Abstract
Telomerase, a ribonucleoprotein complex that includes the telomerase RNA component (hTR) and the telomerase catalytic subunit gene (hTERT) product, has been shown to be activated in the majority of cancer tissues and immortalized cells. To study telomerase activation during the progression of cervical cancer, the expression of hTR and hTERT RNAs in tissues of various stages of cervical cancer was analyzed using the in situ hybridization method and compared with proliferative activity as estimated by Ki-67 immunostaining. To test whether expression of these components is reflected in enzyme activity, we determined the levels of the RNAs in cervical cancer and normal tissues and in primary and immortal keratinocytes by reverse transcription-polymerase chain reaction and RNase protection assays and compared the results to telomerase activities as detected by telomeric repeat amplification protocol assay. In situ hybridization signals of hTR and hTERT were present not only in carcinoma tissues but also in normal epidermal layers. In many adenocarcinoma and fewer squamous cell carcinoma tissues, both signals were focally increased where high proliferative activity was present at the stages of dysplasia/metaplasia, in situ carcinoma, and invasive carcinoma. The level of hTERT, as quantitated by RNase protection assay, was not different between cancer and control tissues or immortal and a subset of primary keratinocytes and did not correlate with telomerase activity. These results indicate that expression of hTR and hTERT is up-regulated in at least a subset of neoplastic cells at an early stage of carcinogenesis and that unidentified factors, such as the modulation or coordination of its protein level with other products, may contribute to the activation of telomerase in cervical cancer.
Telomerase, a ribonucleoprotein enzyme that depends on an RNA template, adds tandem hexanucleotide repeats (TTAGGG)n to the ends of chromosomes, forming telomeres. In most eukaryotic somatic cells, the absence of telomerase activity results in a loss of telomeric repeats with each round of chromosomal DNA replication, resulting in a potential loss of genomic stability. Activation of telomerase, or in some cases the action of undefined recombination mechanisms, 1 reduces or prevents the loss of telomeres. Activity of the enzyme is frequently up-regulated in cancer tissues and immortalized cell lines when compared with normal tissues. 2,3 Numerous efforts have been directed at clarifying the mechanism of enzyme activation, an understanding of which could lead to innovative cancer therapy. Since the successful cloning of the human telomerase RNA component (hTR), 4 the association between its expression and enzyme activity has been studied. Although some studies have shown that hTR expression is higher in more progressive cancers, 5,6 hTR expression is not recognized as a rate-limiting factor for enzyme activity. 7,8 The recently cloned telomerase catalytic subunit gene (hTERT), the product of which (Est2p) forms a stable complex with the telomerase RNA subunit, reflects telomerase activity. 9,10
Telomerase activation has been demonstrated to be a rather late event during multistage carcinogenesis of various cancers. 6,8,11-13 In cervical cancer, human papillomavirus (HPV) infection is considered to be the initiating event of carcinogenesis. Although the HPV-16 E6 gene product induces the activation of telomerase during immortalization of skin or cervical epithelial cells, 14 a recent study indicated that cervical carcinomas have high telomerase activity irrespective of the detection of HPV; 15 however, it is unclear when telomerase is activated during the progression of cervical neoplasia. Based on the evidence that expression of the hTERT gene reflects the activation of telomerase, 9,10 the in situ hybridization technique, using tissues of widely distributed stages of cervical carcinoma, was employed in this study to address this question. Furthermore, because a recent study 16 has shown an association between telomerase activity and proliferative activity, we also estimated proliferative activity by immunohistochemistry in the tissues and compared the level of hTERT expression.
The in situ results demonstrate that expression of hTR and hTERT is focally up-regulated at the regions of invasive and in situ carcinoma of cervical tissues. We also show that both genes are expressed in normal epidermal layers and cultured keratinocytes and that the levels of these RNAs, as measured by RNase protection assay, do not reflect the levels of telomerase activity in cervical tissues and cultured cells.
Materials and Methods
Samples
Paraffin-embedded tissues were obtained from patients with cervical cancer. Forty-eight cases of squamous cell carcinoma (40 cases of invasive carcinoma and 8 cases of microinvasive carcinoma), 24 cases of adenocarcinoma (9 cases of invasive carcinoma and 15 cases of carcinoma in situ), and 1 case of adenosquamous carcinoma were tested. Among these samples, there were 9 cases with regions of cervical intraepithelial neoplasia (CIN3) and 19 cases with cervical intraepithelial neoplasia 1 or 2 (dysplasia) regions. Dysplastic or metaplastic changes of glands were accompanied by 15 cases of in situ adenocarcinoma. Normal cervical tissues were collected from patients who underwent hysterectomy due to leiomyoma of uteri (3 cases), adenomyosis of uteri (1 case), and ovarian cancer (1 case).
