Abstract
Since the early 1950s, the dominant paradigm in the human genetics of infectious diseases postulates that rare monogenic immunodeficiencies confer vulnerability to multiple infectious diseases (one gene, multiple infections), whereas common infections are associated with the polygenic inheritance of multiple susceptibility genes (one infection, multiple genes). Recent studies, since 1996 in particular, have challenged this view. A newly recognised group of primary immunodeficiencies predisposing the individual to a principal or single type of infection is emerging. In parallel, several common infections have been shown to reflect the inheritance of one major susceptibility gene, at least in some populations. This novel causal relationship (one gene, one infection) blurs the distinction between patient-based Mendelian genetics and population-based complex genetics, and provides a unified conceptual frame for exploring the molecular genetic basis of infectious diseases in humans.
Keywords: complex genetics, immunity to infection, major gene, Mendelian genetics, primary immunodeficiencies
Introduction
Although sufficient to ensure reproduction at the species level, the human immune system is weak at the individual level. Indeed, life expectancy at birth did not exceed 20–25 years of age until the advent of Pasteur's microbial theory of disease and the ensuing control of infections by hygiene, vaccines, and antibiotics (Casanova and Abel, 2005). Nevertheless, a striking feature of most infections in human populations world-wide and throughout history is their considerable inter-individual phenotypic variability, ranging from asymptomatic to lethal infections. The field of human genetics of infectious diseases aims to define the genetic variations accounting for inter-individual variability in the course of human infections. From a clinical standpoint, this human genetic view of infectious diseases provides new means of diagnosis, improves the definition of patient prognosis, and paves the way for innovative preventive and curative approaches (Casanova and Abel, 2005). The human model is also important for biological purposes, as infections and immunity occur in natural, as opposed to experimental conditions in this model (Casanova and Abel, 2004). Under the theory of natural selection of living species, the ecologically relevant functions of immune system genes are subject to natural selective pressure (Allison, 1954, 1968, 2002; Lederberg, 1999). It is therefore essential to define the function of immune genes within the setting of their natural ecosystem, within which the organisms and populations concerned live and are selected (Casanova and Abel, 2004).
According to the dominant paradigm, monogenic immunodeficiencies (also known as primary immunodeficiencies, PIDs) are rare and confer vulnerability to multiple infectious diseases (one gene, multiple infections)—that vary in nature and number with the gene affected—(Notarangelo et al, 2006; Ochs et al, 2006), whereas common infections are favoured by the polygenic inheritance of multiple susceptibility genes, most of which if not all making an individually modest contribution to the phenotype (one infection, multiple genes) (Figure 1) (Lander and Schork, 1994; Hill, 2001, 2006). For Galtonian biostatisticians, infectious diseases are seen in populations and reflect polygenic predisposition. In contrast, for Mendelian physician-scientists, severe infections do occur in individuals and result from monogenic PIDs. X-linked recessive agammaglobulinaemia, probably the first PID to be described as such in the English literature, was discovered in 1952 by Ogden Bruton in a few American children with multiple infections (Bruton, 1952, 1962). At about the same period, in 1954, Anthony Allison discovered that the sickle cell trait protects against severe forms of Plasmodium falciparum malaria in African populations (Allison, 1954, 2002), paving the way for acceptance of the notion of multiple-gene involvement in disease susceptibility (Min-Oo and Gros, 2005). Population-based complex genetics and patient-based Mendelian genetics have evolved in parallel for almost 50 years, even though they study the same phenomenon from two ends of a spectrum: the patient and population viewpoints. We argue here that the recent discovery of human genes conferring vulnerability or resistance to a specific infection at the individual or population level (one gene, one infection) bridges the two fields, providing experimental support for a unified theory of the human genetics of infectious diseases (Figure 1, Table I).
Figure 1.
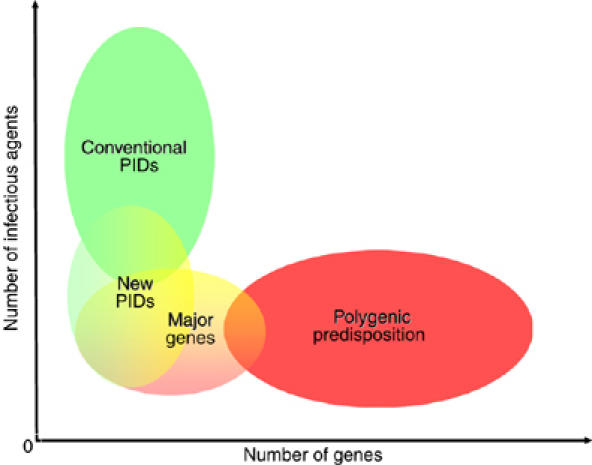
Human genetics of infectious diseases. The spectrum of genetic predisposition to infectious diseases in human patients is represented, according to the number of genes involved (x-axis) and the number of infections (y-axis). The dominant view in human genetics of infectious diseases postulates that rare, ‘conventional', monogenic primary immunodeficiencies (PIDs, in green) predispose the individual to numerous infections (one gene, multiple infections), whereas common infectious diseases are associated with polygenic inheritance (in red) of numerous susceptibility genes (one infection, multiple genes). Novel monogenic PIDs (in yellow/green) predispose the individual to a principal or single type of infection. Major genes (in yellow/red) exert a nearly Mendelian impact at the population level and largely account for common infectious diseases in some individuals. The recent discovery of such human genes conferring vulnerability or resistance to a specific infection at the individual level (one gene, one infection) bridges the gap between the two classical fields of conventional PIDs and polygenic inheritance, as defined in the 50 s. As an example, genetic predisposition to tuberculosis, which was considered to be purely polygenic, was recently shown to reflect both new PID and major gene effects, at least in some patients (see text for details). Overall, these observations provide experimental support for a continuous spectrum of predisposition and a unified theory of the human genetics of infectious diseases.
Table 1.
