Abstract
Chemokines belong to an expanding family of cytokines the primary function of which is recruitment of leukocytes to inflammatory sites. Recent evidence has shown their presence in the central nervous system. Because inflammatory responses have been implicated in the pathogenesis of Alzheimer’s disease (AD), we studied the expression of CCR3, CCR5, and their ligands in normal and AD brains by immunohistochemistry. CCR3 and CCR5 are present on microglia of both control and AD brains, with increased expression on some reactive microglia in AD. Immunohistochemistry for MIP-1β, MIP-1α, RANTES, eotaxin, and MCP-3 (ligands for CCR5 and/or CCR3) revealed the presence of MIP-1β predominantly in a subpopulation of reactive astrocytes, which were more widespread in AD than control brains, and MIP-1α predominantly in neurons and weakly in some microglia in both AD and controls. Many of the CCR3+ or CCR5+ reactive microglia and MIP-1β+ reactive astrocytes were found associated with amyloid deposits. Immunoreactivity for eotaxin, RANTES, and MCP-3 were not detected. Detection of these β-chemokine receptors on microglia and some of their ligands in reactive astrocytes and neurons as well as microglia suggests a role for this system in glial-glial and glial-neuronal interactions, potentially influencing the progression of AD.
Chemokines belong to an expanding family of small cytokines the primary function of which is recruitment of leukocytes to inflammatory sites. Four classes of chemokines, α (CXC), β (CC), γ (C), and δ (CX3C), have been described. 1-3 Chemokine receptors are correspondingly named as CXCR (1 to 4), CCR (1 to 8), CR, and CX3CR. All of the receptors are members of the seven transmembrane domain receptor superfamily. There have been a number of reports showing the presence of some chemokines 4-9 and their receptors 10-13 in the central nervous system (CNS). Recently we have shown the presence of one of the α class of chemokine receptors, interleukin (IL)-8 receptor B (IL-8RB or CXCR2) in hippocampal and cortical neurons in normal brain and also in neuritic plaques of Alzheimer’s disease (AD) brains. 10 Horuk et al 11,12 reported in the CNS the presence of Duffy antigen and similar findings for IL-8RB. Lavi et al 13 demonstrated the expression of CXCR4 in neurons as well as microglia.
β-Chemokine receptors may also have a role in the CNS. CCR3 and CCR5 have drawn considerable attention as the recently found co-receptors for HIV entry into cells, 14-16 including microglia, 17 which are the primary target of HIV infection in the CNS. HIV-infected patients may develop a progressive dementia, with motor and behavioral impairment termed AIDS-dementia complex. This β-chemokine system appeared to be activated and may contribute to this CNS pathology. 18,19 We hypothesized that activation of this system may also occur in AD and may potentially be involved in the disease pathogenesis. Here we describe an immunohistochemical study of CCR3, CCR5, and their ligands in AD and control brains.
Materials and Methods
Monoclonal and Polyclonal Antibodies
One CCR3 monoclonal antibody (MAb) (7B11; IgG) and three CCR5 MAbs (3A9, 5C7, and 2D7; all are IgG) were made at LeukoSite (Cambridge, MA) by immunizing mice using human CCR3 and CCR5 transfectants, respectively. 20,21 For MIP-1β detection, mouse MAbs (1F12; IgG) from LeukoSite was used. For MIP-1α, a mouse MAb (11A3; IgG) 22 from LeukoSite and a mouse MAb (500-M74; IgG) from Peprotech (Rocky Hill, NJ) were used. For eotaxin, mouse MAbs (6H9, 2G6, and 1F5; all IgG) from LeukoSite were used. For RANTES, mouse MAbs from LeukoSite (5B9 and 9H9; both IgM), 22 R&D Systems, Minneapolis, MN (MAb-678; IgG) and Peprotech (500-M75; IgG) were used. For MCP-3, the mouse MAb 6H5 (IgG) from LeukoSite was used. All chemokine MAbs from LeukoSite were highly specific to each specified chemokine as determined by ELISA and Western blotting, and most of them were shown to be good reagents for immunohistochemical study on paraffin or frozen sections. For amyloid-β (Aβ) detection, rabbit polyclonal antibody R1282 (a kind gift of Dr. Selkoe) was used. For glial acidic fibrillary protein (GFAP), a rabbit anti-GFAP MAb (G-9269, Sigma Chemical Co., St. Louis, MO) was used. Antibodies were either purified and used at a concentration of 5 μg/ml or as hybridoma supernatants (1:3 diluted).
