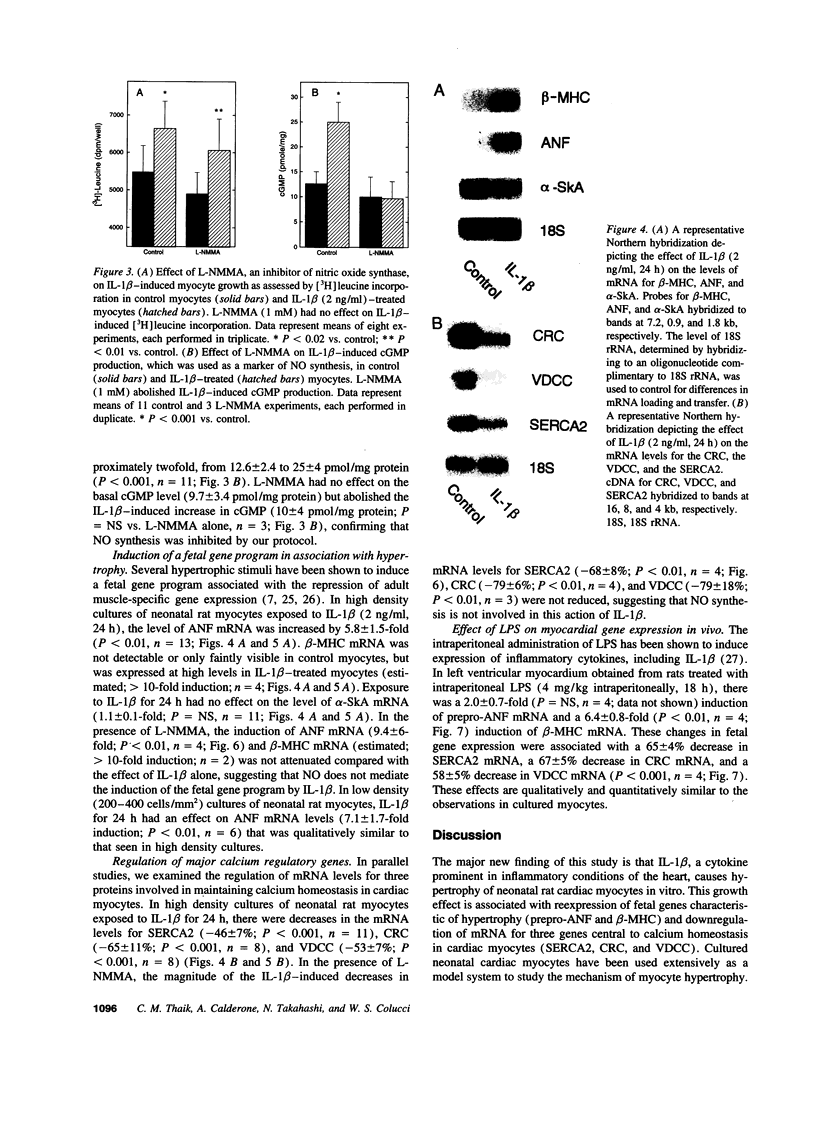
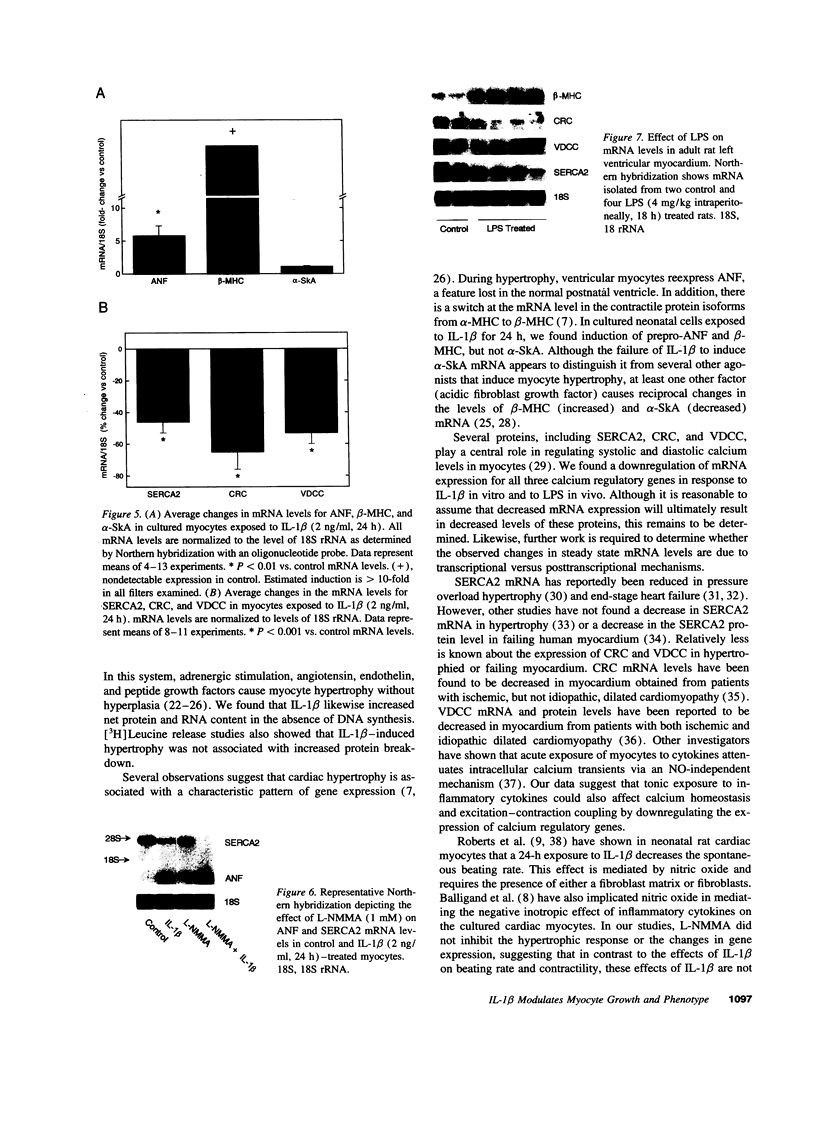
Abstract
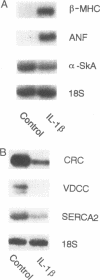
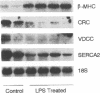
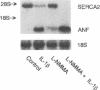
Mononuclear cell infiltration and local cytokine elaboration are hallmarks of inflammatory and immunologic heart diseases. To test the hypothesis that cytokines can modulate cardiac myocyte growth and phenotype, myocytes cultured from neonatal rat hearts were exposed to IL-1 beta, an inflammatory cytokine prevalent in myocardial inflammation. IL-1 beta (2 ng/ml, 24 h) increased [3H]leucine incorporation by 30 +/- 4% (P < 0.001, n = 29) and net cellular protein content by 20 +/- 4% (P < 0.001, n = 27), but had no effect on DNA synthesis. Northern hybridization showed that IL-1 beta increased prepro-atrial natriuretic factor (ANF) mRNA (5.8 +/- 1.5-fold, P < 0.01, n = 13) and beta-myosin heavy chain (beta-MHC) mRNA (> 10-fold, n = 4), and decreased mRNA levels for sarcoplasmic reticulum Ca(2+)-ATPase (SERCA2) (-46 +/- 7%; P < 0.001; n = 11), calcium release channel (CRC) (-65 +/- 11%, P < 0.001, n = 8) and voltage-dependent calcium channel (VDCC) (-53 +/- 7%, P < 0.001, n = 8). NG-monomethyl-L-arginine (1 mM), an inhibitor of nitric oxide (NO) synthesis, did not inhibit the IL-1 beta-induced protein synthesis or changes in mRNA levels. In ventricular myocardium obtained from adult rats treated with lipopolysaccharide (4 mg/kg intraperitoneally 18 h) to stimulate systemic cytokine production, there were changes in the mRNA levels for beta-MHC (6 +/- 1-fold, P < 0.01, n = 4), SERCA2 (-65 +/- 4%, P < 0.0001, n = 4), CRC (-67 +/- 5%, P < 0.001, n = 4), and VDCC (-58 +/- 5%, P < 0.001; n = 4) that were qualitatively similar to those observed in cultured myocytes. Thus, IL-1 beta, acting via an NO-independent mechanism, caused myocyte hypertrophy associated with induction of fetal genes (ANF and beta-MHC) and downregulation of three important calcium regulatory genes (SERCA2, CRC, and VDCC). IL-1 beta may contribute to the abnormal structural and functional alterations of cardiac myocytes in conditions marked by mononuclear cell infiltration.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai M., Alpert N. R., MacLennan D. H., Barton P., Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res. 1993 Feb;72(2):463–469. doi: 10.1161/01.res.72.2.463. [DOI] [PubMed] [Google Scholar]