Genetic predisposition or resistance to specific infections
| Infectious agent | Clinical phenotype | Immunological phenotype | Gene/locus | References |
|---|---|---|---|---|
| Mendelian predisposition | ||||
| Neisseria | Invasive disease | MAC deficiency | C5, C6, C7, C8A, C8B, C8G, C9 | Reviewed in (Mathew and Overturf, 2006) |
| Invasive disease | Properdin deficiency | PFC | Reviewed in (Mathew and Overturf, 2006) | |
| Mycobacteria | MSMD | IL-12/23-IFN-γ deficiency | IFNGR1, IFNGR2, STAT1, NEMO, IL12B, IL12RB1 | Reviewed in (Filipe-Santos et al, 2006a, 2006b) |
| Disseminated tuberculosis | Reviewed in (Alcais et al, 2005a, 2005b) | |||
| Streptococcus pneumoniae | Invasive disease | IRAK-4 deficiency | IRAK4 | (Picard et al, 2003) |
| Epstein-Barr virus | X-linked lympho- proliferative disease | SAP deficiency | SH2D1A | Reviewed in (Picard et al, 2006) |
| XIAP deficiency | XIAP | (Rigaud et al, 2006) | ||
| Human papillomavirus | Epidermodysplasia verruciformis | EVER1/EVER2 deficiency | EVER1, EVER2 | (Ramoz et al, 1999, 2002) |
| Mendelian resistance | ||||
| Plasmodium vivax | Natural resistance | Lack of receptor for pathogen | DARC | (Miller et al, 1975, 1976; Tournamille et al, 1995) |
| Human immunodeficiency virus-1 | Natural resistance | Lack of receptor for pathogen | CCR5 | Reviewed in (Picard et al, 2006) |
| Norovirus | Natural resistance | Lack of receptor for pathogen | FUT2 | Reviewed in (Picard et al, 2006) |
| Major genea | ||||
| Mycobacterium tuberculosis | Pulmonary tuberculosis | To be determined | To be identified (8q12–q13) | (Baghdadi et al, 2006) |
| Mycobacterium leprae | Leprosy per se | To be determined | PARK2/PACRG | (Mira et al, 2003, 2004) |
| Paucibacillary leprosy | To be identified (10p13) | (Siddiqui et al, 2001) | ||
| Schistosoma mansoni | Infection levels | To be determined | To be identified (5q31–q33) | (Marquet et al, 1996; Muller-Myhsok et al, 1997) |
| Hepatic fibrosis | To be identified (6q22–q23) | (Dessein et al, 1999; Blanton et al, 2005) | ||
| Leishmania donovani | Kala-azar | To be determined | To be identified (22q12) | (Bucheton et al, 2003) |
| Major genes presented in this table are those that have been identified by means of a genome-wide linkage analysis. | ||||
Monogenic traits conferring predisposition to specific infections
From idiopathic infectious diseases to novel primary immunodeficiencies
A few idiopathic infections in otherwise healthy patients have been shown to be familial, suggesting a Mendelian mode of inheritance (reviewed by Casanova et al, 2002, 2005; Picard et al, 2006). Defects of the complement membrane attack complex (1974) and properdin (1982) were found to result in selective predisposition to invasive Neisseria disease (reviewed by Mathew and Overturf, 2006). The corresponding germline mutations were identified from 1993 and 1995 onwards. X-linked lymphoproliferative disease (XLP), described clinically in 1975 and predisposing to lethal Epstein-Barr virus (EBV) disease, was found to be heterogeneous at the molecular level, with a first pathogenic gene identified in 1998 and a second in 2006 (Rigaud et al, 2006 and references therein). Neisseria and EBV can also affect children with conventional PIDs, unlike skin-tropic oncogenic papillomaviruses (HPVs), which almost exclusively affect patients with epidermodysplasia verruciformis (EV) (reviewed by Orth, 2006). EV was first described clinically in 1922 as a congenital dermatosis (Lewandowsky and Lutz, 1922). A recessive mode of inheritance for this disease was proposed in 1933 (Cockayne, 1933) and the role of papillomaviruses was established in 1946 (Lutz, 1946). Causal mutations in EVER1 or EVER2 were described in 2002 (Ramoz et al, 1999, 2002). The EVER genes belong to the transmembrane channel-like (TMC) family and may exert their anti-HPV function within keratinocytes. Retrospectively, EV was probably the first PID to be described, although the lack of a detectable immunological phenotype and the narrow range of infections precluded the use of this term at the time.
Mendelian susceptibility to mycobaterial disease
Mendelian susceptibility to mycobacterial disease (MSMD) was probably first clinically described in 1951 and has been thoroughly characterised since 1996 (Mimouni, 1951; Casanova et al, 1996). Patients with MSMD are highly susceptible to weakly virulent mycobacteria, but are apparently resistant to most other infectious agents, with the exception of Salmonella (Casanova et al, 1995, 1996; Levin et al, 1995). Since 1996, disease-causing mutations have been found in five autosomal (IFNGR1, IFNGR2, STAT1, IL12B and IL12RB1) and one X-linked (NEMO) gene. These genes are physiologically related because their products are involved in IL-12/IL-23-dependent, IFN-γ-mediated immunity (Casanova and Abel, 2002; Filipe-Santos et al, 2006a). Extensive allelic heterogeneity at the five autosomal loci accounts for the existence of twelve distinct genetic disorders (Jouanguy et al, 1996, 1997, 1999, 2000; Newport et al, 1996; Altare et al, 1998a, 1998b; Dorman and Holland, 1998; de Jong et al, 1998; Döffinger et al, 2000; Dupuis et al, 2001, 2003; Fieschi et al, 2004; Rosenzweig et al, 2004; Vogt et al, 2005; Chapgier et al, 2006). X-linked MSMD is caused by NEMO mutations impairing the CD40-triggered induction of IL-12 production by monocyte-derived cells upon stimulation by CD40L-expressing T cells (Filipe-Santos et al, 2006b). Incidentally, the study of IFNGR2 revealed that gain-of-glycosylation mutations represent up to 1.4% of disease-causing missense mutations in humans (Vogt et al, 2005). Altogether, studies of MSMD have shown that the IL-12/23-IFN-γ circuit is crucial for host defence against mycobacteria and Salmonella but redundant against most other microorganisms.
Mendelian predisposition to Streptococcus pneumoniae
Patients with PIDs affecting the splenic phagocytosis of opsonised bacteria suffer from multiple pyogenic infections, including invasive pneumococcal disease in particular (Picard et al, 2003b). Patients with inherited interleukin-1 receptor-associated kinase-4 (IRAK-4) deficiency are more specifically vulnerable to pneumococcus infections (Picard et al, 2003a, 2003b). The first patient with IRAK-4 deficiency was described clinically in 1997 (Kuhns et al, 1997), the diagnosis of IRAK-4 deficiency being made in 2003 (Medvedev et al, 2003). Almost 30 other patients have since been identified (Currie et al, 2004; Enders et al, 2004; Chapel et al, 2005; Ku et al, 2005, 2007; Yang et al, 2005; Cardenes et al, 2006; Takada et al, 2006). Clinically, IRAK-4-deficient patients present recurrent infections caused by pyogenic bacteria, such as S. pneumoniae and S. aureus in particular. Only three of the identified patients had invasive disease caused by Gram-negative bacteria. IRAK-4 deficiency is a life-threatening disease in childhood, but the global trend shows a clinical improvement with age. The patients' blood cells fail to produce cytokines upon stimulation with Toll-like receptor (TLR) agonists, IL-1β and IL-18. So far, the only known exception is the induction of IFN-α/β and IFN-λ in response to TLR3 and TLR4 stimulation (Yang et al, 2005). Overall, the TLR and IL-1R signalling pathways that depend on IRAK-4 are critical for protective immunity to a relatively narrow group of pathogens, including pneumococci, but redundant for protective immunity to many other pathogens.
