Abstract
Recognition of the −10 promoter consensus element by region 2 of the bacterial RNA polymerase σ subunit is a key step in transcription initiation. σ also functions as an elongation factor, inducing transcription pausing by interacting with transcribed DNA non-template strand sequences that are similar to the −10 element sequence. Here, we show that the region 1.2 of Escherichia coli σ70, whose function was heretofore unknown, is strictly required for efficient recognition of the non-template strand of −10-like pause-inducing DNA sequence by σ region 2, and for σ-dependent promoter-proximal pausing. Recognition of the fork-junction promoter DNA by RNA polymerase holoenzyme also requires σ region 1.2 and thus resembles the pause-inducing sequence recognition. Our results, together with available structural data, support a model where σ region 1.2 acts as a core RNA polymerase-dependent allosteric switch that modulates non-template DNA strand recognition by σ region 2 during transcription initiation and elongation.
Keywords: formaldehyde crosslinking, promoter melting, RNA polymerase, sigma70 region 1.2, transcription pausing
Introduction
Initiation of transcription by RNA polymerase (RNAP) requires local melting of DNA around the transcription start site. In bacterial cells, a single auxiliary factor, the RNAP σ subunit, directs promoter recognition and opening (Gross et al, 1998; Helmann and deHaseth, 1999). In Escherichia coli, the σ70 subunit is responsible for transcription initiation from most promoters during exponential growth. During transcription initiation, the conserved regions 2 and 4 of σ70 recognize the −10 and −35 promoter elements, respectively. Specific recognition of the non-template strand of the −10 promoter element (consensus sequence 5′-T−12A−11T−10A−9A−8T−7-3′) by σ70 is required for localized melting of promoter DNA (Juang and Helmann, 1994; Roberts and Roberts, 1996; Marr and Roberts, 1997; Panaghie et al, 2000; Young et al, 2001). However, free σ70 does not bind promoter DNA. A coiled-coil domain of the RNAP β′ subunit induces a conformational change in σ70 that allows DNA binding and −10 element recognition by σ region 2 (Callaci et al, 1999; Kulbachinskiy et al, 1999; Young et al, 2001). Detailed mechanism of this conformational change is not known.
Recent studies have shown that σ70 can be retained in early transcription elongation complexes (TECs) in vitro (Bar-Nahum and Nudler 2001; Kapanidis et al, 2005) and in vivo (Raffaelle et al, 2005). This retention occurs when RNAP transcribes through a promoter-proximal sequence resembling the −10 promoter element. In these conditions, σ70 ‘hops' from its initial promoter location and binds the non-template strand of the −10-like sequence, inducing a promoter-proximal pause (Ring et al, 1996; Brodolin et al, 2004; Nickels et al, 2004). The phenomenon of σ70-dependent promoter-proximal pausing reveals that single-stranded DNA binding activity of σ70 region 2, which is strictly essential for promoter DNA recognition and melting, may hinder promoter escape and/or transcription elongation and therefore needs to be suppressed after the open promoter complex has been formed (Chan and Gross, 2001). In support of this idea, crosslinking experiments demonstrate that some σ70 interactions with the −10 promoter element are lost after full-length transcription bubble in the open promoter complex is established (Buckle et al, 1999; Brodolin et al, 2005).
To uncover the mechanism that regulates recognition of the non-template strand of the −10 element during transcription initiation and pausing, we developed a ‘σ-exchange' assay that allows to exchange σ70 bound to paused elongation complexes with σ70 mutants. This assay bypasses the promoter recognition/opening steps and therefore allows one to study σ–TEC interactions even for σ derivatives that are inactive in transcription initiation. We analyzed interactions of different σ70 fragments with paused TEC and found that σ70 region 1.2 enhances intrinsic sequence-specific affinity of σ70 region 2 to single-stranded −10 promoter element DNA in the context of TEC. We propose that modulation of σ region 2 interactions with single-stranded DNA by region 1.2 is part of a regulatory mechanism that operates during transcription initiation and elongation. This mechanism allows RNAP to relinquish initial strong interactions with the −10 element DNA and escape into productive elongation.
Results
σ70 region 2 crosslinks to guanine at position +5 of promoter-proximal pause-inducing sequence
We used formaldehyde crosslinking to identify close σ70-DNA contacts in the transcription bubble of TEC paused at the lacUV5 promoter positions +16/17. Previously, we localized a σ70 crosslink in paused TEC16/17 to between positions +4 and +6 of the non-template DNA strand (Brodolin et al, 2004). Substitution of the non-template guanine at position +5 to adenine (Figure 1A) has no effect on promoter-proximal pause (Figure 1B), but selectively abolishes σ70-DNA crosslink (the β′-DNA crosslink is unaffected by this substitution) (Figure 1C). Thus, G+5 crosslinks to σ70 in TEC16/17 and is also the only base that is crosslinked to σ70 by formaldehyde in TEC16/17.
Figure 1.
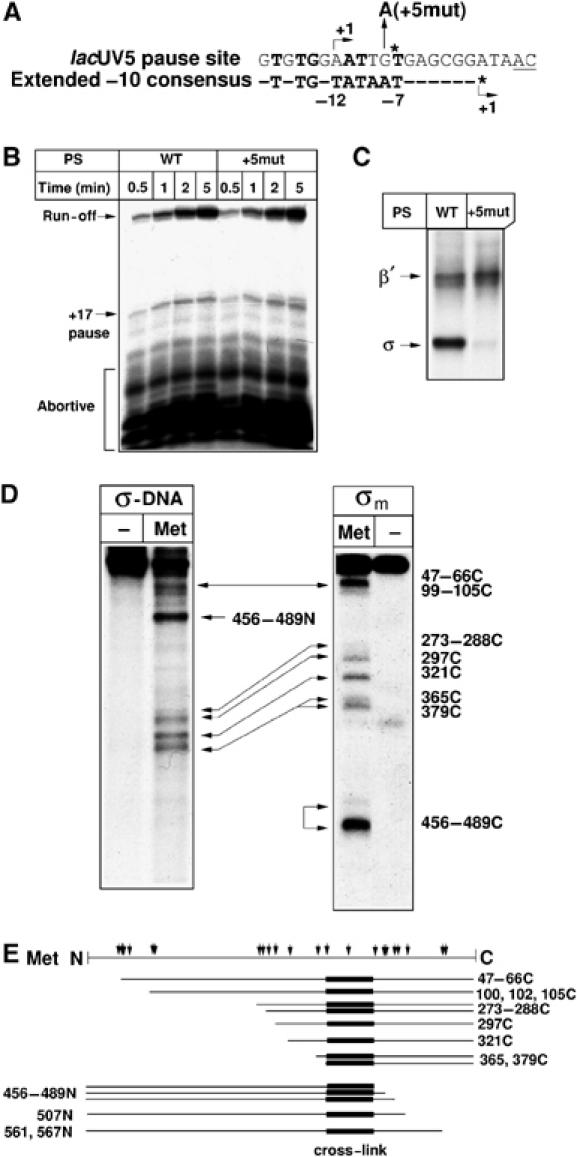
Mapping of the σ-DNA crosslink in paused TEC. (A) The lacUV5 pause-inducing sequence and the −10 promoter element consensus sequence (Keilty and Rosenberg, 1987). Matches to consensus are indicated in bold typeface. The +5G to A substitution is indicated (+5mut). An asterisk marks a position (+6T), which when substituted to A abolishes pausing (Brodolin et al, 2004). Transcription start sites (+1) are shown by arrows. (B) An autoradiogram of a denaturing gel showing 32P-labeled RNA products produced during run-off transcription from the lacUV5 promoter fragments containing either WT or mutant (+5mut) pause-inducing sequence (PS). (C) Formaldehyde crosslinking of the +17 paused complexes formed at the WT or mutant (+5mut) 32P-labeled lacUV5 promoter DNA. Crosslinked σ70-DNA and β′-DNA complexes are labeled as σ and β′. (D) Mapping of the crosslinking site in the σ70 subunit crosslinked to 32P-labeled lacUV5 DNA in TEC16 by Met-specific chemical cleavage (σ-DNA). The panel on the right (σm) shows Met-specific cleavage of the σ70 subunit 32P-labeled at the C terminus. Continuous lines with arrows between panels σm and σ-DNA connect bands corresponding to the identical cleavage products. Positions of σ70 Met residues, which when cleaved give rise to observed cleavage products, are indicated on the right side of the figure. Suffixes C or N refer to C- or N-terminal cleavage product, respectively. (E) The diagram shows the map of the σ70 subunit; the positions of Met cleavage sites are indicated by arrows. 32P-labeled peptides produced by cleavage at indicated σ70 Met residues are shown beneath as lines. σ70 region containing the crosslink site to DNA is indicated by a black bar.