Preabsorption of the anti-MIP-1α antibody 11A3 was carried out by incubating 5 μg/ml 11A3 with 10 μg/ml MIP-1α protein (Peprotech 300-08) for at least 1 hour at room temperature before being used for immunohistochemistry.
Tissue Preparation
Postmortem brain tissues, including temporal lobe (containing the hippocampal formation, parahippocampal gyrus, and adjacent temporal neocortex), visual cortex, caudate, putamen, and cerebellum were obtained from the Massachusetts AD Research Center brain bank, and the diagnosis of AD and control was made using Khachaturian criteria. 23 All brains were processed within 24 (average, 12) hours of death. Fresh tissue blocks were immersion fixed in periodate-lysine-paraformaldehyde (PLP) and processed as previously described. 10 Sections were cut with a sledge freezing microtome at 50 μm thickness.
Temporal lobes from at least 12 age-compatible healthy controls and 18 AD cases, ages from 21 to 104 years, were examined unless indicated otherwise. Sections of visual cortex, putamen, caudate, and cerebellum from several cases were also studied.
Immunohistochemistry
Immunohistochemistry was carried out on free-floating sections using a previously described method. 10 Horseradish peroxidase (HRP)-conjugated goat anti-mouse Ig (Jackson ImmunoResearch Laboratories, West Grove, PA) at 1:200 dilution was used as the secondary antibody. 3′,3′-Diaminobenzidine (DAB) peroxidase substrate kit (Vector Laboratories, Burlingame, CA) was used as chromogen. Video images were captured on a Bioquant (Nashville, TN) image analysis system.
Confocal Microscopic Analysis
Double immunofluorescent staining was carried out essentially as previously described. 10 Bodipy- or Cy3-conjugated anti-mouse or anti-rabbit IgG (1:200 diluted in 1.5% normal goat serum) were used as the secondary antibodies. Confocal images of fluorescent immunostained sections were obtained on a BioRad MRC 1024 confocal microscope with a krypton/argon laser (BioRad, Richmond, CA). The excitation and the emission filters were 488 nm and 522 nm for Bodipy-fluorescein and 568 nm and 605 nm for Cy3.
Results
Elevated CCR3 and CCR5 Expression on Reactive Microglia
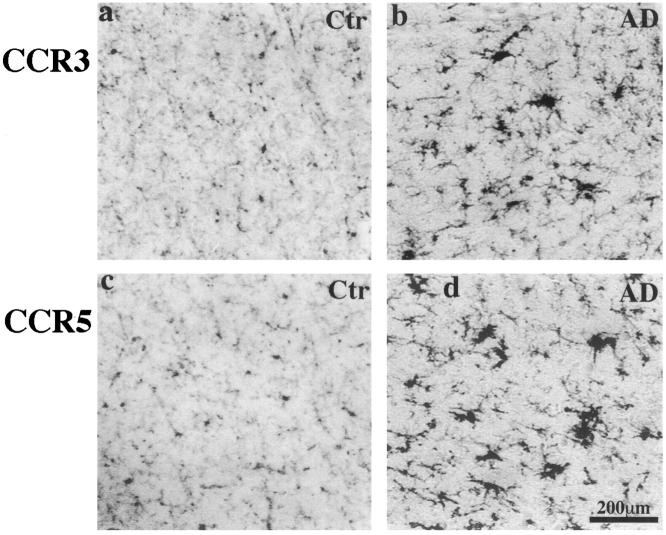
Using well characterized CCR3 (7B11) and CCR5 (3A9) antibodies, CCR3 and CCR5 expression was found on microglia of all tested regions in both control and AD brains (Figure 1) ▶ . Staining by two other CCR5 antibodies, 2D7 and 5C7, gave a similar staining pattern. The intensities of CCR3 and CCR5 staining were very similar. In control brains, most of the CCR3+ or CCR5+ microglia appeared to be in a resting state. However, in AD, the expression of CCR3 and CCR5 were increased on many reactive microglia (Figure 1) ▶ , and clusters of CCR3+ or CCR5+ reactive microglia were frequently found scattered throughout hippocampal formation and neocortex. Double immunostaining in AD revealed that many CCR3+ or CCR5+ reactive microglia were associated with amyloid deposits (Figure 2, a–d) ▶ , although many were also found in apparently plaque-free areas. Case to case variations were attributed in part to postmortem interval (PMI), with weaker staining in longer PMI cases.