Mendelian predisposition to herpes simplex encephalitis
Herpes simplex encephalitis (HSE) provides the best illustration that susceptibility to a single infectious disease may be associated with single-gene lesions (Casrouge et al, 2006). HSE, which was first described in 1941 (Smith et al, 1941), is the most common form of sporadic encephalitis in Western countries. Its occurrence in only a small fraction of individuals infected with the almost ubiquitous HSV-1 and in otherwise healthy individuals remained unexplained until the identification of two children with HSE who produced only low levels of IFN-α/β and -λ in response to viruses and TLR3, TLR7, TLR8, and TLR9 agonists (Casrouge et al, 2006). These children carry homozygous null mutations in UNC93B1. HSV-1 did not trigger the production of optimal amounts of IFN-β and -λ in fibroblasts from these patients, increasing levels of viral replication and cell death. Based on our previous finding that IRAK-4-deficient children did not suffer from severe viral diseases, we concluded that the induction of IFN production via TLR7, TLR8, and TLR9 is redundant for protective immunity to viruses (Yang et al, 2005). The results obtained for these UNC-93B-deficient patients indicate that HSE is caused by the impairment of TLR3-dependent pathways, TLR-independent pathways, or both. The UNC-93B-IFN pathway is critical for primary immunity to HSV-1 in the central nervous system. HSE thus provides the first example of a devastating and sporadic infectious disease, hitherto idiopathic, that is now known to result from a monogenic PID (Casanova et al, 2005).
Major genes predisposing populations to infectious diseases
The concept of major genes in human genetics
Between 1910 and 1930, the studies of Fisher, Haldane, and Wright founded population genetics by developing a mathematical framework that modelled the behaviour of genes in populations (Khoury et al, 1993). Bridging the gap between the Galtonian and the Mendelian approaches, Fisher developed a polygenic model in which familial correlations for quantitative traits resulted from the combined and independent action of a large number of genes, each exerting a small effect (Fisher, 1918). With the development of statistical genetics in the 1960s, models that could explicitly specify the effect of single genes in the expression of common diseases were developed (Edwards, 1969; Lalouel et al, 1983). This led to the concept of ‘major genes/loci', the phenotypic expression of which is influenced by other genes and by the environment, and which was first formalised in the context of complex segregation analysis (Khoury et al, 1993). Several major genes identified by segregation analyses have been reported since the 1970s, for a number of infectious disease-related phenotypes (Abel and Demenais, 1988; Abel et al, 1991, 1992, 1995; Alcais et al, 1997; Plancoulaine et al, 2000, 2003). In the 1990s, with the development of highly polymorphic genetic markers (Dib et al, 1996), the concept of ‘major genes' was applied to loci detected in genome-wide linkage studies. Such loci, including those detected in affected sib-pairs studies, are predicted to have a considerable influence on the phenotype studied (Risch, 1990; Risch and Merikangas, 1996). The first major susceptibility locus for infectious diseases was mapped in 1996, for schistosomiasis (Marquet et al, 1996).
Major genes for parasitic diseases
Schistosomiasis is the second most important parasitic disease world-wide after malaria (Campino et al, 2006). Segregation analysis (Abel et al, 1991) led to the mapping of a major locus controlling levels of gastro-intestinal infection with the nematode Schistosoma mansoni (SM1) to chromosome 5q31–q33 in a Brazilian population (Marquet et al, 1996). This mapping was replicated in a Senegalese population (Muller-Myhsok et al, 1997). In another study combining segregation and linkage analysis, a second major locus predisposing subjects infected with S. mansoni to severe hepatic fibrosis (the SM2 locus) was mapped to chromosome 6q23 in a Sudanese population (Dessein et al, 1999a). This result was subsequently replicated in an Egyptian population (Blanton et al, 2005). Gene variants at these two major loci have yet to be discovered. These studies provide proof-of-principle that major loci may control common infectious phenotypes. They also demonstrate that levels of infection and hepatic disease owing to S. mansoni are under distinct genetic control (Dessein et al, 1999b). A major gene associated with visceral leishamiasis (or kala azar), caused by the protozoa Leishmania donovani, has also recently been identified (Campino et al, 2006). A genome-wide scan conducted in a Sudanese village led to the mapping of a major susceptibility locus to chromosome 22q12 (Bucheton et al, 2003). Interestingly, the effect of this locus was stronger in subjects affected at the start of the outbreak. This suggests that, for a given disease, major genes are more commonly expressed in patients with an early onset of disease.
Major genes for leprosy
Leprosy is a chronic infectious disease that still affects more than 300 000 subjects per year (WHO, 2006). In an effort to combat social stigma, the belief that leprosy was inherited was discredited when Armauer Hansen demonstrated that leprosy was caused by Mycobacterium leprae (Pallamary, 1955). Ironically, we know today that both the development of the disease upon exposure to M. leprae and the pattern of clinical manifestations displayed by leprosy patients (paucibacillary versus multibacillary) are highly dependent on human genes (Casanova and Abel, 2002; Alcais et al, 2005b). Twin studies in the 1960s indicated that leprosy was largely genetic (Beiguelman, 1968), and segregation studies in the 1980s provided strong evidence for the presence of a major gene, particularly in the study carried out on Desirade Island (Abel and Demenais, 1988). Two major regions were recently mapped by genome-wide linkage studies. A major locus was found on chromosome 10p13 in Indians with paucibacillary leprosy (Siddiqui et al, 2001). Another major locus for susceptibility to leprosy per se (i.e. leprosy regardless of its clinical subtype) was mapped to chromosome 6q25 in Vietnamese patients (Mira et al, 2003). Linkage disequilibrium studies in this region identified leprosy susceptibility variants of the regulatory region shared by PARK2, which encodes an E3-ubiquitin ligase called Parkin, and PACRG (Parkin coregulated gene) (Mira et al, 2004). These studies resulted in the first successful positional cloning of a major gene in a common infectious disease, and identified a new pathway of immunity to M. leprae (Schurr et al, 2006).
Major gene for tuberculosis
Tuberculosis, another common mycobacterial disease, is a leading public health problem world-wide (WHO, 2004). It was not until the 1930s that rigorous twin studies provided strong evidence for the contribution of human genetics to tuberculosis (Puffer, 1944; Dubos and Dubos, 1952). No complex segregation studies have been conducted in tuberculosis and, until the late 1990s, all investigations of host genes were based on association studies with candidate genes (Casanova and Abel, 2002; Alcais et al, 2005a). The most consistent results were obtained with some HLA class II and natural resistance-associated macrophage protein 1 (NRAMP1, alias SLC11A1) alleles (Alcais et al, 2005a). The first major locus identified by genome-wide screening was recently mapped to chromosome 8q12–q13 in adult patients with pulmonary tuberculosis from Morocco (Baghdadi et al, 2006). The predisposing allele is dominant, possibly accounting for the rapid decline in tuberculosis mortality rates in Europe during the 19th century, before any specific measures against the disease were taken. Efforts to identify this major gene more precisely are continuing. The other major discovery of recent years has been the demonstration that tuberculosis in children, a distinct disease, may reflect a Mendelian predisposition (Altare et al, 2001; Caragol et al, 2003; Alcais et al, 2005a; Casanova and Abel, 2005; Özbek et al, 2005). The proportion of children with disseminated tuberculosis owing to Mendelian predisposition remains to be experimentally determined, but has been estimated at 3–30% by Bayesian statistics (Alcais et al, 2005a). Overall, these recent studies provide the proof-of-concept that human genetics of common infectious diseases involves both Mendelian and major gene determinism.