The site of σ70 that crosslinks to G+5 was mapped using single-hit Met-specific chemical cleavage of σ70 crosslinked to radioactively labeled DNA (Grachev et al, 1989; Brodolin et al, 2000). In a control reaction, σ70 subunit affinity-labeled between the Met508 and Met561 by 32P-labeled ribonucleotide (Severinov et al, 1994) was used. Incubation with the cleaving reagent CNBr resulted in the appearance of a number of radioactive bands whose mobility on SDS–PAGE was higher than that of the starting crosslinked material (Figure 1D and E). These bands correspond to either N- or C-terminal products of Met-specific cleavage of the crosslinked σ70 subunit. In the case of the control (Figure 1D, panel ‘σm'), radioactive cleavage products corresponded to C-terminal cleavage products and the shortest labeled peptides resulting from cleavage at Met456−489 were clearly visible (labeled 456–489C in Figure 1D). Cleavage of the crosslinked σ-DNA complex (Figure 1D, panel ‘σ-DNA'; Supplementary Figure S1) resulted in a similar pattern of bands arising from cleavages in the central part of the σ70 (Met297–Met379), but bands corresponding to C-terminal cleavage products at Met456−489 were not observed. Instead, a strong band, whose mobility is consistent with that expected for N-terminal product of cleavage at Met456−489 (labeled 456–489N in Figure 1D), was observed. This indicates that the site of crosslinking to DNA is located N-terminal to Met456−489 but C-terminal to Met365,379, as bands corresponding to C-terminal cleavage products at these positions were present in both ‘σ-DNA' and control ‘σm' reactions. The region of σ70 between Met365,379 and Met456−489 includes the entire conserved region 2 of σ70 known to be involved in specific interaction with the −10 element DNA.
To additionally confirm the mapping result, we used partial degradation of crosslinked σ70-DNA complexes with Cys- and Trp-specific chemical proteases (Supplementary Figure S1). The results were consistent with inferences made from Met-specific degradation, and indicated that a residue(s) of σ70 region 2 is within a 2-Å distance from G+5 of the pause-inducing sequence, and that region 2 is the only σ70 region that crosslinks to the non-template DNA strand in TEC16/17.
σ-exchange assay
As promoter-proximal pausing involves ‘hopping' of σ from the promoter to the pause-inducing site, σ70 must transiently relinquish its contacts with DNA at the promoter and then establish new contacts at the pause site. At least some of the contacts with the core should also be broken and then re-established during this process. The σ-exchange assay relies on the ability of exogenously added σ to bind to σ-less TEC stalled in the vicinity of promoter-proximal pause (Figure 2A). As shown previously (Brodolin et al, 2004), ∼50% of transcription complexes stalled at position +16 of the lacUV5 promoter by CTP deprivation retained σ and, upon the addition of full complement of NTPs, paused at position +17 owing to σ70 interactions with the pause-inducing sequence (Figure 2B, lanes 1–4). An additional pause at +18 was also observed, as reported previously (Nickels et al, 2004). Upon σ70 removal by treating the initial TEC16 with heparin, the +17/+18 pause disappeared and the full-sized run-off transcript was observed upon the addition of NTPs (Figure 2B, lanes 5–8). Importantly, the +17 pause was restored upon addition of σ70 (Figure 2B, compare lanes 2–4 and 9–11), indicating that exogenous σ70 binds stalled TEC and induces a promoter-proximal pause, presumably through interactions with the −10 element-like sequence.
Figure 2.
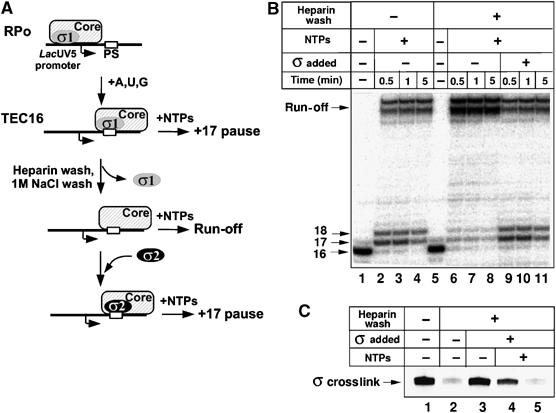
σ-exchange assay using immobilized +16 paused complexes. (A) A scheme demonstrating the principle of ‘σ-exchange' experiments on the lacUV5 promoter-proximal pause. The pausing site (PS) is marked by an open rectangle. Promoter open complex (RPo) and paused elongation complex (TEC16) are shown. An exchange reaction between σ originated from the promoter complex (σ1 in gray) and exogenously added σ (σ2 in black) is illustrated. (B) 32P-labeled RNA transcripts produced by σ70-containing (lanes 1–4 and 9–11) and σ70-less (lanes 5–8) TEC16 immobilized on Ni2+-NTA beads. Transcription complexes were chased by the addition of NTPs and 0.5, 1, or 5-min incubation either before (lanes 2–4) or after heparin wash (lanes 6–11). Lanes 9–11: σ70-less complexes were supplemented with exogenous σ70 (500 nM final concentration) before chase. (C) Crosslinking of the σ70 subunit to 32P-labeled lacUV5 DNA in immobilized TEC16 either before (lane 1) or after (lanes 2–5) heparin wash. Complexes were supplemented with exogenous σ70 (lanes 3–5). NTPs were added before (lane 5) and after (lane 4) the addition of σ70. Crosslinked σ70–DNA complexes resolved on SDS–PAGE are shown (labeled as σ-crosslink).
Crosslinking experiments showed that incubation of heparin-washed TECs with excess σ70 for 5 min at 37°C resulted in the appearance of σ-DNA crosslinks (Brodolin et al, 2004, Figure 2C, lane 3). The σ70-DNA crosslink was also observed when σ70-less TEC16 supplemented with σ70 was incubated with NTPs for 2 min before crosslinking (Figure 2C, lane 4). The result is consistent with the results of transcription experiment that showed that a promoter-proximal pause is formed at these conditions (Figure 2B, lanes 9–11). In contrast, no crosslink was observed when NTPs were added before the addition of σ70 (Figure 2C, lane 5), further supporting an idea that σ70-DNA crosslink in TEC16/17 depends on σ70 interactions with the pause-inducing sequence (see also above). We therefore conclude that TEC16 recruits exogenously added σ70, and that the newly bound σ70 interacts with DNA and blocks RNAP escape into productive elongation. These results strongly suggest that externally added σ70 interacts with the pause-inducing sequence and RNAP core in the same way as ‘endogenous' σ70 that originates from a promoter complex and causes promoter-proximal pausing. These observations form the basis of a ‘σ-exchange' assay, in which different σ70 mutants are tested for their ability to bind σ-less TEC16 and induce promoter-proximal pausing.