Figure 1.
CCR3 (7B11) and CCR5 (3A9) immunoreactivity in the inferior temporal lobes of a 58-year-old control patient and an 81-year-old AD patient with duration of illness for 12 years (PMIs less than 8 hours). CCR3 (a and b) and CCR5 (c and d) immunoreactivities are clearly seen on microglia of both cases. In the control case, the majority of cells stained are resting microglia, whereas in the AD brain both resting and reactive microglia cells are clearly stained, and some reactive microglia appear in clusters. All images have the same scale of magnification. Scale bar, 200 μm.
Figure 2.
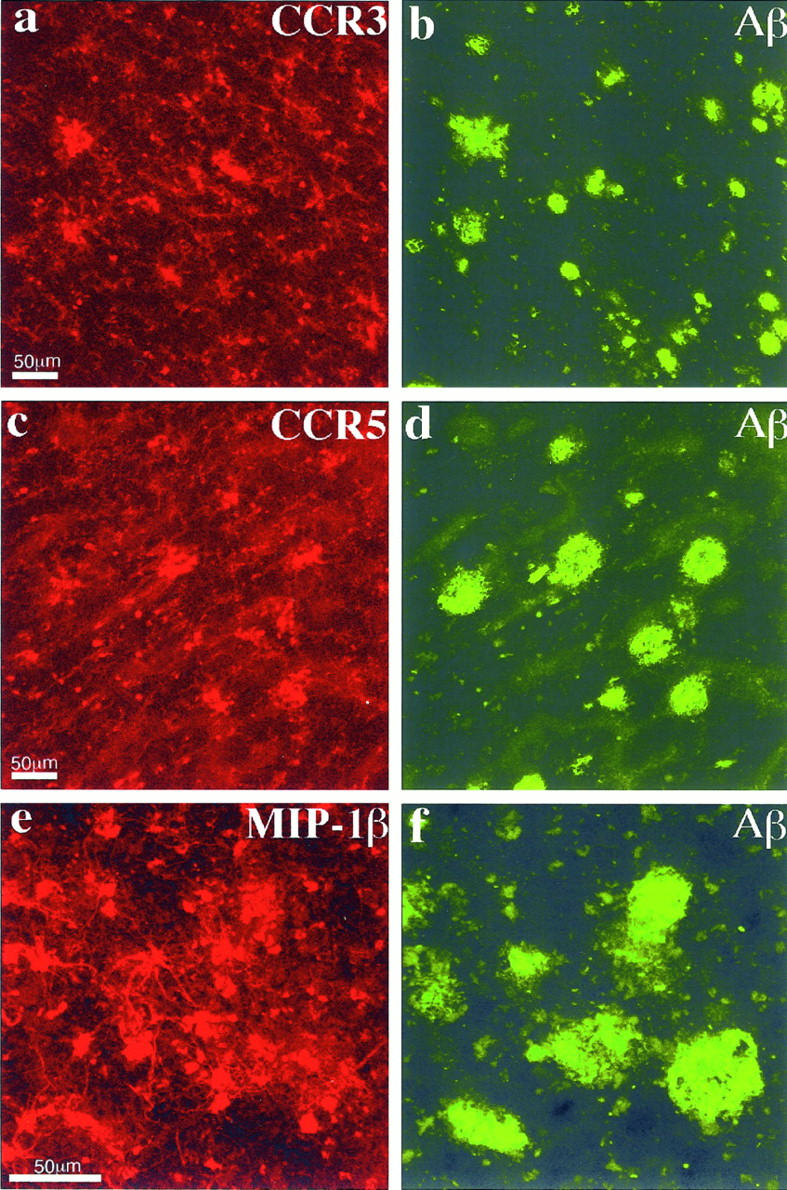
Confocal images of double immunofluorescent staining. a and b: CCR3 (7B11, Cy3-red) versus Aβ (R1282, bodipy-green); c and d: CCR5 (3A9, Cy3-red) versus Aβ (R1282, bodipy-green); e and f: MIP-1β (1F12, Cy3-red) versus Aβ (R1282, bodipy-green). In a to d, many of the CCR3+ and CCR5+ reactive microglia can be seen associated with Aβ deposits. In e to f, some of the MIP-1β+ reactive astrocytes can also been seen in the vicinity of Aβ deposits. Images a to d are from the same AD case shown in Figure 1 ▶ ; images e to f are from an 81-year-old AD patient with duration of illness for 8 years (PMI, 4 hours). Each of the images is a projection of five Z series images 0.7 μm apart. Scale bars, 50 μm.