Concluding remarks
The two related forms of genetic predisposition to infectious diseases reviewed here (one gene, one infection) bridge the gap between PIDs in individuals (one gene, multiple infections) and complex genetics in populations (one infection, multiple genes). Clearly, the concept of pathogen-specific genes applies to some, but not all individuals and populations, as the same gene may be associated with other infections in other individuals or populations. Moreover, pathogen specificity is unlikely to be strict, and degeneracy is almost inevitable, as best illustrated by the occurrence of Salmonella and staphylococcal infections in patients with IL-12Rβ1 and IRAK-4 deficiencies, respectively. One of the major goals of the human genetics of infectious diseases is now to define the relative contributions of conventional PIDs, pathogen-specific monogenic traits, major genes, and purely multigenic inheritance, at both individual and population levels. An important factor to be considered is the virulence of the pathogen. A substantial proportion of predisposing Mendelian defects is expected in infectious diseases that affect only a small proportion of infected individuals (e.g. HSE). Conversely, more common polygenic predisposition is probably involved in diseases caused by more virulent microbes (e.g. HIV). The age of infection may also be a crucial factor to take in consideration, with more Mendelian traits being involved before puberty, when most primary infections do occur and when the impact of infection death on population genetics is expected to be greater (Wright et al, 2003). Future studies in the field should tackle these important questions.
It is often assumed that the contribution of PIDs and Mendelian traits in general, is modest at the population level. However, the emergence of pathogen-specific monogenic susceptibility traits (reviewed here) and pathogen-specific monogenic resistance traits, including defects in genes encoding chemokine receptors such as DARC and CCR5 (reviewed elsewhere (Picard et al, 2006) (Table I)), suggests that there may be more Mendelian disorders than initially thought (Pritchard, 2001; Pritchard and Cox, 2002; Antonarakis and Beckmann, 2006). In recent years, forward genetic studies in the mouse model have revealed a number of pathogen-specific genes, including Mx, Nramp1, Ly49h, and Tlr4 (reviewed in Casanova et al, 2002; Buer and Balling, 2003; Haller and Kochs, 2003; Lam-Yuk-Tseung and Gros, 2003; Papathanasiou and Goodnow, 2005; Yokoyama, 2005; Beutler et al, 2006). These seminal studies paved the way for studies of the utmost importance in immunology. They also provided candidate genes for human infectious diseases and patients bearing mutations in their orthologs are expected to be discovered. Conversely, the impact of polygenic inheritance is often thought to be modest at the individual level. In fact, the demonstration that polygenic inheritance in individuals does confer actual predisposition to infectious diseases has not yet been shown in humans. Its demonstration in mice nonetheless suggests that this may occur in humans too (Buer and Balling, 2003; Lam-Yuk-Tseung and Gros, 2003; Papathanasiou and Goodnow, 2005; Beutler et al, 2006). The ongoing identification of human major genes raises hopes that a simpler, more potent form of causal determinism can be deciphered.
The clinical implications of novel PIDs are already considerable, and prospects for patient care are as promising as for conventional PIDs. Patients with impaired IFN-γ production are susceptible to severe tuberculosis and should be treated with recombinant IFN-γ (Alcais et al, 2005a). Similarly, patients with impaired IFN-α/β production are prone to herpes simplex encephalitis and should be treated with IFN-α (Casrouge et al, 2006). Major genes also hold great promise in terms of public health. The human genetics of infectious diseases also has important biological implications in the fields of immunity to infection and evolutionary immunology. The spread of the deleterious haemoglobin S (Allison, 1954; Lederberg, 1999; Allison, 2002) and DARC (Miller et al, 1975, 1976; Tournamille et al, 1995) alleles within human populations in regions endemic for P. falciparum and Plasmodium vivax, respectively, indicated that microbe-driven natural selection operates on the human genome. Major genes are expected to have important evolutionary implications, as illustrated by the dominant predisposition to tuberculosis, which might account for the rapid selection of resistant individuals (Baghdadi et al, 2006). Finally, studies of the novel PIDs have indicated that certain human genes exert an almost pathogen-specific effect in protective immunity, raising the exciting possibility of coevolution between animal and microbial species, as previously shown between plants and pathogens (Woolhouse et al, 2002; Chisholm et al, 2006).
Acknowledgments
We apologise to our colleagues whose studies were not cited owing to space limitations. We thank our collaborators world-wide, the members of our laboratory, and the patients and their families for their trust and patience. Our laboratory has been supported by grants from the INSERM, the ANR, the University René Descartes, the EU, 3M Pharmaceuticals, the BNP Paribas Foundation, the Schlumberger Foundation, and the March of Dimes. JL Casanova is an international scholar of the Howard Hughes Medical Institute.
References
- Abel L, Casanova JL (2006) Human genetics of infectious diseases: fundamental insights from clinical studies. Semin Immunol 18: 327–329 [DOI] [PubMed] [Google Scholar]
- Abel L, Cot M, Mulder L, Carnevale P, Feingold J (1992) Segregation analysis detects a major gene controlling blood infection levels in human malaria. Am J Hum Genet 50: 1308–1317 [PMC free article] [PubMed] [Google Scholar]
- Abel L, Demenais F (1988) Detection of major genes for susceptibility to leprosy and its subtypes. Am J Hum Genet 42: 256–266 [PMC free article] [PubMed] [Google Scholar]
- Abel L, Demenais F, Prata A, Souza AE, Dessein A (1991) Evidence for the segregation of a major gene in human susceptibility/resistance to infection by Schistosoma mansoni. Am J Hum Genet 48: 959–970 [PMC free article] [PubMed] [Google Scholar]