Region 1.2 of σ70 is essential for −10-like pause-inducing sequence recognition
To determine σ70 regions that are essential for interaction with TEC16/17, a series of recombinant σ70 fragments was prepared (Figure 3A). The boundaries of these fragments were selected such that structural domains of σ70, as defined by biochemical and crystallographic studies (Malhotra et al, 1996; Severinova et al, 1996; Campbell et al, 2002), remained intact. The σ70 fragments used lacked the N-terminal region 1.1 and parts of region 1.2 (σ2−4, amino acids 102–613) or C-terminal regions 3 and 4 (σ1−2, amino acids 1–448). In addition, a fragment missing both N-terminal regions and C-terminal region 4.2 (σ2−3, amino acids 102–574) was created. The shortest fragment used in our experiments, σ2a (amino acids 102–448), corresponds to the previously characterized fragment of σ70, whose structure has been solved and that binds the RNAP core, forming a complex that recognizes the −10 promoter element in a single-stranded form (Malhotra et al, 1996; Severinova et al, 1996; Marr and Roberts, 1997). As deletion of N-terminal 101 amino acids of σ70 disrupts region 1.2, we also prepared an additional fragment σ2b (amino acids 94–448) that has this region intact. None of the σ fragments studied was capable of directing promoter-dependent transcription by the core (data not shown).
Figure 3.
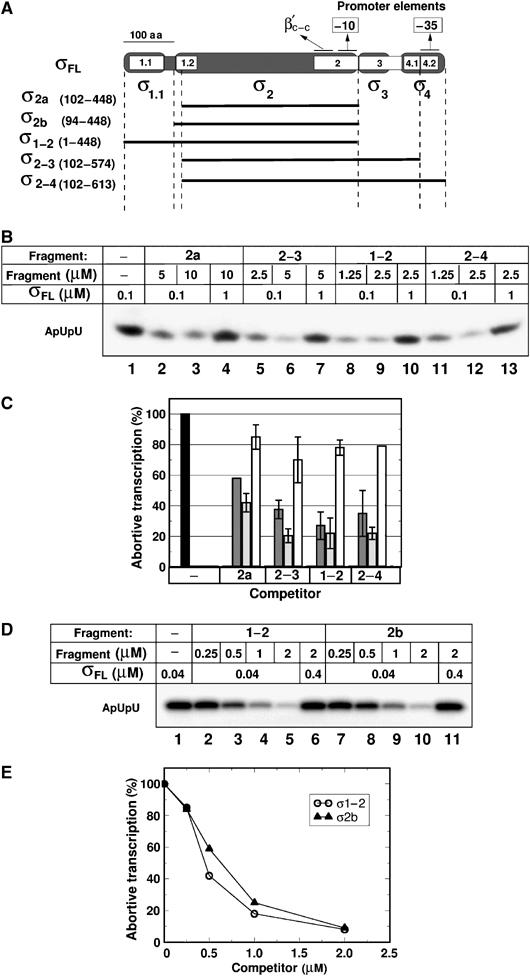
σ70 fragments used and their binding to RNAP core. (A) A scheme of σ70; the universally conserved regions are shown in white and are numbered. The structural domains of σ (σ1.1, σ2, σ3, and σ4; Campbell et al, 2002) are indicated beneath. σ fragments used in this work are shown as simple lines below the σ70 scheme. Contact sites with the −10 and −35 promoter elements and the β′ coiled-coil (β′c–c) are indicated. (B, D) Inhibition of σ70-dependent abortive transcription initiation reaction from the lacUV5 promoter by σ70 fragments. Concentrations of σ70 and its fragments are indicated at the top of the panel. Reactions contained either 50 nM (panel B) or 10 nM (panel D) of RNAP core. 32P-labeled abortive ApUpU RNA product is marked. (C) Quantification of experimental data presented in panel B. The amount of 32P-labeled RNA synthesized in the presence of σ fragments was normalized to the amount synthesized without fragments added (black bar at the left). Gray bars correspond to lanes 2, 5, 8, and 11 of panel B. Light gray bars correspond to lanes 3, 6, 9, and 12. White bars correspond to lanes 4, 7, 10, and 13. Mean values and s.d. from two independent experiments are shown. (E) Quantification of experimental data presented in panel D. The amount of 32P-labeled abortive product synthesized in the presence of indicated concentrations of σ fragments is shown.
The σ-exchange assay is based on σ ability to bind core RNAP in the context of transcription elongation complex. Thus, for every σ fragment, it was essential to determine a concentration range that will allow efficient binding to the core. The relative affinities of σ fragments to RNAP core were measured by determining their ability to competitively inhibit σ70-dependent abortive initiation (Severinova et al, 1996; Sharp et al, 1999), as well as by the native gel analysis, where the formation of RNAP holoenzyme from the core and σ or its fragments is monitored directly (Figure 3 and Supplementary Figure S2). Fragments σ1−2, σ2−4, and σ2b efficiently bound the core, whereas σ2a and σ2−3 bound poorly (Figure 3B and D). In agreement with previous results (Severinova et al, 1996), a 50-fold molar excess of σ2a and σ2−3 over wild-type σ70 inhibited transcription by about 50–70% (Figure 3B, lanes 2, 3, 5, 6 and panel C). The addition of increased amounts of σ70 overcame the inhibition (Figure 3B, lanes 4 and 7), indicating that these fragments retained the binding specificity but lost their affinity. The results also indicate that region 1.1 does not contribute to the strength of σ70–core interactions, as σ1−2 and σ2b bind core with similar affinity (Figure 3D and E). On the other hand, the poor binding of σ2a shows that region 1.2 amino acids 94–101 are important for efficient binding to the core. However, the presence of region 4 in the σ2−4 fragment allows efficient core binding even in the absence of these amino acids (Figure 3B, compare lanes 8, 9 and 11, 12). Thus, both regions 1.2 and 4 of σ appear to independently contribute to the interaction with the RNAP core.
To define regions of σ70 required for pause-inducing sequence recognition, we used the fragments described above in the σ-exchange assay. The exchange was followed by the appearance of crosslinks between σ fragments and promoter DNA. The concentrations of σ fragments were adjusted to allow equal efficiency of complex formation with the RNAP core. As can be seen, σ1−2 fragment was crosslinked to pause-inducing sequence as efficiently as σ70, whereas fragments σ2a, σ2−3, and σ2−4 were not crosslinked (Figure 4A). Therefore, the N-terminal 101 amino acids of σ70, which include the entire region 1.1 and a portion of region 1.2, are necessary for recognition of the pause-inducing sequence, whereas the C-terminal regions 3 and 4 are dispensable. In contrast to σ2a, the σ2b fragment (lacking 93 N-terminal amino acids, which constitute the entire region 1.1, but having intact region 1.2) recognized the pause site as efficiently as σ1−2 (Figure 4B, compare lanes 3 and 4). Efficient crosslinking was also observed when this fragment was added to σ-less TEC16 complexes before the addition of NTPs (Figure 4B, lanes 5 and 6), whereas no crosslinking was observed when NTPs were added before σ2b (Figure 4B, lanes 8 and 9). Therefore, σ2b blocked RNAP escape from the pause site with the same efficiency as σ1−2. Thus, region 1.2 amino acids 94–101 are essential for formation of a contact between σ region 2 and the −10-like pause-inducing sequence and formation of a promoter-proximal pause.
Figure 4.
