. Kidney Int. 1990 Apr;37(4):1048–1054. doi: 10.1038/ki.1990.84. [DOI] [PubMed] [Google Scholar]
- Adler S., Striker L. J., Striker G. E., Perkinson D. T., Hibbert J., Couser W. G. Studies of progressive glomerular sclerosis in the rat. Am J Pathol. 1986 Jun;123(3):553–562. [PMC free article] [PubMed] [Google Scholar]
- Anderson S., Meyer T. W., Rennke H. G., Brenner B. M. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1985 Aug;76(2):612–619. doi: 10.1172/JCI112013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border W. A., Noble N. A., Yamamoto T., Harper J. R., Yamaguchi Y. u., Pierschbacher M. D., Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992 Nov 26;360(6402):361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Languino L. R., Ruoslahti E. Transforming growth factor-beta regulates production of proteoglycans by mesangial cells. Kidney Int. 1990 Feb;37(2):689–695. doi: 10.1038/ki.1990.35. [DOI] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Languino L. R., Sporn M. B., Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990 Jul 26;346(6282):371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Nakamura T., Languino L. R., Ruoslahti E. Role of TGF-beta 1 in experimental glomerulonephritis. Ciba Found Symp. 1991;157:178–193. [PubMed] [Google Scholar]
- Border W. A., Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992 Jul;90(1):1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chansel D., Czekalski S., Pham P., Ardaillou R. Characterization of angiotensin II receptor subtypes in human glomeruli and mesangial cells. Am J Physiol. 1992 Mar;262(3 Pt 2):F432–F441. doi: 10.1152/ajprenal.1992.262.3.F432. [DOI] [PubMed] [Google Scholar]
- Coimbra T., Wiggins R., Noh J. W., Merritt S., Phan S. H. Transforming growth factor-beta production in anti-glomerular basement membrane disease in the rabbit. Am J Pathol. 1991 Jan;138(1):223–234. [PMC free article] [PubMed] [Google Scholar]
- Diamond J. R., Karnovsky M. J. Focal and segmental glomerulosclerosis: analogies to atherosclerosis. Kidney Int. 1988 May;33(5):917–924. doi: 10.1038/ki.1988.87. [DOI] [PubMed] [Google Scholar]
- Dzau V. J., Gibbons G. H. Vascular remodeling: mechanisms and implications. J Cardiovasc Pharmacol. 1993;21 (Suppl 1):S1–S5. [PubMed] [Google Scholar]
- Dzau V. J., Ingelfinger J. R. Molecular biology and pathophysiology of the intrarenal renin-angiotensin system. J Hypertens Suppl. 1989 Sep;7(7):S3–S8. doi: 10.1097/00004872-198909007-00002. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J., Alpers C. E., Burns M. W., Pritzl P., Gordon K., Couser W. G., Johnson R. J. Glomerular cells, extracellular matrix accumulation, and the development of glomerulosclerosis in the remnant kidney model. Lab Invest. 1992 Apr;66(4):485–497. [PubMed] [Google Scholar]
- Floege J., Burns M. W., Alpers C. E., Yoshimura A., Pritzl P., Gordon K., Seifert R. A., Bowen-Pope D. F., Couser W. G., Johnson R. J. Glomerular cell proliferation and PDGF expression precede glomerulosclerosis in the remnant kidney model. Kidney Int. 1992 Feb;41(2):297–309. doi: 10.1038/ki.1992.42. [DOI] [PubMed] [Google Scholar]
- Floege J., Topley N., Resch K. Regulation of mesangial cell proliferation. Am J Kidney Dis. 1991 Jun;17(6):673–676. doi: 10.1016/s0272-6386(12)80349-0. [DOI] [PubMed] [Google Scholar]
- Fogo A., Ichikawa I. Evidence for the central role of glomerular growth promoters in the development of sclerosis. Semin Nephrol. 1989 Dec;9(4):329–342. [PubMed] [Google Scholar]
- Gibbons G. H., Pratt R. E., Dzau V. J. Vascular smooth muscle cell hypertrophy vs. hyperplasia. Autocrine transforming growth factor-beta 1 expression determines growth response to angiotensin II. J Clin Invest. 1992 Aug;90(2):456–461. doi: 10.1172/JCI115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A. W., Resink T. J., Bernhardt J., Ferracin F., Bühler F. R. Stimulation of autocrine platelet--derived growth factor AA-homodimer and transforming growth factor beta in vascular smooth muscle cells. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1451–1458. doi: 10.1016/0006-291x(91)91056-i. [DOI] [PubMed] [Google Scholar]