- Abel L, Vu DL, Oberti J, Nguyen VT, Van VC, Guilloud-Bataille M, Schurr E, Lagrange PH (1995) Complex segregation analysis of leprosy in southern Vietnam. Genet Epidemiol 12: 63–82 [DOI] [PubMed] [Google Scholar]
- Alcais A, Abel L, David C, Torrez ME, Flandre P, Dedet JP (1997) Evidence for a major gene controlling susceptibility to tegumentary leishmaniasis in a recently exposed Bolivian population. Am J Hum Genet 61: 968–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcais A, Fieschi C, Abel L, Casanova JL (2005a) Tuberculosis in children and adults: two distinct genetic diseases. J Exp Med 202: 1617–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcais A, Mira M, Casanova JL, Schurr E, Abel L (2005b) Genetic dissection of immunity in leprosy. Curr Opin Immunol 17: 44–48 [DOI] [PubMed] [Google Scholar]
- Allison AC (1954) Protection afforded by sickle cell trait against subtertian malarian infection. Brit Med J 1: 290–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison AC (1968) Genetics and infectious diseases. In Haldane and Modern Miology, Dronamraju, KR (ed) pp 179–201. Baltimore: Johns Hopkins Press [Google Scholar]
- Allison AC (2002) The discovery of resistance to malaria of sickle-cell heterozygotes. Biochem Mol Biol Education 30: 279–287 [Google Scholar]
- Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Döffinger R, Bernaudin F, Jeppsson O, Gollob JA, Meinl E, Segal AW, Fischer A, Kumararatne D, Casanova JL (1998a) Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280: 1432–1435 [DOI] [PubMed] [Google Scholar]
- Altare F, Ensser A, Breiman A, Reichenbach J, Baghdadi JE, Fischer A, Emile JF, Gaillard JL, Meinl E, Casanova JL (2001) Interleukin-12 receptor beta1 deficiency in a patient with abdominal tuberculosis. J Infect Dis 184: 231–236 [DOI] [PubMed] [Google Scholar]
- Altare F, Lammas D, Revy P, Jouanguy E, Döffinger R, Lamhamedi S, Drysdale P, Tollner D, Girdlestone J, Darbyshire P, Wadhwa M, Dockrel H, Salmon M, Fischer A, Durandy A, Casanova JL, Kumararatne D (1998b) Inherited interleukin 12 deficiency in a child with bacille Calmette-Guérin and Salmonella enteritidis disseminated infection. J Clin Invest 102: 2035–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis SE, Beckmann JS (2006) Mendelian disorders deserve more attention. Nat Rev Genet 7: 277–282 [DOI] [PubMed] [Google Scholar]
- Baghdadi JE, Orlova M, Alter A, Ranque B, Chentoufi M, Lazrak F, Archane MI, Casanova JL, Benslimane A, Schurr E, Abel L (2006) An autosomal dominant major gene confers predisposition to pulmonary tuberculosis in adults. J Exp Med 203: 1679–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiguelman B (1968) Some remarks on the genetics of leprosy resistance. Acta Genet Med Gemellol (Roma) 17: 584–594 [DOI] [PubMed] [Google Scholar]
- Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K (2006) Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol 24: 353–389 [DOI] [PubMed] [Google Scholar]
- Blanton RE, Salam EA, Ehsan A, King CH, Goddard KA (2005) Schistosomal hepatic fibrosis and the interferon gamma receptor: a linkage analysis using single-nucleotide polymorphic markers. Eur J Hum Genet 13: 660–668 [DOI] [PubMed] [Google Scholar]
- Bruton OC (1952) Agammaglobulinemia. Pediatrics 9: 722–728 [PubMed] [Google Scholar]
- Bruton OC (1962) A decade with agammaglobulinemia. J Pediatr 60: 672–676 [DOI] [PubMed] [Google Scholar]
- Bucheton B, Abel L, El-Safi S, Kheir MM, Pavek S, Lemainque A, Dessein AJ (2003) A major susceptibility locus on chromosome 22q12 plays a critical role in the control of kala-azar. Am J Hum Genet 73: 1052–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer J, Balling R (2003) Mice, microbes and models of infection. Nat Rev Genet 4: 195–205 [DOI] [PubMed] [Google Scholar]
- Campino S, Kwiatkowski D, Dessein A (2006) Mendelian and complex genetics of susceptibility and resistance to parasitic infections. Semin Immunol 18: 411–422 [DOI] [PubMed] [Google Scholar]
- Caragol I, Raspall M, Fieschi C, Feinberg J, Larrosa MN, Hernandez M, Figueras C, Bertran JM, Casanova JL, Espanol T (2003) Clinical tuberculosis in 2 of 3 siblings with interleukin-12 receptor beta1 deficiency. Clin Infect Dis 37: 302–306 [DOI] [PubMed] [Google Scholar]
- Cardenes M, von Bernuth H, Garcia-Saavedra A, Santiago E, Puel A, Ku CL, Emile JF, Picard C, Casanova JL, Colino E, Bordes A, Garfia A, Rodriguez-Gallego C (2006) Autosomal recessive interleukin-1 receptor-associated kinase 4 deficiency in fourth-degree relatives. J Pediatr 148: 549–551 [DOI] [PubMed] [Google Scholar]
- Casanova JL, Abel L (2002) Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol 20: 581–620 [DOI] [PubMed] [Google Scholar]
- Casanova JL, Abel L (2004) The human model: a genetic dissection of immunity to infection in natural conditions. Nat Rev Immunol 4: 55–66 [DOI] [PubMed] [Google Scholar]
- Casanova JL, Abel L (2005) Inborn errors of immunity to infection: the rule rather than the exception. J Exp Med 202: 197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova JL, Blanche S, Emile JF, Jouanguy E, Lamhamedi S, Altare F, Stephan JL, Bernaudin F, Bordigoni P, Turck D, Lachaux A, Albertini M, Bourrillon A, Dommergues JP, Pocidalo MA, Le Deist F, Gaillard JL, Griscelli C, Fischer A (1996) Idiopathic disseminated bacillus Calmette-Guerin infection: a French national retrospective study. Pediatrics 98: 774–778 [PubMed] [Google Scholar]
- Casanova JL, Fieschi C, Bustamante J, Reichenbach J, Remus N, von Bernuth H, Picard C (2005) From idiopathic infectious diseases to novel primary immunodeficiencies. J Allergy Clin Immunol 116: 426–430 [DOI] [PubMed] [Google Scholar]
- Casanova JL, Jouanguy E, Lamhamedi S, Blanche S, Fischer A (1995) Immunological conditions of children with BCG disseminated infection. Lancet 346: 581. [DOI] [PubMed] [Google Scholar]
- Casanova JL, Schurr E, Abel L, Skamene E (2002) Forward genetics of infectious diseases: immunological impact. Trends Immunol 23: 469–472 [DOI] [PubMed] [Google Scholar]
- Casrouge A, Zhang S, Eidenschenk C, Jouanguy E, Puel A, Yang K, Alcais A, Picard C, Mahfoufi N, Nicolas N, Lorenzo L, Plancoulaine S, Sénéchal B, Geissmann F, Tabeta K, Hoebe K, Du X, Miller RL, Héron B, Mignot C, Billette de Villemeur T, Lebon P, Dulac O, Rozenberg F, Beutler B, Tardieu M, Abel L, Casanova JL (2006) Herpes simplex encephalitis in human UNC-93B deficiency. Science 314: 308–312 [DOI] [PubMed] [Google Scholar]