- Balligand J. L., Ungureanu D., Kelly R. A., Kobzik L., Pimental D., Michel T., Smith T. W. Abnormal contractile function due to induction of nitric oxide synthesis in rat cardiac myocytes follows exposure to activated macrophage-conditioned medium. J Clin Invest. 1993 May;91(5):2314–2319. doi: 10.1172/JCI116461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brillantes A. M., Allen P., Takahashi T., Izumo S., Marks A. R. Differences in cardiac calcium release channel (ryanodine receptor) expression in myocardium from patients with end-stage heart failure caused by ischemic versus dilated cardiomyopathy. Circ Res. 1992 Jul;71(1):18–26. doi: 10.1161/01.res.71.1.18. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cozzolino F., Torcia M., Aldinucci D., Ziche M., Almerigogna F., Bani D., Stern D. M. Interleukin 1 is an autocrine regulator of human endothelial cell growth. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6487–6491. doi: 10.1073/pnas.87.17.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dec G. W., Jr, Palacios I. F., Fallon J. T., Aretz H. T., Mills J., Lee D. C., Johnson R. A. Active myocarditis in the spectrum of acute dilated cardiomyopathies. Clinical features, histologic correlates, and clinical outcome. N Engl J Med. 1985 Apr 4;312(14):885–890. doi: 10.1056/NEJM198504043121404. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Feldman A. M., Weinberg E. O., Ray P. E., Lorell B. H. Selective changes in cardiac gene expression during compensated hypertrophy and the transition to cardiac decompensation in rats with chronic aortic banding. Circ Res. 1993 Jul;73(1):184–192. doi: 10.1161/01.res.73.1.184. [DOI] [PubMed] [Google Scholar]
- Gustafson T. A., Markham B. E., Morkin E. Analysis of thyroid hormone effects on myosin heavy chain gene expression in cardiac and soleus muscles using a novel dot-blot mRNA assay. Biochem Biophys Res Commun. 1985 Aug 15;130(3):1161–1167. doi: 10.1016/0006-291x(85)91737-1. [DOI] [PubMed] [Google Scholar]
- Han R. O., Ray P. E., Baughman K. L., Feldman A. M. Detection of interleukin and interleukin-receptor mRNA in human heart by polymerase chain reaction. Biochem Biophys Res Commun. 1991 Dec 16;181(2):520–523. doi: 10.1016/0006-291x(91)91219-3. [DOI] [PubMed] [Google Scholar]
- Hosenpud J. D., Campbell S. M., Pan G. Indirect inhibition of myocyte RNA and protein synthesis by interleukin-1. J Mol Cell Cardiol. 1990 Feb;22(2):213–225. doi: 10.1016/0022-2828(90)91117-p. [DOI] [PubMed] [Google Scholar]