- Ikoma M., Kawamura T., Kakinuma Y., Fogo A., Ichikawa I. Cause of variable therapeutic efficiency of angiotensin converting enzyme inhibitor on glomerular lesions. Kidney Int. 1991 Aug;40(2):195–202. doi: 10.1038/ki.1991.200. [DOI] [PubMed] [Google Scholar]
- Itoh H., Mukoyama M., Pratt R. E., Gibbons G. H., Dzau V. J. Multiple autocrine growth factors modulate vascular smooth muscle cell growth response to angiotensin II. J Clin Invest. 1993 May;91(5):2268–2274. doi: 10.1172/JCI116454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Iida H., Alpers C. E., Majesky M. W., Schwartz S. M., Pritzi P., Gordon K., Gown A. M. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991 Mar;87(3):847–858. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kifor I., Dzau V. J. Endothelial renin-angiotensin pathway: evidence for intracellular synthesis and secretion of angiotensins. Circ Res. 1987 Mar;60(3):422–428. doi: 10.1161/01.res.60.3.422. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Denhez F., Kim K. Y., Holt J. T., Sporn M. B., Roberts A. B. Activation of the second promoter of the transforming growth factor-beta 1 gene by transforming growth factor-beta 1 and phorbol ester occurs through the same target sequences. J Biol Chem. 1989 Nov 15;264(32):19373–19378. [PubMed] [Google Scholar]
- Lafayette R. A., Mayer G., Park S. K., Meyer T. W. Angiotensin II receptor blockade limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1992 Sep;90(3):766–771. doi: 10.1172/JCI115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie G. W., Horikoshi S., Killen P. D., Segui-Real B., Yamada Y. In situ hybridization reveals temporal and spatial changes in cellular expression of mRNA for a laminin receptor, laminin, and basement membrane (type IV) collagen in the developing kidney. J Cell Biol. 1989 Sep;109(3):1351–1362. doi: 10.1083/jcb.109.3.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. K., Pollock A. S., Lovett D. H. Asymmetric origins of the mature glomerular basement membrane. J Cell Physiol. 1993 Oct;157(1):169–177. doi: 10.1002/jcp.1041570122. [DOI] [PubMed] [Google Scholar]
- MacKay K., Kondaiah P., Danielpour D., Austin H. A., 3rd, Brown P. D. Expression of transforming growth factor-beta 1 and beta 2 in rat glomeruli. Kidney Int. 1990 Dec;38(6):1095–1100. doi: 10.1038/ki.1990.318. [DOI] [PubMed] [Google Scholar]
- MacKay K., Striker L. J., Stauffer J. W., Doi T., Agodoa L. Y., Striker G. E. Transforming growth factor-beta. Murine glomerular receptors and responses of isolated glomerular cells. J Clin Invest. 1989 Apr;83(4):1160–1167. doi: 10.1172/JCI113996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer G., Lafayette R. A., Oliver J., Deen W. M., Myers B. D., Meyer T. W. Effects of angiotensin II receptor blockade on remnant glomerular permselectivity. Kidney Int. 1993 Feb;43(2):346–353. doi: 10.1038/ki.1993.52. [DOI] [PubMed] [Google Scholar]
- Meyer T. W., Anderson S., Rennke H. G., Brenner B. M. Reversing glomerular hypertension stabilizes established glomerular injury. Kidney Int. 1987 Mar;31(3):752–759. doi: 10.1038/ki.1987.62. [DOI] [PubMed] [Google Scholar]
- Miller P. L., Meyer T. W. Effects of tissue preparation on glomerular volume and capillary structure in the rat. Lab Invest. 1990 Dec;63(6):862–866. [PubMed] [Google Scholar]
- Nagata M., Kriz W. Glomerular damage after uninephrectomy in young rats. II. Mechanical stress on podocytes as a pathway to sclerosis. Kidney Int. 1992 Jul;42(1):148–160. doi: 10.1038/ki.1992.272. [DOI] [PubMed] [Google Scholar]