The ligands for CCR3 include eotaxin, RANTES, MCP-3, and MCP-4; the ligands for CCR5 include MIP-1β, MIP-1α, and RANTES. 2 As both CCR3 and CCR5 were found to be present on microglia in both AD and non-AD brains, we further examined whether some of their ligands are also expressed in the brain. Immunohistochemical study for MIP-1β, MIP-1α, RANTES, eotaxin, and MCP-3 were then carried out.
Elevated MIP-1β Expression in Reactive Astrocytes
MIP-1β expression was found in a subpopulation of astrocytes in all brain tissues tested. Although some apparently resting astrocytes were weakly positive for MIP-1β, most of the strongly stained astrocytes appeared to be in a reactive state as judged by morphology. In control brains, weak MIP-1β staining appeared in a subpopulation of resting astrocytes (Figure 3, a and b) ▶ , with some reactive astrocytes mainly seen in subpial areas. In contrast, in AD brains, more widespread and much stronger MIP-1β staining of astrocyte was observed (Figure 3, c and d) ▶ . Generally, in AD brains, the astrocyte staining appeared to be more abundant in the hippocampal formation and the entorhinal cortex than in the neocortex. MIP-1β+ astrocytes were frequently seen associated with amyloid deposits (Figure 2, e and f) ▶ .
Figure 3.
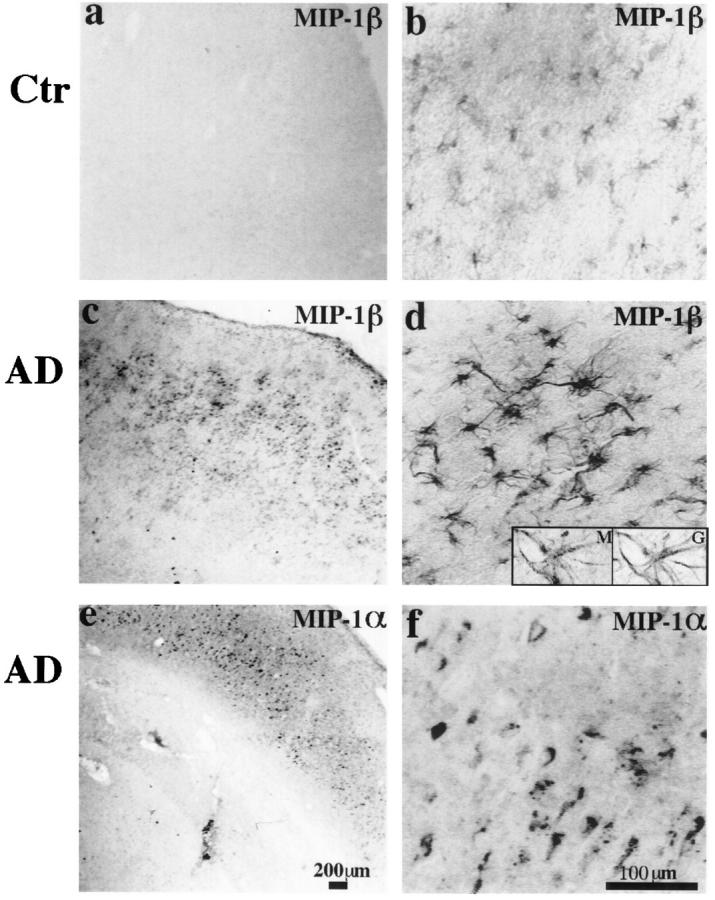
a to d: MIP-1β (1F12) immunoreactivity in the inferior temporal lobes of a 63-year-old control patient and a 58-year-old AD patient (PMIs less than 8 hours). In the control case, weak MIP-1β staining can be found on a small population of resting and some occasional reactive astrocytes. However, in the AD case, much more widespread and stronger astrocyte staining is observed, and most of the astrocytes appear to be reactive. Insets are high-power images (inverted images) of double staining of MIP-1β (M, Cy3) versus GFAP (G, bodipy), showing a MIP-1β-positive cell is also clearly positive for GFAP. e to f: MIP-1α (11A3) immunoreactivity in the hippocampal formation of an 84-year-old AD patient (PMI, 7 hours). A diffused pattern of neuronal staining can be seen. Both neurons and neuropil were stained. Some neurons showed increased expression of MIP-1α. This pattern of immunoreactivity can be completely blocked by preabsorption with MIP-1α protein. a, c, and e: Low-power images (scale bar, 200 μm); b, d, and f are higher-power images (scale bar, 100 μm).