We obtained matched cervical cancer and adjacent nontumor tissues from seven patients with invasive cervical cancer (6 cases of squamous cell carcinoma and 1 case of adenosquamous carcinoma) and normal cervix from four patients with leiomyoma of uteri, who gave informed consent for this study. The samples were immediately frozen and stored at −80°C until use for telomeric repeat amplification protocol (TRAP), reverse transcription-polymerase chain reaction (RT-PCR), and RNase protection assays. Primary (mortal) cervical keratinocytes (from two different sources: passages 5 and 6), foreskin keratinocytes (from five different sources: passages 2, 2, 2, 4, and 5), immortalized cervical keratinocytes transfected with HPV-16 E6/E7 genes (E6/E7: passage 57), maintained as previously described, 17 and HeLa cervical cancer cells were passaged and harvested simultaneously under subconfluent conditions to analyze the correlation between telomerase activity and its RNA expression.
hTR and hTERT Clonings
The hTR and hTERT clones were generated as follows: cDNA was synthesized with a SuperScript II kit (Life Technologies Inc., Grand Island, NY) using 3 μg of total RNA isolated from HeLa cells. The cDNA was subjected to PCR amplification using primers for the hTR gene (20-bp published sequence 7 from 1 to 20 and 19-bp sequence from 176 to 194; GenBank accession no. U86046) and primers for the hTERT gene (23-bp published sequence 9 from 1682 to 1704; 23-bp sequence from 2036 to 2058; GenBank accession no. AF015950). The resulting cDNA fragments were inserted into pBlueScript SK+ (Stratagene, La Jolla, CA) and pGEM-T Easy Vectors (Promega, Madison, WI), respectively. Sequences were confirmed using cycle sequencing with dye-labeled terminators.
In Situ Hybridization
Digoxigenin-labeled hTR and hTERT anti-sense probes were generated from HindIII- and SpeI-linearized plasmids according to the protocol of Riboprobe in vitro Transcription Systems (Promega) using digoxigenin RNA labeling mixture (Boehringer Mannheim, Indianapolis, IN) and sense-probes from BamHI- and NcoI-linearized plasmids using T7 and SP6 RNA polymerase, respectively. Formalin-fixed, paraffin-embedded tissue sections (5-μm thickness) were deparaffinized in xylene and graded alcohol series and pretreated with Target Retrieval Solution (DAKO, Carpinteria, CA) at 95°C for 20 minutes and 0.01% pepsin in 0.2 N HCl solution at room temperature for 20 minutes. The tissues were then fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) at 4°C for 5 minutes, acetylated, dehydrated through graded alcohol series and air-dried. Digoxigenin-labeled anti-sense and sense probes were denaturated at 85°C for 3 minutes and placed over the tissues. After hybridization at 47°C overnight, the slides were washed in 50% formamide/2× standard saline citrate at 52°C for 30 minutes, treated with RNase A in TNE solution at 37°C for 30 minutes, and washed in 2× standard saline citrate and 0.2× standard saline citrate solution twice at 50°C for 20 minutes each. After the incubation in 2% blocking solution (Boehringer Mannheim), the tissues were incubated with a sheep monoclonal anti-digoxigenin antibody (Boehringer Mannheim) diluted 1:500 in 0.1 mol/L Tris-HCl, pH 7.5/0.15 mol/L NaCl buffer at room temperature for 30 minutes. After washing with 0.1 mol/L Tris-HCl, pH 7.5/0.15 mol/L NaCl buffer, the color detection was carried out by incubating with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium solution containing levamisole (Vector Laboratories, Burlingame, CA) overnight. The sections were counterstained with fast green FCF (Aldrich Chemical Corp., Milwaukee, WI) and then mounted with Crystal Mount (Biomeda Corp., Foster City, CA). Differential diagnosis of the stages in adjacent regions and evaluation of signal intensity were independently confirmed by Dr. Ming-Gang Lin (Fred Hutchinson Cancer Research Center).