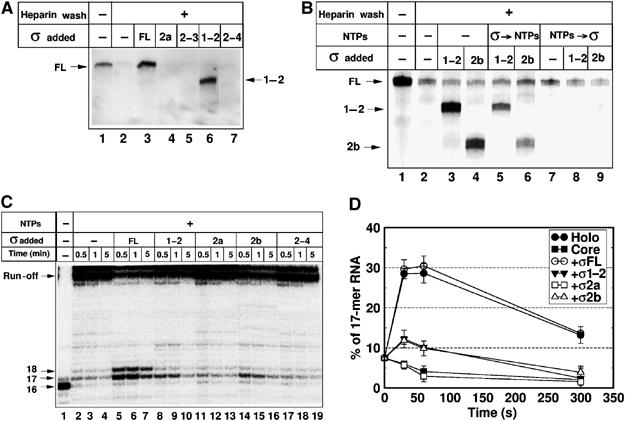
Binding of the exogenously added σ70 fragments to paused TEC16. (A) Crosslinking of TEC16 complexes to 32P-labeled lacUV5 DNA before (lane 1) and after (lanes 2–7) heparin wash. Heparin-washed σ70-less complexes were supplemented with 0.5 μM of wild-type σ70 (FL) or fragments: 5 μM of σ2a, 3 μM of σ2−3, 1.5 μM of σ1−2, and 2.5 μM of σ2.4 (lanes 3–7). Crosslinked complexes were resolved by SDS–PAGE and revealed by autoradiography. Crosslinked subunits are indicated. (B) Crosslinking of TEC16 complexes washed with heparin (lanes 2–9) and supplemented with 1.5 μM of σ2b or σ1−2 (lanes 3–6, 8, and 9). NTPs were added before (lanes 7–9) or after (lanes 5 and 6) the addition of σ70 fragments. Lane 1: TEC16 before heparin wash. Reaction products were analyzed as in panel A. (C) 32P-labeled RNA transcripts produced upon addition of NTPs to immobilized TEC16. Complexes were chased by the addition of NTPs for 0.5, 1, and 5 min. Lanes 5–19: complexes were supplemented with wild-type σ70 (FL) or indicated σ70 fragments before addition of NTPs. (D) Quantification of the results of experiment shown in panel C. The amount of +17 RNA was calculated as percentage of the initial amount of the starting 16-mer RNA present before the addition of NTPs. Mean values and s.d. from two independent experiments are shown.
To directly show that σ70 residues 94–101 are required for promoter-proximal pausing, we compared the ability of σ1−2, σ2a, σ2b, and σ2−4 fragments to induce pausing at the +17 position (Figure 4C). Heparin-washed TEC16 were incubated with σ70 fragments, supplemented with NTPs, and RNA products were analyzed at different time points after NTP addition (0.5, 1, and 5 min). As can be seen, σ1−2 and σ2b induced a pause at +17 (Figure 4C, lanes 8–10 and 14–16), whereas fragments lacking the first 101 amino acids did not (Figure 4C, lanes 11–13 and 17–19). Noticeably, the +18 pause that was observed upon addition of σ70 was not observed with the fragments (Figure 4C, compare lanes 5–7 and 8–10). The reason(s) for this difference was not further investigated. Quantification revealed that in the case of σ70 fragments, a fraction of TEC16 retained in the +17 pause during the first 60 s of the chase was ∼30% of that observed with full-length σ70 (Figure 4D). This lower efficiency of pausing is likely caused by lower affinity of σ701−2 and σ702b for the elongation complex, either RNAP core or DNA (or both). In summary, we conclude that σ70 region 1.2 amino acids 94–101 are required for promoter-proximal pausing, whereas σ70 region 1.1 (amino acids 1–93) is dispensable.
Disruption of region 1.2 abolishes a conformational switch in σ70
Specific recognition of the non-template DNA strand of the −10 promoter element is accomplished by residues of σ region 2 and requires a conformational transition in σ induced by the β′ subunit coiled-coil (Callaci and Heyduk 1998; Young et al, 2001). A plausible hypothesis is that deletion of region 1.2 residues 94–101 abolishes such a transition and therefore makes specific binding to single-stranded −10 element DNA impossible. To test this model, we performed UV crosslinking of RNAP holoenzymes containing σ70 or σ fragments with an oligonucleotide corresponding to the non-template strand of the −10 promoter element (−10 oligo) of the lacUV5 promoter. σ70 effectively crosslinks to the −10 oligo in the context of the holoenzyme, whereas isolated σ70 crosslinks weakly (Marr and Roberts, 1997; Kulbachinskiy et al, 1999; Young et al, 2001). The ‘activation effect' of RNAP core on the interaction of σ70 or its fragments with the −10 oligo was measured as a ratio of the amount of DNA crosslinked to σ70 or its fragments in the presence or absence of the core (Figure 5A and B). At concentrations less than 500 nM, σ1−2 and σ2b exhibited a ∼10-fold activation effect comparable with that observed with σ70, whereas only two-fold activation by σ2a was observed, indicating that region 1.2 is required for −10 oligo recognition in the context of the holoenzyme. At higher concentrations (>1.5 μM), efficient crosslinks between the −10 oligo and σ70 or its fragments were observed even in the absence of RNAP core. Quantitative analysis demonstrated that free σ2a and σ1−2 bound the −10 oligo with similar affinities (Supplementary Figure S3), suggesting that N-terminal 101 amino acids do not affect region 2 affinity for single-stranded DNA in the context of free σ. The crosslinks resulted from sequence-specific interactions, as they were not observed with control oligonucleotide corresponding to the −10 element template strand (Figure 5C, lanes 5–12). Therefore, region 2 of σ70 specifically recognizes the non-template strand of the −10 promoter element in the absence of RNAP core, albeit with low efficiency, and this interaction does not depend on region 1.2. However, only when region 1.2 is present σ70 can undergo a core-dependent conformational change(s) that leads to increased affinity of region 2 for the −10 element single-stranded DNA.
Figure 5.
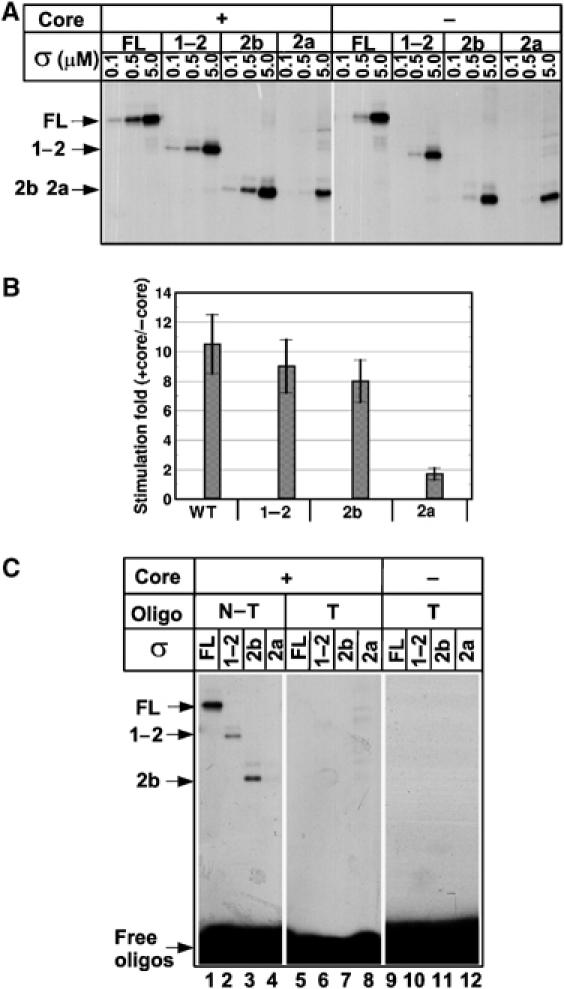
Probing of the interactions between σ70 or its fragments and the-10 oligonucleotide using UV crosslinking assay. (A) Crosslinking of the −10 non-template oligonucleotide with the wild-type σ70 (FL) or σ70 fragments (1–2, 2b, and 2a) present at the indicated concentrations in the absence or in the presence of 20 nM of RNAP core. Crosslinked complexes were resolved by 8% SDS–PAGE. (B) Quantification of data from panel A obtained in the presence of 0.1 μM σ70 or its fragments ‘Stimulation fold' is a ratio of crosslinking signals observed in presence and in the absence of RNAP core. Mean values and standard deviations from three independent experiments are shown. (C) Crosslinking of the non-template (NT) (lanes 1–4) and control template (T) (lanes 5–12) −10 element oligonucleotides with RNAP holoenzymes (lanes 1–8) containing either wild-type σ70 (FL) or indicated σ70 fragments. Lanes 9–12: crosslinking of σ70 and σ70 fragments without core RNAP. The samples contained 0.5 μM (lanes 1–4) or 5 μM of σ (lanes 5–12).