Double immunostaining with GFAP showed that the MIP-1β immunoreactive cells were indeed astrocytes (Figure 3d ▶ , insets) and MIP-1β+ cells represent a subpopulation of the reactive astrocytes found in AD brains (not shown).
There was rather large case to case variation in the abundance and intensity of MIP-1β staining of astrocytes, probably due to technical factors, including postmortem interval, as well as differences potentially attributable to different stages of the disease process. In some cases, cortical neurons were also weakly stained for MIP-1β.
Detection of MIP-1α in Neurons and Microglia
MIP-1α immunoreactivity (detected by MAb 11A3) was found in the gray matter in both AD and control brains. A diffuse pattern of neuronal staining was observed. Neurons and neuropil of dentate gyrus, CA regions, and cortex were all positive (Figure 3, e and f) ▶ . The clarity of neuronal staining differed greatly among cases. Some CA region neurons and cortical neurons showed increased expression of MIP-1α. Weak microglia staining mainly in the white matter was also observed, and stronger microglia staining was seen in some AD brains. A similar pattern of staining was also observed using another MIP-1α MAb from a different source (Peprotech). Specificity of the antibody 11A3 was tested by inhibition assay. Its immunoreactivity was completely abolished by preabsorption with MIP-1α protein (data not shown), supporting the specificity of its immunostaining pattern.
Evaluation of Eotaxin, RANTES, and MCP-3 Expression
Five cases that showed the strongest MIP-1β and MIP-1α staining were tested for the other known ligands, eotaxin, RANTES, and MCP-3 expression. Despite using antibodies from several sources, we were unable to detect immunoreactivity for these ligands in brain.
Discussion
The primary roles of chemokine receptors are to mediate activation and migration of leukocytes. In the periphery, CCR3 is expressed on human eosinophils, basophils, T helper 2 cells, and dendritic cells. 20,24-26 CCR5 is expressed on a subset of effector/memory T cells in blood as well as monocytes/macrophages. 21,27 On exposure to its ligands, each receptor is believed to be able to mediate chemotaxis of the corresponding cells through a common G-protein-coupled signaling mechanism, and for CCR5, its signaling pathway seems independent of its ability to support HIV-1 infection in host cells. 28,29 Here we demonstrate immunohistochemical expression of these β-chemokine receptors in the human CNS and illustrate their localization on reactive microglia surrounding senile plaques in AD. CCR3 and CCR5 are the only β-chemokine receptors documented on microglia, and in vitro experiments have demonstrated an increased migratory response of this cell type to β-chemokines. 30
There are several known ligands for these receptors. Ligands for CCR3 include eotaxin, RANTES, MCP-3, and MCP-4; ligands for CCR5 include MIP-1β, MIP-1α, and RANTES. 1,2 Some of those ligands have previously been reported in the CNS. In rodent models of spinal cord injury, MIP-1β and MIP-1α mRNA were found to be up-regulated within the first hour after injury, 31 and resident CNS cells were the main source for these chemokine mRNAs. Immunohistochemical staining has revealed a diffuse expression of both RANTES and MIP-1β in necrotic tissue after brain trauma and of MIP-1β on reactive astrocytes near the lesion site. 32 In rodent experimental autoimmune encephalomyelitis (EAE) models, expression of RANTES by astrocytes and microglia has also been observed. 33,34 The capacity of the human CNS to express some β-chemokines under pathophysiological conditions has also been demonstrated. Northern blot data have suggested the constitutive presence of MIP-1α in the brain. 35 Elevated MIP-1α and MIP-1β have been detected by reverse transcriptase polymerase chain reaction, and reverse transcriptase in situ polymerase chain reaction in brain tissue from patients with AIDS dementia compared with infected patients without dementia 18,19 and cells expressing these chemokine mRNAs were identified morphologically as astrocytes and microglia. In vitro experiments have also shown that MIP-1α, MIP-1β, and RANTES can be released by mixed brain cell cultures by tumor necrosis factor-α treatment. 36 There has been no report so far on the expression of eotaxin, MCP-3, or MCP-4 in CNS cells either in rodents or in primates.