Immunohistochemistry
Formalin-fixed, paraffin-embedded sections (4-μm thickness) were deparaffinized and rehydrated through xylenes and a series of graded alcohols. Immunohistochemistry was carried out with an automated processor (TechMate 1000, Ventana Medical Systems, Inc., Tucson, AZ). The tissue sections were subjected to steaming in 10 mmol/L citrate buffer, pH 6.0 for 20 minutes and allowed to cool at room temperature. Anti-Ki-67 clone MIB-1 (Immunotech, Westbrook, ME) was applied for 1 hour at room temperature at a concentration of 1:200 in PBS containing 1% bovine serum albumin and 0.03% Tween 20 in the automated stainer, followed by an incubation with a secondary biotinylated antibody, avidin-biotinylated peroxidase complex, and 3,3-diaminobenzidine with NiCl2 enhancement as a substrate. The slides were counterstained with methyl green and mounted with Histmount (National Diagnostics, Atlanta, GA).
RT-PCR
Total RNA isolated from frozen tissues and cultured cells using RNeasy total RNA kit (Qiagen, Chatsworth, CA) was reverse transcribed by random primer and Super-Script II reverse transcriptase. The resulting cDNA was subjected to PCR with the primers as follows: hTERT, 5′-CGGAAGAGTGTCTGGAGCAA and 5′-GGATGAAG-CGGAGTCTGGA, 10 hTR, 5′-GGGTTGCGGAGGGTGGG-CCT and 5′-ACGGGCCAGCAGCTGACAT, and 36B4 mRNA 18 as an internal control, 5′-TGGCAGATGGATCAGC and 5′-AGTGTCTCTCTGCA. The amplification reactions were performed with an initial incubation step at 94°C for 3 minutes followed by 31 cycles at 94°C for 45 seconds, 57°C for 1 minute, and 72°C for 1 minute for hTERT; 30 cycles at 94°C for 1 minute, 60°C for 45 seconds, and 72°C for 45 seconds for hTR; and 30 cycles at 94°C for 1 minute, 50°C for 1 minute, and 72°C for 1 minute for 36B4, with a final incubation at 72°C for 7 minutes for all cDNAs. The reaction products were subjected to electrophoresis in 1.8% agarose gel and visualized by ethidium bromide staining.
RNase Protection Assay
Radiolabeled hTERT (345-bp length) and β-actin (340-bp length) probes that protect 285- and 200-bp fragments in RNase protection assay were synthesized from AspI- and HindIII-linearized plasmids (β-actin, a gift from Dr. Scott Foster, Fred Hutchinson Cancer Research Center) according to the protocol of Riboprobe in vitro Transcription Systems (Promega) using [α-32P]UTP and T7 RNA polymerase, respectively. The RNase protection assay was carried out using the RPA II kit (Ambion, Austin, Texas) according to the manufacturer’s protocol. Briefly, total RNA samples (20 μg from tissues and 30 μg from cultured keratinocytes) and radiolabeled hTERT (specific radioactivity, 0.9 to 1.0 × 10 9 cpm/μg) and internal control β-actin (1.6 to 2.2 × 10 7 cpm/μg) probes were coprecipitated in ethanol, resuspended in hybridization buffer, hybridized at 42°C overnight, and then treated with RNase A and T1. Samples were precipitated, resuspended in loading buffer and analyzed on 5% denaturating polyacrylamide gel, after which the gel was dried and exposed to X-OMAT film (Kodak, Rochester, NY) at −80°C.
TRAP Assay
Frozen tissues were homogenized on ice using sterile dounce homogenizer with TRAP lysis buffer as described. 3 The homogenized solution was centrifuged at 14,000 × g for 30 minutes at 4°C, and the supernatant was collected and stored at −80°C until used for the TRAP assay. The TRAP assay was performed as described. 3 Telomerase synthesizes telomeric repeats onto a nontelomeric [γ-32P]ATP end-labeled repeat TS (5′-AATCCGTCGAGCAGAGTT-3′). The reaction was carried out using 5 μg of protein extractions in 50 μL of reaction mixture, to which an internal telomerase assay standard was added for estimation of the levels of telomerase activity and identification of any false-negative samples that contain Taq polymerase inhibitors. These products are specifically amplified by PCR with the downstream primer CX (5′-(CCCTTA)3CCCTAA-3′) and upstream labeled primer TS. An aliquot of the reaction was run on a 10% nondenaturating acrylamide gel, which was dried and exposed to X-OMAT film overnight.