- Chapel H, Puel A, von Bernuth H, Picard C, Casanova JL (2005) Shigella sonnei meningitis due to interleukin-1 receptor-associated kinase-4 deficiency: first association with a primary immune deficiency. Clin Infect Dis 40: 1227–1231 [DOI] [PubMed] [Google Scholar]
- Chapgier A, Boisson-Dupuis S, Jouanguy E, Vogt G, Feinberg J, Prochnicka-Chalufour A, Casrouge A, Yang K, Soudais C, Fieschi C, Filipe-Santos O, Bustamante J, Picard C, de Beaucoudrey L, Emile J-F, Arkwright P, Schreiber RD, Rolinck-Werninghaus C, Rösen-Wolff A, Magdorf K, Roesler J, Casanova J-L (2006) Novel STAT1 alleles in otherwise healthy patients with mycobacterial disease. PLoS Genetics 2: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124: 803–814 [DOI] [PubMed] [Google Scholar]
- Cockayne EA (1933) Epidermodysplasia verruciformis. In Inherited Abnormalities of the Skin and its Appendages. O.U. (ed) p 156 London: Oxford University Press [Google Scholar]
- Currie AJ, Davidson DJ, Reid GS, Bharya S, MacDonald KL, Devon RS, Speert DP (2004) Primary immunodeficiency to pneumococcal infection due to a defect in Toll-like receptor signaling. J Pediatr 144: 512–518 [DOI] [PubMed] [Google Scholar]
- de Jong R, Altare F, Haagen IA, Elferink DG, Boer T, van Breda Vriesman PJ, Kabel PJ, Draaisma JM, van Dissel JT, Kroon FP, Casanova JL, Ottenhoff TH (1998) Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 280: 1435–1438 [DOI] [PubMed] [Google Scholar]
- Dessein AJ, Hillaire D, Elwali NE, Marquet S, Mohamed-Ali Q, Mirghani A, Henri S, Abdelhameed AA, Saeed OK, Magzoub MM, Abel L (1999a) Severe hepatic fibrosis in Schistosoma mansoni infection is controlled by a major locus that is closely linked to the interferon-gamma receptor gene. Am J Hum Genet 65: 709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessein AJ, Marquet S, Henri S, El Wali NE, Hillaire D, Rodrigues V, Prata A, Ali QM, Gharib B, de Reggi M, Magzoub MM, Saeed OK, Abdelhameed AA, Abel L (1999b) Infection and disease in human schistosomiasis mansoni are under distinct major gene control. Microbes Infect 1: 561–567 [DOI] [PubMed] [Google Scholar]
- Dib C, Faure S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, Marc S, Hazan J, Seboun E, Lathrop M, Gyapay G, Morissette J, Weissenbach J (1996) A comprehensive genetic map of the human genome based on 5264 microsatellites. Nature 380: 152–154 [DOI] [PubMed] [Google Scholar]
- Döffinger R, Jouanguy E, Dupuis S, Fondanèche MC, Stéphan JL, Emile JF, Lamhamedi S, Altare F, Pallier A, Barcenas-Morales G, Krause C, Pestka S, Schreiber RD, Novelli F, Casanova JL (2000) Partial interferon gamma receptor signalling chain deficiency in a patient with bacille Calmette-Guérin and Mycobacterium abscessus infection. J Infect Dis 181: 379–384 [DOI] [PubMed] [Google Scholar]
- Dorman SE, Holland SM (1998) Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J Clin Invest 101: 2364–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos RJ, Dubos J (1952) The White Plague; Tuberculosis, Man, and Society. Little Brown, Boston [Google Scholar]
- Dupuis S, Dargemont C, Fieschi C, Thomassin N, Rosenzweig S, Harris J, Holland SM, Schreiber RD, Casanova JL (2001) Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science 293: 300–303 [DOI] [PubMed] [Google Scholar]
- Dupuis S, Jouanguy E, Al-Hajjar S, Fieschi C, Al-Mohsen IZ, Al-Jumaah S, Yang K, Chapgier A, Eidenschenk C, Eid P, Al Ghonaium A, Tufenkeji H, Frayha H, Al-Gazlan S, Al-Rayes H, Schreiber RD, Gresser I, Casanova JL (2003) Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat Genet 33: 388–391 [DOI] [PubMed] [Google Scholar]
- Edwards JH (1969) Familial predisposition in man. Br Med Bull 25: 58–64 [DOI] [PubMed] [Google Scholar]
- Enders A, Pannicke U, Berner R, Henneke P, Radlinger K, Schwarz K, Ehl S (2004) Two siblings with lethal pneumococcal meningitis in a family with a mutation in Interleukin-1 receptor-associated kinase 4. J Pediatr 145: 698–700 [DOI] [PubMed] [Google Scholar]
- Fieschi C, Bosticardo M, De Beaucoudrey L, Boisson-Dupuis S, Feinberg J, Santos OF, Bustamante J, Levy J, Candotti F, Casanova JL (2004) A novel form of complete IL-12/IL-23 receptor {beta}1 deficiency with cell surface-expressed nonfunctional receptors. Blood 104: 2095–2101 [DOI] [PubMed] [Google Scholar]
- Filipe-Santos O, Bustamante J, Chapgier A, Vogt G, De Beaucoudrey L, Feinberg J, Jouanguy E, Dupuis-Boisson S, Fieschi C, Picard C, Casanova JL (2006a) Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol 18: 347–361 [DOI] [PubMed] [Google Scholar]
- Filipe-Santos O, Bustamante J, Haverkamp MH, Vinolo E, Ku CL, Puel A, Frucht DM, Christel K, von Bernuth H, Jouanguy E, Feinberg J, Durandy A, Senechal B, Chapgier A, Vogt G, de Beaucoudrey L, Fieschi C, Picard C, Garfa M, Chemli J, Bejaoui M, Tsolia MN, Kutukculer N, Plebani A, Notarangelo L, Bodemer C, Geissmann F, Israel A, Veron M, Knackstedt M, Barbouche R, Abel L, Magdorf K, Gendrel D, Agou F, Holland SM, Casanova JL (2006b) X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. J Exp Med 203: 1745–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA (1918) The correlation between relatives on the supposition of Mendelian inheritance. Trans R Soc Edinb 52: 399–433 [Google Scholar]
- Haller O, Kochs G (2003) Interferon-induced Mx proteins: dynamin-like GTPases with antiviral activity. Traffic 3: 710–717 [DOI] [PubMed] [Google Scholar]
- Hill AV (2001) The genomics and genetics of human infectious disease susceptibility. Annu Rev Genomics Hum Genet 2: 373–400 [DOI] [PubMed] [Google Scholar]
- Hill AVS (2006) Aspects of genetic susceptibility to human infectious diseases. Annu Rev Genet 40: 469–486 [DOI] [PubMed] [Google Scholar]
- Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile JF, Newport M, Levin M, Blanche S, Seboun E, Fischer A, Casanova JL (1996) Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N Engl J Med 335: 1956–1961 [DOI] [PubMed] [Google Scholar]