Our current study detects two of the β-chemokines, MIP-1α (also a ligand for CCR1 in addition to CCR5) and MIP-1β (a ligand only for CCR5), on specific populations of cells in the human brain. MIP-1α appears to be constitutively expressed at a low level by neurons and microglia, whereas MIP-1β is predominantly expressed by a subpopulation of reactive astrocytes. These results are largely in accord with the data on their mRNA expression. 18,19 Although the MIP-1α immunoreactivity does not apparently differ between AD and control cases, the amount of its expression remains to be quantified. The observation of more abundant MIP-1β-positive astrocytes in AD brains and their association with amyloid deposits suggests an involvement in plaque-associated responses. We speculate that these chemokines could contribute to the recruitment of microglia to Aβ deposits. Interestingly, MIP-1β and MIP-1α are the major β-chemokines found selectively induced from monocytes after active HIV-1 infection and have been found elevated in brains of AIDS dementia patients compared with those HIV-1-infected patients without dementia. 18,19 The potentially detrimental role of these chemokines to neuronal functions requires further investigation.
It is known that cytokines are difficult to detect by immunohistochemistry in human brain, so that the failure to detect RANTES, eotaxin, and MCP-3 in our study may reflect a sensitivity issue and does not rule out their potential presence in brain nor their up-regulation in AD. Antibodies for the other CCR3 ligand, MCP-4, are not currently available.
The presence of both β-chemokines and their receptors in the control brains suggest a physiological role for these molecules, which are important in cell-cell communication in the periphery. Although it is unclear what effect these β-chemokines have on neuronal functions, the elevated expression of CCR3/5 on reactive microglia and MIP-1β in reactive astrocytes of AD brains and their association with AD pathology suggest their involvement in AD pathogenesis. Taken together with earlier studies demonstrating α-chemokine receptors CXCR2 and CXCR4 on neurons, the presence of the β-chemokine receptors CCR3 and CCR5 on microglia and CCR5 ligands in glia as well as in neurons implies a new role for this class of molecules in mediating glial-glial and glial-neuronal interactions.
Acknowledgments
We thank Dr. Walter Newman (LeukoSite) for his support of this work and Massachusetts Disease Research Center brain bank (Dr. E.T. Hedley-Whyte, Director) for brain tissues. We also thank Dr. Roger Knowles for his careful reading of this manuscript.
Footnotes
Address reprint requests to Dr. Bradley T. Hyman, Alzheimer’s Research Unit, Massachusetts General Hospital-East, 149 13th Street (CNY 6405), Charlestown, MA 02129. E-mail: B_hyman@helix.mgh.harvard.edu.
Supported by NIH grants AG05134 and AG08487.