Results
Expression of hTERT and hTR in Cervical Tissue
As shown in Figure 1 ▶ , expression of hTR RNA and hTERT mRNA was co-localized not only in carcinoma regions but also in normal to dysplastic epidermal layers and dysplastic/metaplastic glandular epithelia in almost all cases. In histologically normal epidermal layers, hTR and hTERT expression was limited to basal and a couple of suprabasal cell layers. Dysplastic epidermis with expanded cell layers expressed both signals, and the level of expression appeared to be higher than in histologically normal epidermis. In some cases of squamous cell carcinoma, higher levels of expression of both signals were detected compared with adjacent noncancerous epidermal layers, whereas there was no apparent difference in signal intensity in other cases between the regions. In histologically normal glands, in contrast, there was a faint signal or no signal of hTR and hTERT. Both signals were detected in dysplastic/metaplastic glands, and in in situ and invasive adenocarcinomas. The level of expression was heterogenous within the carcinoma regions in the same tissue. Among stromal cells, both signals were mainly present in lymphocytes of normal and cancer tissues, in which signal intensity was similar, although a precise quantitative comparison of in situ signal was difficult. An hTR but not hTERT signal was detected in endothelial cells.
Figure 1.
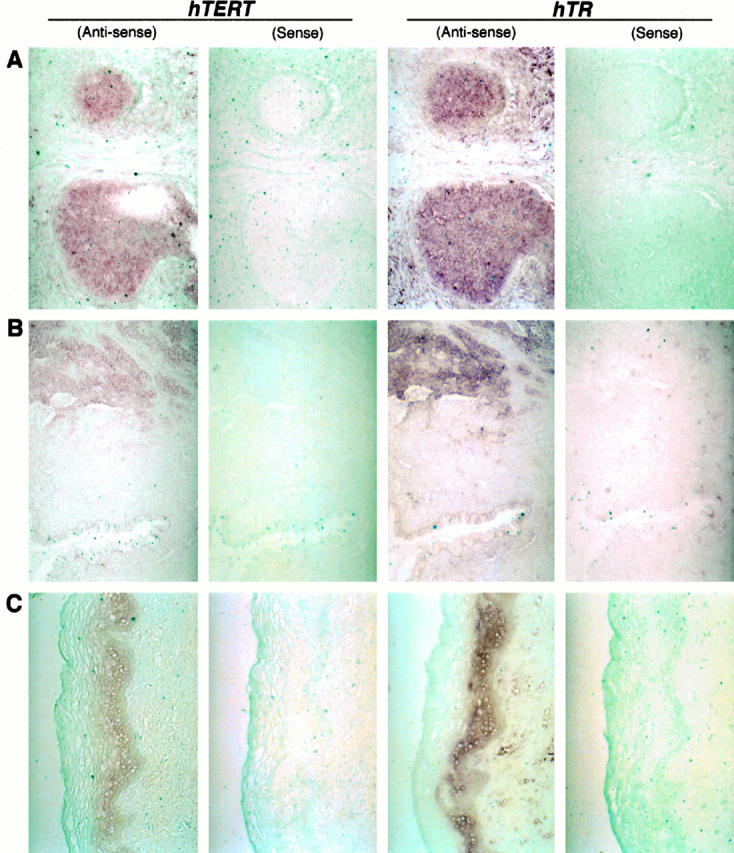
Histological distribution of hTR and hTERT RNA signals in cervical tissues detected by the in situ hybridization using digoxigenin-labeled probes. Both signals are present in not only carcinomas (invasive squamous cell carcinoma (A) and invasive adenocarcinoma (B)) but also normal epidermal layers (C). In contrast, no or faint signals are detected in normal glands (B, lower field). Original magnification, ×200 (A and C) and ×100 (B).
Association between hTERT and hTR Expression and Proliferation in Cervical Tissues
High proliferative activity estimated by positivity of Ki-67 >50% was seen in carcinoma regions (74.6%; 44 of 59 cases). A similar result (Ki-67 >50%) was observed in dysplastic/metaplastic epidermal and glandular regions (67.6%; 23 of 34 cases), where hTERT signal was increased compared with normal tissues. Even in histologically normal epidermal layers, the intense signal was detected in the regions with high proliferative activity, as represented in Figure 2 ▶ , whereas the incidence of Ki-67 >50% was fewer (21.1%; 4 of 19 cases). Up-regulation of hTR expression was also accompanied by the high proliferative activity in many cases (data not shown).
Figure 2.

Corresponding distribution of hTERT signal and Ki-67 staining in cervical tissues. Intense signal of hTERT is seen in normal (A) and dysplastic (B) epidermal layers, dysplastic/metaplastic glands (C), and adenocarcinoma in situ (D) in which strong Ki-67 staining is present. On the other hand, carcinoma cells (A, right field) without Ki-67 reactivity contain a faint hTERT signal. Original magnification, ×200. Hematoxylin and eosin staining.