- Jouanguy E, Dupuis S, Pallier A, Döffinger R, Fondanèche MC, Lamhamedi-Cherradi S, Altare F, Emile JF, Lutz P, Bordigoni P, Cokugras H, Akcakaya N, Landman-Parker J, Donnadieu J, Camcioglu Y, Casanova JL (2000) In a novel form of complete IFNγR1 deficiency, cell-surface receptors fail to bind IFNγ. J Clin Invest 105: 1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanguy E, Lamhamedi-Cherradi S, Altare F, Fondaneche MC, Tuerlinckx D, Blanche S, Emile JF, Gaillard JL, Schreiber R, Levin M, Fischer A, Hivroz C, Casanova JL (1997) Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guerin infection and a sibling with clinical tuberculosis. J Clin Invest 100: 2658–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanguy E, Lamhamedi-Cherradi S, Lammas D, Dorman SE, Fondanèche M-C, Dupuis S, Döffinger R, Altare F, Emile J-F, Girdelstone J, Ducoulombier H, Edgar D, Clarke J, Oxelius VA, Brai M, Novelli V, Heyne K, Fischer A, Holland SH, Kumararatne DS, Schreiber RD, Casanova J-L (1999) A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nature Genet 21: 370–378 [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Beaty TH, Cohen BH (1993) Fundamentals of Genetic Epidemiology. Oxford University Press, New York [Google Scholar]
- Ku CL, Picard C, Erdös M, Jeurissen A, Bustamante J, Puel A, von Bernuth H, Filipe Santos O, Chang HH, Lawrence T, Raes M, Marodi L, Bossuyt X, Casanova JL (2007) IRAK-4 and NEMO mutations in two otherwise healthy children with recurrent invasive pneumococcal disease. J Med Genet 44: 16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku CL, Yang K, Bustamante J, Puel A, von Bernuth H, Dos Santos O, Lawrence T, Chang HH, Al-Mousa H, Picard C, Casanova JL (2005) Inherited disorders of human Toll-like receptor signalling: immunological implications. Immunol Rev 203: 10–20 [DOI] [PubMed] [Google Scholar]
- Kuhns DB, Long Priel DA, Gallin JI (1997) Endotoxin and IL-1 hyporesponsiveness in a patient with recurrent bacterial infections. J Immunol 158: 3959–3964 [PubMed] [Google Scholar]
- Lalouel JM, Rao DC, Morton NE, Elston RC (1983) A unified model for complex segregation analysis. Am J Hum Genet 35: 816–826 [PMC free article] [PubMed] [Google Scholar]
- Lam-Yuk-Tseung S, Gros P (2003) Genetic control of susceptibility to bacterial infections in mouse models. Cell Microbiol 5: 299–313 [DOI] [PubMed] [Google Scholar]
- Lander ES, Schork NJ (1994) Genetic dissection of complex traits. Science 265: 2037–2048 [DOI] [PubMed] [Google Scholar]
- Lederberg J (1999) J.B.S. Haldane (1949) on infectious disease and evolution. Genetics 153: 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Newport MJ, D'Souza S, Kalabalikis P, Brown IN, Lenicker HM, Agius PV, Davies EG, Thrasher A, Klein N, Blackwell J (1995) Familial disseminated atypical mycobacterial infection in childhood: a human mycobacterial susceptibility gene? Lancet 345: 79–83 [DOI] [PubMed] [Google Scholar]
- Lewandowsky F, Lutz W (1922) Ein Fall einer bisher nicht beschriebenen Hauterkrankung (Epidermodysplasia verruciformis). Arch Dermatol Syphilol 141: 193–203 [Google Scholar]
- Lutz W (1946) A propos de l'épidermodysplasie verruciforme. Dermatologica 92: 30–43 [PubMed] [Google Scholar]
- Marquet S, Abel L, Hillaire D, Dessein H, Kalil J, Feingold J, Weissenbach J, Dessein AJ (1996) Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31-q33. Nat Genet 14: 181–184 [DOI] [PubMed] [Google Scholar]
- Mathew S, Overturf GD (2006) Complement and properidin deficiencies in meningococcal disease. Pediatr Infect Dis J 25: 255–256 [DOI] [PubMed] [Google Scholar]
- Medvedev AE, Lentschat A, Kuhns DB, Blanco JC, Salkowski C, Zhang S, Arditi M, Gallin JI, Vogel SN (2003) Distinct mutations in IRAK-4 confer hyporesponsiveness to lipopolysaccharide and interleukin-1 in a patient with recurrent bacterial infections. J Exp Med 198: 521–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LH, Mason SJ, Clyde DF, McGinniss MH (1976) The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med 295: 302–304 [DOI] [PubMed] [Google Scholar]
- Miller LH, Mason SJ, Dvorak JA, McGinniss MH, Rothman IK (1975) Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science 189: 561–563 [DOI] [PubMed] [Google Scholar]
- Mimouni J (1951) Notre expérience de trois années de vaccination à Constantine; étude de 25 cas de complications. Alger Médicale 55: 1138–1147 [PubMed] [Google Scholar]
- Min-Oo G, Gros P (2005) Erythrocyte variants and the nature of their malaria protective effect. Cell Microbiol 7: 753–763 [DOI] [PubMed] [Google Scholar]
- Mira MT, Alcais A, Nguyen VT, Moraes MO, Di Flumeri C, Vu HT, Mai CP, Nguyen TH, Nguyen NB, Pham XK, Sarno EN, Alter A, Montpetit A, Moraes ME, Moraes JR, Dore C, Gallant CJ, Lepage P, Verner A, Van De Vosse E, Hudson TJ, Abel L, Schurr E (2004) Susceptibility to leprosy is associated with PARK2 and PACRG. Nature 427: 636–640 [DOI] [PubMed] [Google Scholar]
- Mira MT, Alcais A, Van Thuc N, Thai VH, Huong NT, Ba NN, Verner A, Hudson TJ, Abel L, Schurr E (2003) Chromosome 6q25 is linked to susceptibility to leprosy in a Vietnamese population. Nat Genet 33: 412–415 [DOI] [PubMed] [Google Scholar]
- Muller-Myhsok B, Stelma FF, Guisse-Sow F, Muntau B, Thye T, Burchard GD, Gryseels B, Horstmann RD (1997) Further evidence suggesting the presence of a locus, on human chromosome 5q31–q33, influencing the intensity of infection with Schistosoma mansoni. Am J Hum Genet 61: 452–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, Levin M (1996) A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med 335: 1941–1949 [DOI] [PubMed] [Google Scholar]
- Notarangelo L, Casanova JL, Conley ME, Chapel H, Fischer A, Puck J, Roifman C, Seger R, Geha RS (2006) Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee Meeting in Budapest, 2005. J Allergy Clin Immunol 117: 883–896 [DOI] [PubMed] [Google Scholar]
- Ochs H, Smith CIE, Puck J (2006) Primary Immunodeficiencies: a Molecular and Genetic Approach. Oxford University Press, New York [Google Scholar]
- Orth G (2006) Genetics of Epidermodysplasia verruciformis: insights into host defense against papillomaviruses. Semin Immunol 18: 362–374 [DOI] [PubMed] [Google Scholar]
- Özbek N, Fieschi C, Yilmaz BT, De Beaucoudrey L, Bikmaz YE, Feinberg J, Casanova JL (2005) Interleukin-12 receptor beta 1 chain deficiency in a child with disseminated tuberculosis. Clin Infect Dis 40: e55–e58 [DOI] [PubMed] [Google Scholar]
- Pallamary P (1955) Translation of Gerhard Armauer Hansen. Spedalskhedens Aarsager [causes of leprosy]. Int J Lepr 23: 307–309 [Google Scholar]
- Papathanasiou P, Goodnow CC (2005) Connecting mammalian genome with phenome by ENU mouse mutagenesis: gene combinations specifying the immune system. Annu Rev Genet 39: 241–262 [DOI] [PubMed] [Google Scholar]