- Ito H., Hirata Y., Hiroe M., Tsujino M., Adachi S., Takamoto T., Nitta M., Taniguchi K., Marumo F. Endothelin-1 induces hypertrophy with enhanced expression of muscle-specific genes in cultured neonatal rat cardiomyocytes. Circ Res. 1991 Jul;69(1):209–215. doi: 10.1161/01.res.69.1.209. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988 Jan;85(2):339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo N. J., Jr, Tulenko T. N., Colucci W. S. Phorbol esters and norepinephrine destabilize alpha 1B-adrenergic receptor mRNA in vascular smooth muscle cells. J Biol Chem. 1994 Jan 21;269(3):1705–1710. [PubMed] [Google Scholar]
- Jones S. R., Herskowitz A., Hutchins G. M., Baughman K. L. Effects of immunosuppressive therapy in biopsy-proved myocarditis and borderline myocarditis on left ventricular function. Am J Cardiol. 1991 Aug 1;68(4):370–376. doi: 10.1016/0002-9149(91)90834-8. [DOI] [PubMed] [Google Scholar]
- Lane J. R., Neumann D. A., Lafond-Walker A., Herskowitz A., Rose N. R. Role of IL-1 and tumor necrosis factor in coxsackie virus-induced autoimmune myocarditis. J Immunol. 1993 Aug 1;151(3):1682–1690. [PubMed] [Google Scholar]
- Lenfant C. NHLBI funding policies. Enhancing stability, predictability, and cost control. Circulation. 1994 Jul;90(1):1–1. doi: 10.1161/01.cir.90.1.1. [DOI] [PubMed] [Google Scholar]
- Levitsky D., de la Bastie D., Schwartz K., Lompré A. M. Ca(2+)-ATPase and function of sarcoplasmic reticulum during cardiac hypertrophy. Am J Physiol. 1991 Oct;261(4 Suppl):23–26. doi: 10.1152/ajplung.1991.261.4.L23. [DOI] [PubMed] [Google Scholar]
- Libby P., Warner S. J., Friedman G. B. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest. 1988 Feb;81(2):487–498. doi: 10.1172/JCI113346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C. S., Henrich C. J., Simpson P. C. A growth factor for cardiac myocytes is produced by cardiac nonmyocytes. Cell Regul. 1991 Dec;2(12):1081–1095. doi: 10.1091/mbc.2.12.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D. L., Young J. B. Basic mechanisms in congestive heart failure. Recognizing the role of proinflammatory cytokines. Chest. 1994 Mar;105(3):897–904. doi: 10.1378/chest.105.3.897. [DOI] [PubMed] [Google Scholar]
- Marczin N., Papapetropoulos A., Catravas J. D. Tyrosine kinase inhibitors suppress endotoxin- and IL-1 beta-induced NO synthesis in aortic smooth muscle cells. Am J Physiol. 1993 Sep;265(3 Pt 2):H1014–H1018. doi: 10.1152/ajpheart.1993.265.3.H1014. [DOI] [PubMed] [Google Scholar]