Region 1.2 is required for fork-junction DNA binding
Two recently published studies suggested that σ70 region 1.2 interacts sequence specifically with the non-template DNA downstream of the −10 element in the context of open promoter complex (Feklistov et al, 2006; Haugen et al, 2006). Thus, deletion of σ70 residues 94–101 may abolish the −10 element recognition by disrupting this interaction. To explore this possibility, we used a fork-junction DNA template that mimic interactions of RNAP with upstream part of the transcription bubble within the open promoter complex (Guo and Gralla, 1998).
The −10 promoter element bases −7 and −11, that are critical for formation of stable RNAP holoenzyme fork-junction DNA complexes (Guo and Gralla, 1998; Matlock and Heyduk, 2000; Fenton and Gralla 2001), correspond to promoter-proximal pause-inducing sequence bases +2 and +6 that are critical for pause formation (see Figure 1A; Ring et al, 1996; Brodolin et al, 2004). To increase a similarity between fork-junction DNA complex and complex paused at promoter-proximal pause, we used a fork-junction template that lacked a −35 promoter element sequence but contained an extended −10 element sequence corresponding to pause-inducing sequence of lacUV5 (Figure 6A). Formaldehyde crosslinking experiments revealed that fork-junction DNA efficiently crosslinked to σ70 in context of the holoenzyme, whereas no crosslinks were observed with RNAP core or free σ70 (Figure 6B, lanes 1 and 2 and data not shown). The crosslink was formed when a G residue was present at position −8 of the −10 element. Substitution of this G by C abolished the crosslink (Figure 6B, lane 3), but had no effect on RNAP holoenzyme affinity for the fork-junction DNA (data not shown). Taking into account that formaldehyde specifically targets unpaired guanines (Brodolin et al, 2000; K Brodolin, unpublished results), the result strongly suggests that G−8 is crosslinked to the σ70 in the fork-junction DNA–RNAP σ70 holoenzyme complex. Recall that a corresponding guanine at position +5 located in the pause-inducing sequence was also crosslinked to σ70 in TEC16 (Figure 1A and C). Therefore, the process of recognition of the −10 element in the context of fork-junction DNA complexes resembles the recognition of pause-inducing sequence in the context of TEC, and is therefore expected to be dependent on σ region 1.2. The requirement for region 1.2 should be independent of its recently proposed direct role in DNA binding (Feklistov et al, 2006; Haugen et al, 2006), as the non-template DNA strand of the fork-junction template ends at position −7 and hence does not contain region 1.2 binding site.
Figure 6.
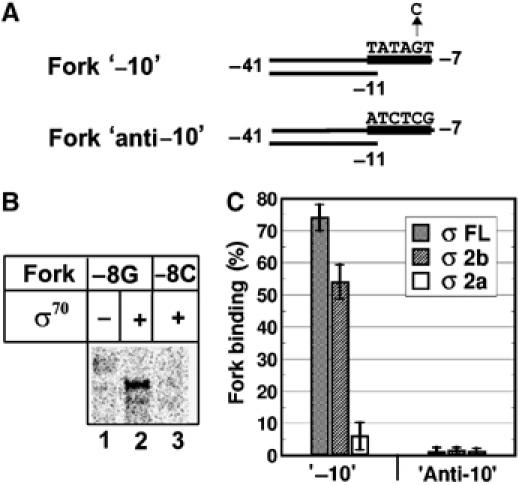
Interaction of fork-junction templates with holoenzymes containing the wild-type σ70 subunit or σ fragments. (A) A scheme of the fork-junction templates. The positions of −10 elements are marked by black rectangle and corresponding DNA sequences are shown. Arrow indicates substitution of the −8G to C. (B) Crosslinking of the core RNAP or σ70 containing holoenzyme to fork DNA. Templates contained at position −8 either G (−8G) or C (−8C). Crosslinked complexes were resolved by SDS–PAGE and revealed by autoradiography. (C) Complex formation between RNAP and forks bearing −10 element (‘−10') or anticonsensus −10 sequence (‘anti-10') was detected by nitrocellulose filtration method as described in Materials and methods. Binding is shown as percentage of total DNA in the sample. Mean values and s.d. from two independent experiments are shown.
To explore the role of region 1.2 in fork-junction DNA binding, we analyzed interactions of RNAP holoenzymes containing fragments σ2a or σ2b with fork-junction DNA using a filter binding assay (Figure 6C). Preliminary experiments showed that recognition of fork-junction DNA by the σ70 holoenzyme strictly depended on the presence of the −10 element, as fork-junction templates containing the anticonsensus sequence instead of the −10 element sequence were not bound by RNAP (Figure 6C, ‘anti-10'). Furthermore, holoenzymes containing σ70 or the σ2b fragment efficiently bound fork-junction DNA, whereas binding by the holoenzyme containing σ2a was much weaker (5–10% of that observed with σ70 or σ2b holoenzymes, Figure 6C). Free σ subunit or its fragments did not bind fork-junction DNA, as expected. These results show that (1) RNAP binding to the −10 element in the context of fork DNA template is stimulated by region 1.2 and (2) the stimulation is independent of region 1.2 interactions with DNA downstream of the −10 element.
Discussion
Recognition of the −10 element non-template DNA
Our study of interactions between the bacterial RNAP σ70 subunit and transcription elongation complex revealed that σ70 region 1.2 amino acids 94–101 are required for specific interaction with the −10 promoter element (or −10-like pause-inducing sequence) non-template strand in the context of RNAP holoenzyme. On the other hand, region 1.2 is not required for specific, but low-affinity binding to single-stranded −10 promoter element DNA by free σ. Therefore, region 1.2 does not interact with the −10 element DNA by itself, but modulates the recognition of the −10 element by the σ subunit region 2 indirectly, that is, allosterically. We propose that region 1.2 stabilizes a conformation of region 2 that is required for optimal binding of the −10 element. One known allosteric effector that activates region 2 ability to bind and melt DNA is the β′ coiled-coil element (Young et al, 2001, 2004). We suggest that region 1.2 is also part of this activation mechanism that functions during transcription initiation and promoter-proximal pausing.
Our interpretation is supported by available structures of RNAP holoenzymes and promoter complexes. In Figure 7, a model of the transcription complex paused at a promoter-proximal site is presented. The model is based on the crystal structure of the Thermus aquaticus RNAP holoenzyme complexed with a fork-junction DNA template (Murakami et al, 2002a). The model takes into consideration the following data. First, our crosslink mapping data show that σ region 2 contacts the non-template G+5 in paused TEC16. Second, we assume, based on mutagenesis and biochemical data, that RNAP complex with fork-junction DNA mimic the σ-dependent interactions of RNAP with the pause-inducing sequence. These considerations suggest that the DNA melting region 2.3 of σ that contacts the position −8 of the fork-junction DNA also contacts (and crosslinks to) the +5 position of the paused complex (Figures 6 and 7B). Our results show that formation of this contact is strictly dependent on region 1.2. According to the structure, there are no direct interactions between region 1.2 and the −10 element (Figure 7B). Furthermore, the fork-junction DNA complex is devoid of the non-template DNA strand downstream of the −10 element that might interact with region 1.2 in E. coli RNAP promoter complex (Haugen et al, 2006) or T. aquaticus σA–aptamer complex (Feklistov et al, 2006). Thus, a direct binding of region 1.2 to DNA is not required for core RNAP-dependent recognition of the −10 element.
Figure 7.