References
- 1.Premack BA, Schall TJ: Chemokine receptors: gateways to inflammation and infection. Nature Med 1996, 2:1174-1178 [DOI] [PubMed] [Google Scholar]
- 2.Baggiolini M, Dewald B, Moser B: Human chemokines: an update. Annu Rev Immunol 1997, 15:675-705 [DOI] [PubMed] [Google Scholar]
- 3.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ: A new class of membrane-bound chemokine with a CX(3)C motif. Nature 1997, 385:640-644 [DOI] [PubMed] [Google Scholar]
- 4.Fisher SN, Vanguri P, Shin HS, Shin ML: Regulatory mechanisms of MuRantes and CRG-2 chemokine gene induction in central nervous system glial cells by virus. Brain Behav Immun 1995, 9:331-344 [DOI] [PubMed] [Google Scholar]
- 5.Glabinski AR, Tani M, Tuohy VK, Tuthill RJ, Ransohoff RM: Central nervous system chemokine mRNA accumulation follows initial leukocyte entry at the onset of acute murine experimental autoimmune encephalomyelitis. Brain Behav Immun 1995, 9:315-330 [DOI] [PubMed] [Google Scholar]
- 6.Glabinski AR, Balasingam V, Tani M, Kunkel SL, Strieter RM, Yong VW, Ransohoff RM: Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J Immunol 1996, 156:4363-4368 [PubMed] [Google Scholar]
- 7.Calvo CF, Yoshimura T, Gelman M, Mallat M: Production of monocyte chemotactic protein-1 by rat brain macrophages. Eur J Neurosci 1996, 8:1725-1734 [DOI] [PubMed] [Google Scholar]
- 8.Ransohoff RM, Glabinski A, Tani M: Chemokines in immune-mediated inflammation of the central nervous system. Cytokine Growth Factor Rev 1996, 7:35-46 [DOI] [PubMed] [Google Scholar]
- 9.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos JC, Gearing D: Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature 1997, 387:611-617 [DOI] [PubMed] [Google Scholar]
- 10.Xia MQ, Qin SX, McNamara M, Mackay C, Hyman BT: Interleukin-8 receptor B immunoreactivity in brain and neuritic plaques of Alzheimers disease. Am J Pathol 1997, 150:1267-1274 [PMC free article] [PubMed] [Google Scholar]
- 11.Horuk R, Martin AW, Wang ZX, Schweitzer L, Gerassimides A, Guo HH, Lu ZH, Hesselgesser J, Perez HD, Kim J, Parker J, Hadley TJ, Peiper SC: Expression Of chemokine receptors by subsets of neurons in the central nervous system. J Immunol 1997, 158:2882-2890 [PubMed] [Google Scholar]
- 12.Horuk R, Martin A, Hesselgesser J, Hadley T, Lu ZH, Wang ZX, Peiper SC: The Duffy antigen receptor for chemokines: structural analysis and expression in the brain. J Leukocyte Biol 1996, 59:29-38 [DOI] [PubMed] [Google Scholar]
- 13.Lavi E, Strizki JM, Ulrich AM, Zhang W, Fu L, Wang Q, Oconnor M, Hoxie JA, Gonzalezscarano F: CXCR-4 (Fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Pathol 1997, 151:1035-1042 [PMC free article] [PubMed] [Google Scholar]
- 14.Deng HK, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Dimarzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR: Identification of a major co-receptor for primary isolates Of HIV-1. Nature 1996, 381:661-666 [DOI] [PubMed] [Google Scholar]
- 15.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu LJ, Mackay CR, Larosa G, Newman W, Gerard N, Gerard C, Sodroski J: The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 1996, 85:1135-1148 [DOI] [PubMed] [Google Scholar]
- 16.Broder CC, Collman RG: Chemokine receptors and HIV. J Leukocyte Biol 1997, 62:20-29 [DOI] [PubMed] [Google Scholar]
- 17.He JL, Chen YZ, Farzan M, Choe HY, Ohagen A, Gartner S, Busciglio J, Yang XY, Hofmann W, Newman W, Mackay CR, Sodroski J, Gabuzda D: CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature 1997, 385:645-649 [DOI] [PubMed] [Google Scholar]
- 18.Schmidtmayerova H, Nottet HS, Nuovo G, Raabe T, Flanagan CR, Dubrovsky L, Gendelman HE, Cerami A, Bukrinsky M, Sherry B: Human immunodeficiency virus type 1 infection alters chemokine β peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci USA 1996, 93:700-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nuovo GJ, Alfieri ML: AIDS dementia is associated with massive, reactive HIV-1 infection and concomitant expression of several cytokines. Mol Med 1996, 2:358-366 [PMC free article] [PubMed] [Google Scholar]