- Mayani H., Dragowska W., Lansdorp P. M. Cytokine-induced selective expansion and maturation of erythroid versus myeloid progenitors from purified cord blood precursor cells. Blood. 1993 Jun 15;81(12):3252–3258. [PubMed] [Google Scholar]
- Meidell R. S., Sen A., Henderson S. A., Slahetka M. F., Chien K. R. Alpha 1-adrenergic stimulation of rat myocardial cells increases protein synthesis. Am J Physiol. 1986 Nov;251(5 Pt 2):H1076–H1084. doi: 10.1152/ajpheart.1986.251.5.H1076. [DOI] [PubMed] [Google Scholar]
- Mercadier J. J., Lompré A. M., Duc P., Boheler K. R., Fraysse J. B., Wisnewsky C., Allen P. D., Komajda M., Schwartz K. Altered sarcoplasmic reticulum Ca2(+)-ATPase gene expression in the human ventricle during end-stage heart failure. J Clin Invest. 1990 Jan;85(1):305–309. doi: 10.1172/JCI114429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P. Abnormal intracellular modulation of calcium as a major cause of cardiac contractile dysfunction. N Engl J Med. 1991 Aug 29;325(9):625–632. doi: 10.1056/NEJM199108293250906. [DOI] [PubMed] [Google Scholar]
- Morishita R., Gibbons G. H., Pratt R. E., Tomita N., Kaneda Y., Ogihara T., Dzau V. J. Autocrine and paracrine effects of atrial natriuretic peptide gene transfer on vascular smooth muscle and endothelial cellular growth. J Clin Invest. 1994 Aug;94(2):824–829. doi: 10.1172/JCI117402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movsesian M. A., Karimi M., Green K., Jones L. R. Ca(2+)-transporting ATPase, phospholamban, and calsequestrin levels in nonfailing and failing human myocardium. Circulation. 1994 Aug;90(2):653–657. doi: 10.1161/01.cir.90.2.653. [DOI] [PubMed] [Google Scholar]
- Parker T. G., Chow K. L., Schwartz R. J., Schneider M. D. Differential regulation of skeletal alpha-actin transcription in cardiac muscle by two fibroblast growth factors. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7066–7070. doi: 10.1073/pnas.87.18.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker T. G., Packer S. E., Schneider M. D. Peptide growth factors can provoke "fetal" contractile protein gene expression in rat cardiac myocytes. J Clin Invest. 1990 Feb;85(2):507–514. doi: 10.1172/JCI114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Roche N. S., Winokur T. S., Burmester J. K., Sporn M. B. Role of transforming growth factor-beta in maintenance of function of cultured neonatal cardiac myocytes. Autocrine action and reversal of damaging effects of interleukin-1. J Clin Invest. 