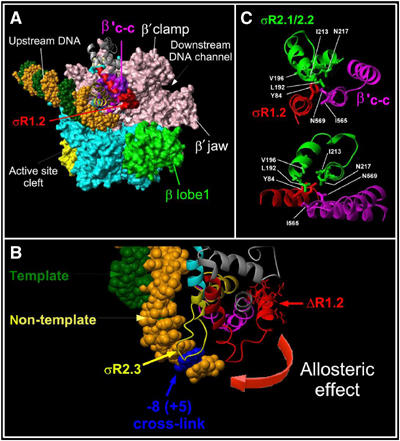
Modeling of the σ2b fragment interactions with RNAP core on the crystallographic structures of T. aquaticus and T. thermophilus RNAP. (A) The structure of T. thermophilus RNAP (PDB accession number 1IW7) fitted with the T. aquaticus RNAP structure in the complex with fork-junction DNA (PDB accession number 1L9Z). RNAP is shown as a molecular surface and DNA is shown as a CPK structure with the non-template strand in orange and the template strand in green. The large non-conserved domain present in Thermus β′ is omitted for clarity. The σ2b fragment is shown as ribbons with region 1.2 amino acids 94–101 shown as a red molecular surface. RNAP core subunits are colored as follows: β′ in rose and β in cyan; the β′ coiled-coil in violet. The β lobe that interacts with σ region 1.1 and forms the upper jaw is shown in green. (B) Modeling of σ crosslink in TEC16 on the T. aquaticus RNAP and fork-junction DNA complex structure. Color codes are as in panel A. The position corresponding to crosslinked +5G is shown in blue. (C) Interactions between σ region 1.2 (red) and 2.1–2.2 (green) and the β′ coiled-coil (magenta) in the T. thermophilus RNAP holoenzyme structure. Two projections are shown. The molecular modeling and figures were acquired using MOLMOL package (Koradi et al, 1996).
As our data clearly indicate that region 1.2 is required for this recognition, region 1.2 may modulate the DNA-binding affinity of the region 2.3 through protein–protein interactions. Indeed, analysis of crystal structures of the σA RNAP holoenzymes from Thermus thermophilus and T. aquaticus (Vassylyev et al, 2002; Murakami et al, 2002a, 2002b) reveals that region 1.2 amino acids 77–84 of T. thermophilus σA (T. aquaticus σA amino acids 92–99) that correspond to σ70 amino acids 94–101 form an α-helix (shown in red on Figure 7A) that contacts the β′ coiled-coil (shown in violet). In particular, conserved Tyr84 of T. thermophilus σA (E. coli σ70-Tyr101) interacts with the β′ coiled-coil Ile565 and Asn569 (E. coli Ile290 and Asn294, correspondingly), σA region 2.1 Leu192 and Val196 (E. coli σ70 Leu384 and Val388, correspondingly), and region 2.2 Ile213 and Asn217 (E. coli σ70 Ile405 and Asn409, correspondingly) (Figure 7C). These residues of regions 2.1–2.2 also form a contact surface with conserved residues of the β′ coiled-coil. Thus, region 1.2 amino acids 94–101 interacts both with σ region 2 and the β′ coiled-coil and therefore may regulate the DNA binding activity of σ region 2.3.
The mechanism of activation of σ region 2 DNA binding by the β′ coiled-coil is not known. Even though the overall fold of σ domain 2 (regions 1.2 and 2) does not change significantly upon core binding (Campbell et al, 2002; Vassylyev et al, 2002; Murakami et al, 2002b), relative movement of α-helices of σ regions 2.1 and 2.2 is required for efficient promoter melting and open complex formation (Anthony and Burgess, 2002), and conformational changes within the ‘DNA melting' region 2.3 were detected upon the holoenzyme formation (Callaci and Heyduk, 1998), suggesting that this region must adopt a specific conformation required for DNA binding. Region 1.2 is well positioned to act in concert with the β′ coiled-coil to lock region 2.3 in a conformation that is capable of strong interaction with the −10 element sequence. This interaction is required for initiation of promoter DNA melting at the −10 element (Guo and Gralla 1998; Fenton et al, 2000; Panaghie et al, 2000; Lim et al, 2001). In support of this idea, amino-acid substitutions in the region 1.2 blocked isomerization from closed to open promoter complex and promoter escape (Baldwin & Dombroski, 2001; Hsu et al, 2004).
Interestingly, our results show that free σ can specifically (but with low affinity) recognize non-template −10 oligo even without core RNAP. This finding contrasts with the results of previous studies showing that −10 oligo recognition is possible only in the RNAP holoenzyme complex (Marr and Roberts 1997; Kulbachinskiy et al, 1999; Young et al, 2001). We suggest that the reason for this difference is that interactions between σ and non-template −10 oligo have never been studied at the high concentrations of σ (1–5 μM) that we have used. Furthermore, the in-solution method of detection used by us, UV crosslinking, allows to detect weak interactions that could have escaped detection by non-equilibrium methods, that is, filer binding assay or gel shift, used previously.
Function of region 1.2 in pausing and promoter escape
We show that a minimal fragment of σ70 that is sufficient for binding to transcribing RNAP and causing promoter-proximal pause corresponds to the σ2 structural domain (σ70 regions 1.2–2), which is conserved in all σ70 family proteins (Severinova et al, 1996, Campbell et al, 2002). Therefore, it appears that all σ70 family proteins may act as elongation factors, recognizing specific −10-like sequences within transcription units in vivo. Interestingly, pausing is induced by σ70 or σ70 fragments at concentrations (0.5–2 μM) that are below the concentration (∼11 μM) estimated for σ70 in vivo (Mooney and Landick, 2003).
An important conclusion that follows from our results is that disruption of contacts between σ regions 3–4 and core RNAP does not prevent the −10 element recognition, and is therefore not sufficient to allow promoter escape. This indicates that a different mechanism that disrupts σ interactions with the single-stranded promoter DNA at later stages of transcription initiation may exist. We speculate that region 1.2 is involved in this mechanism, activating/inactivating the −10 element binding during transcription initiation. As was noted by Vassylyev et al (2002), the α-helix of region 1.2 and the β subunit Lobe 1 block the entry of double-stranded downstream DNA into its binding site. Therefore, RNAP interactions with downstream DNA may influence the conformation/position of σ region 1.2. In addition, σ region 1.1 that undergoes a large-scale movement upon open promoter complex formation (Mekler et al, 2002) may physically pull the adjacent region 1.2, and thus influence its orientation relative to σ region 2 and the β′ coiled-coil. Disruption of contacts between region 1.2 and β′ caused by either of these processes may result in weakening of interactions between region 2.3 and the −10 element. Weakening the interactions with the −10 element creates a prerequisite for σ dissociation after the growing nascent RNA chain disrupts contacts with the core made by C-terminal domains of σ. The proposed mechanism of region 1.2-mediated −10 element binding is functioning in all experimental systems that require specific recognition of the −10 promoter element single-stranded DNA by σ region 2 (fork-junction DNA–RNAP holoenzyme complex, −10 oligonucleotide–RNAP holoenzyme complex, and elongation complex paused at a promoter-proximal pause site) and should operate for all σ70 family of transcription factors.
Materials and methods
Proteins and DNA
E. coli RNAP containing N-terminally His6-tagged β′ was purified as described (Brodolin et al, 2000). The wild-type σ70 subunit was purified from an overexpression strain as described (Gribskov and Burgess, 1983). To construct σ70 deletions, the rpoD gene was cloned in the pET28 vector at NdeI–EcoRI sites. PCR mutagenesis was performed by amplifying the required fragments with primers containing the NdeI site at the N terminus and the EcoRI site at the C terminus and recloned at the same vector. Expression in E.coli BL21(DE3) strain and purification using chromatography on Ni2+-NTA agarose column (Qiagen) of the σ fragments were performed as described (Wilson and Dombroski, 1997). All the fragments had His6 tags at the N terminus that did not interfere with binding to the core (Severinova et al, 1996). The lacUV5 promoter fragments (−59 to +58), either wild type or containing a +5G to A substitution, were prepared by PCR, 32P-labeled as described (Brodolin et al, 2004), and purified on 7% PAGE.
Transcription in vitro, core-binding assay and σ-exchange crosslinking assay
Transcription was performed in 10 μl of transcription buffer (TB) (40 mM HEPES pH 8.0, 50 mM NaCl, 5 mM MgCl2, and 5% glycerol) containing 200 nM RNAP and 20 nM lacUV5 fragment incubated at 37°C for 10 min to form an open complex. Transcription was initiated by addition of the mixture of 0.5 mM ApA and 40 μM NTPs. In order to label RNA, 2 μCi of [α-32P]UTP was added per reaction.