- Picard C, Casanova JL, Abel L (2006) Mendelian traits that confer predisposition or resistance to specific infections in humans. Curr Opin Immunol 18: 383–390 [DOI] [PubMed] [Google Scholar]
- Picard C, Puel A, Bonnet M, Ku CL, Bustamante J, Yang K, Soudais C, Dupuis S, Feinberg J, Fieschi C, Elbim C, Hitchcock R, Lammas D, Davies G, Al-Ghonaium A, Al-Rayes H, Al-Jumaah S, Al-Hajjar S, Al-Mohsen IZ, Frayha HH, Rucker R, Hawn TR, Aderem A, Tufenkeji H, Haraguchi S, Day NK, Good RA, Gougerot-Pocidalo MA, Ozinsky A, Casanova JL (2003a) Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 299: 2076–2079 [DOI] [PubMed] [Google Scholar]
- Picard C, Puel A, Ku CL, Casanova JL (2003b) Primary immunodeficiencies associated with pneumococcal disease. Curr Opin Allergy Clin Immunol 3: 451–459 [DOI] [PubMed] [Google Scholar]
- Plancoulaine S, Gessain A, Joubert M, Tortevoye P, Jeanne I, Talarmin A, de The G, Abel L (2000) Detection of a major gene predisposing to human T lymphotropic virus type I infection in children among an endemic population of African origin. J Infect Dis 182: 405–412 [DOI] [PubMed] [Google Scholar]
- Plancoulaine S, Gessain A, van Beveren M, Tortevoye P, Abel L (2003) Evidence for a recessive major gene predisposing to human herpesvirus 8 (HHV-8) infection in a population in which HHV-8 is endemic. J Infect Dis 187: 1944–1950 [DOI] [PubMed] [Google Scholar]
- Pritchard JK (2001) Are rare variants responsible for susceptibility to complex diseases? Am J Hum Genet 69: 124–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Cox NJ (2002) The allelic architecture of human disease genes: common disease-common variant…or not? Hum Mol Genet 11: 2417–2423 [DOI] [PubMed] [Google Scholar]
- Puffer R (1944) Familial Susceptibility to Tuberculosis; its Importance as a Public Health Problem. Harvard University Press, Cambridge, MA [Google Scholar]
- Ramoz N, Rueda LA, Bouadjar B, Favre M, Orth G (1999) A susceptibility locus for Epidermodysplasia verruciformis, an abnormal predisposition to infection with the oncogenic human papillomavirus type 5, maps to chromosome 17qter in a region containing a psoriasis locus. J Invest Dermatol 112: 259–263 [DOI] [PubMed] [Google Scholar]
- Ramoz N, Rueda LA, Bouadjar B, Montoya LS, Orth G, Favre M (2002) Mutations in two adjacent novel genes are associated with Epidermodysplasia verruciformis. Nat Genet 32: 579–581 [DOI] [PubMed] [Google Scholar]
- Rigaud S, Fondaneche MC, Lambert N, Pasquier B, Mateo V, Soulas P, Galicier L, Le Deist F, Rieux-Laucat F, Revy P, Fischer A, De Saint Basile G, Latour S (2006) XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature 444: 110–114 [DOI] [PubMed] [Google Scholar]
- Risch N (1990) Linkage strategies for genetically complex traits. III. The effect of marker polymorphism on analysis of affected relative pairs. Am J Hum Genet 46: 242–253 [PMC free article] [PubMed] [Google Scholar]
- Risch N, Merikangas K (1996) The future of genetic studies of complex human diseases. Science 273: 1516–1517 [DOI] [PubMed] [Google Scholar]
- Rosenzweig SD, Dorman SE, Uzel G, Shaw S, Scurlock A, Brown MR, Buckley RH, Holland SM (2004) A novel mutation in IFN-gamma receptor 2 with dominant negative activity: biological consequences of homozygous and heterozygous states. J Immunol 173: 4000–4008 [DOI] [PubMed] [Google Scholar]
- Schurr E, Alcais A, de Leseleuc L, Abel L (2006) Genetic predisposition to leprosy: a major gene reveals novel pathways of immunity to Mycobacterium leprae. Semin Immunol 18: 404–410 [DOI] [PubMed] [Google Scholar]
- Siddiqui MR, Meisner S, Tosh K, Balakrishnan K, Ghei S, Fisher SE, Golding M, Shanker Narayan NP, Sitaraman T, Sengupta U, Pitchappan R, Hill AV (2001) A major susceptibility locus for leprosy in India maps to chromosome 10p13. Nat Genet 27: 439–441 [DOI] [PubMed] [Google Scholar]
- Smith MG, Lennette EH, Reames HR (1941) Isolation of the virus of herpes simplex and the demonstration of intranuclear inclusions in a case of acute encephalitis. Am J Pathol 17: 55–68 [PMC free article] [PubMed] [Google Scholar]
- Takada H, Yoshikawa H, Imaizumi M, Kitamura T, Takeyama J, Kumaki S, Nomura A, Hara T (2006) Delayed separation of the umbilical cord in two siblings with interleukin-1 receptor-associated kinase 4 deficiency: rapid screening by flow cytometer. J Pediatr 148: 546–548 [DOI] [PubMed] [Google Scholar]
- Tournamille C, Colin Y, Cartron JP, Le Van Kim C (1995) Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals. Nat Genet 10: 224–228 [DOI] [PubMed] [Google Scholar]
- Vogt G, Chapgier A, Yang K, Chuzhanova N, Feinberg J, Fieschi C, Boisson-Dupuis S, Alcais A, Filipe-Santos O, Bustamante J, de Beaucoudrey L, Al-Mohsen I, Al-Hajjar S, Al-Ghonaium A, Adimi P, Mirsaeidi M, Khalilzadeh S, Rosenzweig S, de la Calle Martin O, Bauer TR, Puck JM, Ochs HD, Furthner D, Engelhorn C, Belohradsky B, Mansouri D, Holland SM, Schreiber RD, Abel L, Cooper DN, Soudais C, Casanova JL (2005) Gains of glycosylation comprise an unexpectedly large group of pathogenic mutations. Nat Genet 37: 692–700 [DOI] [PubMed] [Google Scholar]
- WHO (2006) Global tuberculosis control. Surveillance, planning, financing. WHO report, www.who.int/tb/publications/global_ report/2006/download_centre/en/inde x.html [Google Scholar]
- WHO (2006) Global leprosy situation, 2006. Wkly Epidemiol Rec 81: 309–316 [PubMed] [Google Scholar]
- Woolhouse ME, Webster JP, Domingo E, Charlesworth B, Levin BR (2002) Biological and biomedical implications of the coevolution of pathogens and their hosts. Nat Genet 32: 569–577 [DOI] [PubMed] [Google Scholar]
- Wright A, Charlesworth B, Rudan I, Carothers A, Campbell H (2003) A polygenic basis for late-onset disease. Trends Genet 19: 97–106 [DOI] [PubMed] [Google Scholar]
- Yang K, Puel A, Zhang S, Eidenschenk C, Ku CL, Casrouge A, Picard C, von Bernuth H, Senechal B, Plancoulaine S, Al-Hajjar S, Al-Ghonaium A, Marodi L, Davidson D, Speert D, Roifman C, Garty BZ, Ozinsky A, Barrat FJ, Coffman RL, Miller RL, Li X, Lebon P, Rodriguez-Gallego C, Chapel H, Geissmann F, Jouanguy E, Casanova JL (2005) Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda Is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity 23: 465–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama WM (2005) Specific and non-specific natural killer cell responses to viral infection. Adv Exp Med Biol 560: 57–61 [DOI] [PubMed] [Google Scholar]