1992 Nov;90(5):2056–2062. doi: 10.1172/JCI116087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Vodovotz Y., Roche N. S., Sporn M. B., Nathan C. F. Role of nitric oxide in antagonistic effects of transforming growth factor-beta and interleukin-1 beta on the beating rate of cultured cardiac myocytes. Mol Endocrinol. 1992 Nov;6(11):1921–1930. doi: 10.1210/mend.6.11.1282674. [DOI] [PubMed] [Google Scholar]
- Sadoshima J., Izumo S. Molecular characterization of angiotensin II--induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993 Sep;73(3):413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- Schulz R., Nava E., Moncada S. Induction and potential biological relevance of a Ca(2+)-independent nitric oxide synthase in the myocardium. Br J Pharmacol. 1992 Mar;105(3):575–580. doi: 10.1111/j.1476-5381.1992.tb09021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Takahashi N., Calderone A., Izzo N. J., Jr, Mäki T. M., Marsh J. D., Colucci W. S. Hypertrophic stimuli induce transforming growth factor-beta 1 expression in rat ventricular myocytes. J Clin Invest. 1994 Oct;94(4):1470–1476. doi: 10.1172/JCI117485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Allen P. D., Lacro R. V., Marks A. R., Dennis A. R., Schoen F. J., Grossman W., Marsh J. D., Izumo S. Expression of dihydropyridine receptor (Ca2+ channel) and calsequestrin genes in the myocardium of patients with end-stage heart failure. J Clin Invest. 1992 Sep;90(3):927–935. doi: 10.1172/JCI115969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulich T. R., Guo K. Z., Irwin B., Remick D. G., Davatelis G. N. Endotoxin-induced cytokine gene expression in vivo. II. Regulation of tumor necrosis factor and interleukin-1 alpha/beta expression and suppression. Am J Pathol. 1990 Nov;137(5):1173–1185. [PMC free article] [PubMed] [Google Scholar]
- Yokoyama T., Vaca L., Rossen R. D., Durante W., Hazarika P., Mann D. L. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J Clin Invest. 1993 Nov;92(5):2303–2312. doi: 10.1172/JCI116834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbe T. R. Contrast of mediators: identification of cells and cytokines in human heart tissue with acute rejection and myocarditis. Transplant Proc. 1989 Feb;21(1 Pt 1):443–444. [PubMed] [Google Scholar]
- van Bilsen M., Chien K. R. Growth and hypertrophy of the heart: towards an understanding of cardiac specific and inducible gene expression. Cardiovasc Res. 1993 Jul;27(7):1140–1149. doi: 10.1093/cvr/27.7.1140. [DOI] [PubMed] [Google Scholar]