- 20.Heath H, Qin SX, Rao P, Wu LJ, Larosa G, Kassam N, Ponath PD, Mackay CR: Chemokine receptor usage by human eosinophils: the importance Of CCR3 demonstrated using an antagonistic monoclonal antibody. J Clin Invest 1997, 99:178-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu LJ, Paxton WA, Kassam N, Ruffing N, Rottman JB, Sullivan N, Choe H, Sodroski J, Newman W, Koup RA, Mackay CR: CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1 in vitro. J Exp Med 1997, 185:1681-1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasseville VG, Smith MM, Mackay CR, Pauley DR, Mansfield KG, Ringler DJ, Lackner AA: Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am J Pathol 1996, 149:1459-1467 [PMC free article] [PubMed] [Google Scholar]
- 23.Khachaturian Z: Diagnosis of Alzheimer’s disease. Arch Neurol 1985, 52:1097-1105 [DOI] [PubMed] [Google Scholar]
- 24.Uguccioni M, Mackay CR, Ochensberger B, Loetscher P, Rhis S, Larosa GJ, Rao P, Ponath PD, Baggiolini M, Dahinden CA: High expression of the chemokine receptor CCR3 in human blood basophils: role in activation by eotaxin, MCP-4, and other chemokines. J Clin Invest 1997, 100:1137-1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sallusto F, Mackay CR, Lanzavecchia A: Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science 1997, 277:2005-2007 [DOI] [PubMed] [Google Scholar]
- 26.Sozzani S, Luini W, Borsatti A, Polentarutti N, Zhou D, Piemonti L, Damico G, Power CA, Wells TNC, Gobbi M, Allavena P, Mantovani A: Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J Immunol 1993, 159:1993-2000 [PubMed] [Google Scholar]
- 27.Bleul CC, Wu LJ, Hoxie JA, Springer TA, Mackay CR: The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA 1997, 94:1925-1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gosling J, Monteclaro FS, Atchison RE, Arai H, Tsou CL, Goldsmith MA, Charo IF: Molecular uncoupling of C-C chemokine receptor 5-induced chemotaxis and signal transduction from HIV-1 coreceptor activity. Proc Natl Acad Sci USA 1997, 94:5061-5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aramori I, Zhang J, Ferguson SSG, Bieniasz PD, Cullen BR, Caron MG: Molecular mechanism Of desensitization of the chemokine receptor CCR-5. Receptor signaling and internalization are dissociable from its role as an HIV-1 co-receptor. EMBO J 1997, 16:4606-4616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson PK, Hu SX, Salakjohnson J, Molitor TW, Chao CC: Differential production of and migratory response to β chemokines by human microglia and astrocytes. J Infect Dis 1997, 175:478-481 [DOI] [PubMed] [Google Scholar]
- 31.Bartholdi D, Schwab ME: Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci 1997, 9:1422-1438 [DOI] [PubMed] [Google Scholar]
- 32.Ghirnikar RS, Lee YL, He TR, Eng LF: Chemokine expression in rat stab wound brain injury. J Neurosci Res 1996, 46:727-733 [DOI] [PubMed] [Google Scholar]
- 33.Miyagishi R, Kikuchi S, Takayama C, Inoue Y, Tashiro K: Identification of cell types producing RANTES, MIP-1α and MIP-1β in rat experimental autoimmune encephalomyelitis by in situ hybridization. J Neuroimmunol 1997, 77:17-26 [DOI] [PubMed] [Google Scholar]
- 34.Sun Dm, Hu XZ, Liu XH, Whitaker JN, Walker WS: Expression of chemokine genes in rat glial cells: the effect of myelin basic protein-reactive encephalitogenic T cells. J Neuroci Res 1997, 48:192-200 [PubMed] [Google Scholar]
- 35.Ishizuka K, Igatayi R, Kimura T, Hieshima K, Kukita T, Kin Y, Misumi Y, Yamamoto M, Nomiyama H, Miura R, Takamatsu J, Katsuragi S, Miyakawa T: Expression and distribution of CC chemokine macrophage inflammatory protein-1-α/LD78 in the human brain. Neuroreport 1997, 8:1215-1218 [DOI] [PubMed] [Google Scholar]
- 36.Lokensgard JR, Gekker G, Ehrlich LC, Hu SX, Chao CC, Peterson PK: Proinflammatory cytokines inhibit HIV-1(Sf162) expression in acutely infected human brain cell cultures. J Immunol 1997, 158:2449-2455 [PubMed] [Google Scholar]