- Nagata M., Schärer K., Kriz W. Glomerular damage after uninephrectomy in young rats. I. Hypertrophy and distortion of capillary architecture. Kidney Int. 1992 Jul;42(1):136–147. doi: 10.1038/ki.1992.271. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Miller D., Ruoslahti E., Border W. A. Production of extracellular matrix by glomerular epithelial cells is regulated by transforming growth factor-beta 1. Kidney Int. 1992 May;41(5):1213–1221. doi: 10.1038/ki.1992.183. [DOI] [PubMed] [Google Scholar]
- Nathan C., Sporn M. Cytokines in context. J Cell Biol. 1991 Jun;113(5):981–986. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S., Languino L. R., Ruoslahti E., Border W. A. Elevated expression of transforming growth factor-beta and proteoglycan production in experimental glomerulonephritis. Possible role in expansion of the mesangial extracellular matrix. J Clin Invest. 1990 Aug;86(2):453–462. doi: 10.1172/JCI114731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J. L., Heptinstall R. H. Nonimmunologic mechanisms of glomerular injury. Lab Invest. 1988 Nov;59(5):564–578. [PubMed] [Google Scholar]
- Rakugi H., Jacob H. J., Krieger J. E., Ingelfinger J. R., Pratt R. E. Vascular injury induces angiotensinogen gene expression in the media and neointima. Circulation. 1993 Jan;87(1):283–290. doi: 10.1161/01.cir.87.1.283. [DOI] [PubMed] [Google Scholar]
- Rennke H. G., Klein P. S. Pathogenesis and significance of nonprimary focal and segmental glomerulosclerosis. Am J Kidney Dis. 1989 Jun;13(6):443–456. doi: 10.1016/s0272-6386(89)80001-0. [DOI] [PubMed] [Google Scholar]
- Resnick N., Collins T., Atkinson W., Bonthron D. T., Dewey C. F., Jr, Gimbrone M. A., Jr Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4591–4595. doi: 10.1073/pnas.90.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Engvall E., Butkowski R., Hunter D. D. Molecular heterogeneity of basal laminae: isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J Cell Biol. 1990 Oct;111(4):1685–1699. doi: 10.1083/jcb.111.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariola H., Timpl R., von der Mark K., Mayne R., Fitch J. M., Linsenmayer T. F., Ekblom P. Dual origin of glomerular basement membrane. Dev Biol. 1984 Jan;101(1):86–96. doi: 10.1016/0012-1606(84)90119-2. [DOI] [PubMed] [Google Scholar]
- Schunkert H., Ingelfinger J. R., Jacob H., Jackson B., Bouyounes B., Dzau V. J. Reciprocal feedback regulation of kidney angiotensinogen and renin mRNA expressions by angiotensin II. Am J Physiol. 1992 Nov;263(5 Pt 1):E863–E869. doi: 10.1152/ajpendo.1992.263.5.E863. [DOI] [PubMed] [Google Scholar]
- Schwartz M. M., Bidani A. K. Comparison of glomerular injury in juvenile versus mature rats in a remnant kidney model. J Lab Clin Med. 1993 Feb;121(2):348–355. [PubMed] [Google Scholar]
- Stouffer G. A., Owens G. K. Angiotensin II-induced mitogenesis of spontaneously hypertensive rat-derived cultured smooth muscle cells is dependent on autocrine production of transforming growth factor-beta. Circ Res. 1992 Apr;70(4):820–828. doi: 10.1161/01.res.70.4.820. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel G. B., Abrahamson D. R. Quantitation and localization of laminin A, B1, and B2 chain RNA transcripts in developing kidney. Am J Physiol. 1993 Aug;265(2 Pt 2):F293–F299. doi: 10.1152/ajprenal.1993.265.2.F293. [DOI] [PubMed] [Google Scholar]
- Waldherr R., Gretz N. Natural course of the development of histological lesions after 5/6 nephrectomy. Contrib Nephrol. 1988;60:64–72. doi: 10.1159/000414791. [DOI] [PubMed] [Google Scholar]
- Walpola P. L., Gotlieb A. I., Langille B. L. Monocyte adhesion and changes in endothelial cell number, morphology, and F-actin distribution elicited by low shear stress in vivo. Am J Pathol. 1993 May;142(5):1392–1400. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Nakamura T., Noble N. A., Ruoslahti E., Border W. A. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]