Core-binding competition assay was performed as follows. Core RNAP (10 nM or 50 nM) and σ70 or σ70 fragments in amounts indicated in the figures were incubated in 10 μl of TB for 10 min at 37°C. Then the DNA fragment (3 nM) containing the lacUV5 promoter, 0.5 mM ApA, and 40 μM [α-32P]UTP were added and the samples were incubated for 5 min at 37°. RNA was analyzed on denaturing 24% PAGE.
Transcription complexes stalled at position +16 of the lacUV5 promoter were prepared and immobilized on Ni2+-NTA agarose (Qiagen) via the His6 tag, essentially as described (Brodolin et al, 2004). To remove σ70 subunit from the stalled complexes, they were washed with TB containing 200 μg/ml heparin for 5 min at 37°C. Heparin was removed by extensive washing with TB containing 1 M NaCl and then with TB. A 0.5 μM portion of σ70, 5 μM of σ102−448, 3 μM of σ102−574, 1.5 μM of σ1−448, or 2.5 μM of σ102−613 or 1.5 μM of σ94−448 were added to σ70-less complexes where indicated. NTPs were added to 100 μM before or after σ70 or σ70 fragments and the samples were incubated for 5 min at 37°C. Crosslinking was performed for 30 s with 30 mM formaldehyde (Brodolin et al, 2000). Crosslinked complexes were analyzed on 5% SDS–PAGE.
Limited chemical cleavage of crosslinked complexes
Cleavage at Met residues with CNBr and at Cys with TNCBA was performed as described (Brodolin et al, 2000). Cleavage at Trp with N-bromosuccinimide was performed as follows. Crosslinked complexes eluted from gel were dissolved in 0.5% SDS and pH was adjusted to 4.0 with the HCOOH-Na buffer. N-bromosuccinimide was added at 0.1 mM and the samples were incubated for 5 min at room temperature. Reaction was terminated by addition of equal volume of a Laemmli loading buffer and immediately loaded on SDS–PAGE.
UV light crosslinking of −10 oligonucleotides
Non-template and template oligonucleotides used in the crosslinking experiments were closely related to the lacUV5 promoter −10 element (Supplementary data) Labeling, purification, and crosslinking of the oligonucleotides was performed as described (Kulbachinskiy et al 1999). Crosslinked product were resolved on 8% SDS–PAGE.
Fork-junction DNA crosslinking and filter binding
Fork-junction DNA was prepared by annealing two oligonucleotides corresponding to the template and non-template DNA strands (Supplementary data). The template oligonucleotide was labeled at the 5′-end by [γ-32P]ATP. The resulting fork-junction contained a 30 nt long double-stranded region followed by a 5-nt single-stranded extension of the non-template strand corresponding to the −10 promoter element. In control experiments, we used a fork-junction that contained C instead of G at the fifth position of the −10 element (TATACT) or the ‘anticonsensus' sequence of the −10 element (ATCTCG). In the binding assays, core enzyme RNAP (3 nM) was incubated with either the full-length σ70 subunit (100 nM) or σ70 fragments 2a (5 μM) and 2b (2 μM) for 10 min in 50 μl of the binding buffer (20 mM Tris–HCl, pH 7.9, 240 mM NaCl, 60 mM KCl, and 10 mM MgCl2) and fork-junction DNA was added at 3 nM. The incubation continued at 25°C for another 30 min. The samples were filtered through 0.45 μm nitrocellulose filters (HAWP, Millipore). The filters were washed with 5 ml of the buffer and quantified with PhosphorImager.
Formaldehyde crosslinking of RNAP holoenzyme (200 nM) in complex with fork-junction template (10 nM) was performed in TB containing 200 mM NaCl (Supplementary data).
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary data
Acknowledgments
This work was supported by the Russian Foundation for Basic Research grants RFBR-02-04-48525 to KB and RFBR 05-04-49405 to IB; NIH RO1 grant GM64530 to KS and GM30717 to AM. AK was supported by a grant from the Russian Science Support Foundation and by the grant of the President of Russian Federation MK-952.2005.4.
References
- Anthony LC, Burgess RR (2002) Conformational flexibility in sigma70 region 2 during transcription initiation. J Biol Chem 277: 46433–46441 [DOI] [PubMed] [Google Scholar]
- Baldwin NE, Dombroski AJ (2001) Isolation and characterization of mutations in region 1.2 of Escherichia coli sigma70. Mol Microbiol 42: 427–437 [DOI] [PubMed] [Google Scholar]
- Bar-Nahum G, Nudler E (2001) Isolation and characterization of σ(70)-retaining transcription elongation complexes from Escherichia coli. Cell 106: 443–451 [DOI] [PubMed] [Google Scholar]
- Brodolin K, Mustaev A, Severinov K, Nikiforov V (2000) Identification of RNA polymerase β′ subunit segment contacting the melted region of the lacUV5 promoter. J Biol Chem 275: 3661–3666 [DOI] [PubMed] [Google Scholar]
- Brodolin K, Zenkin N, Mustaev A, Mamaeva D, Heumann H (2004) The σ 70 subunit of RNA polymerase induces lacUV5 promoter-proximal pausing of transcription. Nat Struct Mol Biol 11: 551–557 [DOI] [PubMed] [Google Scholar]
- Brodolin K, Zenkin N, Severinov K (2005) Remodeling of the sigma70 subunit non-template DNA strand contacts during the final step of transcription initiation. J Mol Biol 350: 930–937 [DOI] [PubMed] [Google Scholar]
- Buckle M, Pemberton IK, Jacquet MA, Buc H (1999) The kinetics of σ subunit directed promoter recognition by E. coli RNA polymerase. J Mol Biol 285: 955–964 [DOI] [PubMed] [Google Scholar]
- Callaci S, Heyduk E, Heyduk T (1999) Core RNA polymerase from E. coli induces a major change in the domain arrangement of the σ 70 subunit. Mol Cell 3: 229–238 [DOI] [PubMed] [Google Scholar]
- Callaci S, Heyduk T (1998) Conformation and DNA binding properties of a single-stranded DNA binding region of σ 70 subunit from Escherichia coli RNA polymerase are modulated by an interaction with the core enzyme. Biochemistry 37: 3312–3320 [DOI] [PubMed] [Google Scholar]
- Campbell EA, Muzzin O, Chlenov M, Sun JL, Olson CA, Weinman O, Trester-Zedlitz ML, Darst SA (2002) Structure of the bacterial RNA polymerase promoter specificity σ subunit. Mol Cell 9: 527–539 [DOI] [PubMed] [Google Scholar]
- Chan CL, Gross CA (2001) The anti-initial transcribed sequence, a portable sequence that impedes promoter escape, requires sigma70 for function. J Biol Chem 276: 38201–38209 [DOI] [PubMed] [Google Scholar]
- Feklistov A, Barinova N, Sevostyanova A, Heyduk E, Bass I, Vvedenskaya I, Kuznedelov K, Merkiene E, Stavrovskaya E, Klimašauskas S, Nikiforov V, Heyduk T, Severinov K, Kulbachinskiy K (2006) A basal promoter element recognized by free RNA polymerase sigma subunit determines promoter recognition by RNA polymerase holoenzyme. Mol Cell 23: 97–107 [DOI] [PubMed] [Google Scholar]
- Fenton MS, Gralla JD (2001) Function of the bacterial TATAAT −10 element as single-stranded DNA during RNA polymerase isomerization. Proc Natl Acad Sci USA 98: 9020–9025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton MS, Lee SJ, Gralla JD (2000) Escherichia coli promoter opening and −10 recognition: mutational analysis of sigma70. EMBO J 19: 1130–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grachev MA, Lukhtanov EA, Mustaev AA, Zaychikov EF, Abdukajumov MN, Rabinov IV, Richter VI, Skoblov, Yu S, Chistyakov PG (1989) Studies of the functional topography of Escherichia coli RNA polymerase. A method for localization of the sites of affinity labelling. Eur J Biochem 180: 577–585 [DOI] [PubMed] [Google Scholar]
- Gribskov M, Burgess RR (1983) Overexpression and purification of the σ subunit of Escherichia coli RNA polymerase. Gene 26: 109–118 [DOI] [PubMed] [Google Scholar]
- Gross CA, Chan C, Dombroski A, Gruber T, Sharp M, Tupy J, Young B (1998) The functional and regulatory roles of σ factors in transcription. Cold Spring Harb Symp Quant Biol 63: 141–155 [DOI] [PubMed] [Google Scholar]
- Guo Y, Gralla JD (1998) Promoter opening via a DNA fork junction binding activity. Proc Natl Acad Sci USA 95: 11655–11660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen SP, Berkmen MB, Ross W, Gaal T, Ward C, Gourse RL (2006) rRNA promoter regulation by nonoptimal binding of sigma region 1.2: an additional recognition element for RNA polymerase. Cell 125: 1069–1082 [DOI] [PubMed] [Google Scholar]
- Helmann JD, deHaseth PL (1999) Protein–nucleic acid interactions during open complex formation investigated by systematic alteration of the protein and DNA binding partners. Biochemistry 38: 5959–5967 [DOI] [PubMed] [Google Scholar]
- Hsu HH, Huang WC, Chen JP, Huang LY, Wu CF, Chang BY (2004) Properties of Bacillus subtilis sigma A factors with region 1.1 and the conserved Arg-103 at the N terminus of region 1.2 deleted. J Bacteriol 186: 2366–2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juang YL, Helmann JD (1994) A promoter melting region in the primary σ factor of Bacillus subtilis. Identification of functionally important aromatic amino acids. J Mol Biol 235: 1470–1488 [DOI] [PubMed] [Google Scholar]
- Kapanidis AN, Margeat E, Laurence TA, Doose S, Ho SO, Mukhopadhyay J, Kortkhonjia E, Mekler V, Ebright RH, Weiss S (2005) Retention of transcription initiation factor sigma70 in transcription elongation: single-molecule analysis. Mol Cell 20: 347–356 [DOI] [PubMed] [Google Scholar]
- Keilty S, Rosenberg M (1987) Constitutive function of a positively regulated promoter reveals new sequences essential for activity. J Biol Chem 262: 6389–6395 [PubMed] [Google Scholar]
- Koradi R, Billeter M, Wüthrich K (1996) MOLMOL: a program for display and analysis of macromolecular structures. J Mol Graph 14: 51–55 [DOI] [PubMed] [Google Scholar]
- Kulbachinskiy A, Mustaev A, Goldfarb A, Nikiforov V (1999) Interaction with free β′ subunit unmasks DNA-binding domain of RNA polymerase σ subunit. FEBS Lett 454: 71–74 [DOI] [PubMed] [Google Scholar]
- Lim HM, Lee HJ, Roy S, Adhya S (2001) A ‘master' in base unpairing during isomerization of a promoter upon RNA polymerase binding. Proc Natl Acad Sci USA 98: 14849–14852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A, Severinova E, Darst SA (1996) Crystal structure of a σ 70 subunit fragment from E. coli RNA polymerase. Cell 87: 127–136 [DOI] [PubMed] [Google Scholar]
- Marr MT, Roberts JW (1997) Promoter recognition as measured by binding of polymerase to nontemplate strand oligonucleotide. Science 276: 1258–1260 [DOI] [PubMed] [Google Scholar]
- Matlock DL, Heyduk T (2000) Sequence determinants for the recognition of the fork junction DNA containing the −10 region of promoter DNA by E. coli RNA polymerase. Biochemistry 39: 12274–12283 [DOI] [PubMed] [Google Scholar]
- Mekler V, Kortkhonjia E, Mukhopadhyay J, Knight J, Revyakin A, Kapanidis AN, Niu W, Ebright YW, Levy R, Ebright RH (2002) Structural organization of bacterial RNA polymerase holoenzyme and the RNA polymerase–promoter open complex. Cell 108: 599–614 [DOI] [PubMed] [Google Scholar]
- Mooney RA, Landick R (2003) Tethering sigma70 to RNA polymerase reveals high in vivo activity of sigma factors and sigma70-dependent pausing at promoter-distal locations. Genes Dev 17: 2839–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami KS, Masuda S, Campbell EA, Muzzin O, Darst SA (2002a) Structural basis of transcription initiation: an RNA polymerase holoenzyme–DNA complex. Science 296: 1285–1290 [DOI] [PubMed] [Google Scholar]
- Murakami KS, Masuda S, Darst SA (2002b) Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 A resolution. Science 296: 1280–1284 [DOI] [PubMed] [Google Scholar]
- Nickels BE, Mukhopadhyay J, Garrity SJ, Ebright RH, Hochschild A (2004) The σ 70 subunit of RNA polymerase mediates a promoter-proximal pause at the lac promoter. Nat Struct Mol Biol 11: 544–550 [DOI] [PubMed] [Google Scholar]
- Panaghie G, Aiyar SE, Bobb KL, Hayward RS, de Haseth PL (2000) Aromatic amino acids in region 2.3 of Escherichia coli σ 70 participate collectively in the formation of an RNA polymerase–promoter open complex. J Mol Biol 299: 1217–1230 [DOI] [PubMed] [Google Scholar]
- Raffaelle M, Kanin EI, Vogt J, Burgess RR, Ansari AZ (2005) Holoenzyme switching and stochastic release of σ factors from RNA polymerase in vivo. Mol Cell 20: 357–366 [DOI] [PubMed] [Google Scholar]
- Ring BZ, Yarnell WS, Roberts JW (1996) Function of E. coli RNA polymerase σ factor σ 70 in promoter-proximal pausing. Cell 86: 485–493 [DOI] [PubMed] [Google Scholar]
- Roberts CW, Roberts JW (1996) Base-specific recognition of the nontemplate strand of promoter DNA by E. coli RNA polymerase. Cell 86: 495–501 [DOI] [PubMed] [Google Scholar]
- Severinov K, Fenyo D, Severinova E, Mustaev A, Chait BT, Goldfarb A, Darst SA (1994) The σ subunit conserved region 3 is part of ‘5′-face' of active center of Escherichia coli RNA polymerase. J Biol Chem 269: 20826–20828 [PubMed] [Google Scholar]
- Severinova E, Severinov K, Fenyo D, Marr M, Brody EN, Roberts JW, Chait BT, Darst SA (1996) Domain organization of the Escherichia coli RNA polymerase σ 70 subunit. J Mol Biol 263: 637–647 [DOI] [PubMed] [Google Scholar]
- Sharp MM, Chan CL, Lu CZ, Marr MT, Nechaev S, Merritt EW, Severinov K, Roberts JW, Gross CA (1999) The interface of σ with core RNA polymerase is extensive, conserved, and functionally specialized. Genes Dev 13: 3015–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassylyev DG, Sekine S, Laptenko O, Lee J, Vassylyeva MN, Borukhov S, Yokoyama S (2002) Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 A resolution. Nature 417: 712–719 [DOI] [PubMed] [Google Scholar]
- Wilson C, Dombroski AJ (1997) Region 1 of sigma70 is required for efficient isomerization and initiation of transcription by Escherichia coli RNA polymerase. J Mol Biol 267: 60–74 [DOI] [PubMed] [Google Scholar]
- Young BA, Anthony LC, Gruber TM, Arthur TM, Heyduk E, Lu CZ, Sharp MM, Heyduk T, Burgess RR, Gross CA (2001) A coiled-coil from the RNA polymerase β′ subunit allosterically induces selective nontemplate strand binding by σ(70). Cell 105: 935–944 [DOI] [PubMed] [Google Scholar]
- Young BA, Gruber TM, Gross CA (2004) Minimal machinery of RNA polymerase holoenzyme sufficient for promoter melting. Science 303: 1382–1384 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary data