Levels of hTR and hTERT Expression in Cervical Tissues and Cultured Keratinocytes Determined by RT-PCR and RNase Protection Assays
We also examined hTERT and hTR expression using frozen cervical cancer and adjacent nontumor tissues and cultured keratinocytes by RT-PCR. Some of the cancer tissues showed an increased expression of hTERT and hTR compared with the control tissues, but almost all nontumor tissues and some primary keratinocytes expressed both RNAs (Figure 3) ▶ . To quantitate hTERT expression more accurately, RNase protection assay was carried out (Figure 4A) ▶ . Nontumor control tissues and primary cultured keratinocytes (four of seven) expressed levels of hTERT similar to those of cancer tissues, HPV-16 E6/E7 immortalized keratinocytes, and HeLa cells. Although it was not possible to detect hTERT expression in tissues by Northern blot analysis because of limited tissue availability and the low level of expression, the same level of hTERT-specific transcript was identified in primary and immortal keratinocytes (data not shown).
Figure 3.
hTERT and hTR expression in cervical tissues and cultured keratinocytes determined by RT-PCR. Total RNA was amplified using hTERT and hTR primers (top and middle, respectively). Amplification of 36B4 (bottom) was used to demonstrate the integrity of cDNA templates. Numbers above lanes are patient identifiers; T, cancer; N, nontumor tissues; HCK(CX) and HCK, primary cervical keratinocytes; HFK, HFK11696, NHK1, NHK2, and NHK3; primary foreskin keratinocytes; E6/E7; HPV-16 E6/E7-immortalized cervical keratinocytes; HeLa, cervical carcinoma cell line.
Figure 4.
Comparison between hTERT expression level and telomerase activity in cervical tissues and cultured keratinocytes. hTERT expression was quantitated by RNase protection assay (A) using total RNA from tissues and cultured keratinocytes (20 and 30 μg, respectively), a hTERT riboprobe that protects a 285-bp fragment (ph-hTERT), and a β-actin riboprobe with a protected fragment of about 200 bp (pβ-actin) as an internal standard. Telomerase activity was analyzed by TRAP assay (B). Internal telomerase assay standard (ITAS) band is an internal control for PCR amplification. Yeast tRNA and TRAP lysis buffer without protein extract were used as a negative control for RNase protection and TRAP assays, respectively. Numbers above lanes are patient identifiers; T, cancer; N, nontumor tissues; HCK(CX) and HCK, primary cervical keratinocytes; HFK, HFK11696, NHK1, NHK2, and NHK3, primary foreskin keratinocytes; E6/E7, HPV-16 E6/E7-immortalized cervical keratinocytes; HeLa, cervical carcinoma cell line.
Relation between the Level of hTERT Expression and Telomerase Activity
Telomerase enzyme activity was detected in cancer tissues and immortal keratinocytes by TRAP assay but was either not detectable or significantly lower in noncancer tissues and primary keratinocytes, as depicted in Figure 4B ▶ . Comparison of the results in RNase protection and TRAP assays demonstrated no consistent association between the enzyme activity and total level of hTERT expression in cervical tissues and cultured keratinocytes.
Discussion
We have shown in this study that expression of hTR RNA and hTERT mRNA are co-localized in cervical carcinomas and in normal epidermal layers, and that their up-regulation is focally induced in not only carcinomas but also precancerous stage regions, such as dysplastic/metaplastic epidermis and glands. These results also indicate a dissociation between the levels of hTR and hTERT and telomerase activity, suggesting the presence of other regulators for telomerase activity.
Activation of telomerase has been shown to be an important event for immortalization and carcinogenesis. Up-regulation of hTR expression has been shown to be an early event in various cancers. 6,8,13 In cervical carcinoma, the relation between up-regulation of hTR and hTERT expression and the progression of disease has not been investigated, although Soder et al 5 have demonstrated amplification of the hTR gene on chromosome 3q in cervical cancer. In this study, signals of the both RNAs were focally increased in carcinoma regions, dysplastic/metaplastic epidermis, and glands. These results indicate that not only hTR but also hTERT up-regulation are early events during cervical carcinogenesis. hTR expression has previously been demonstrated to correlate with proliferation index in astrocytomas 19 and ependymomas. 20 The expression of hTERT seems to be coordinated with proliferation activity, because dysplastic/metaplastic epidermal and glandular cells as well as carcinoma cells possess high proliferative activity, whereas normal epidermal epithelia exhibit less activity. On the other hand, correlation between Ki-67 staining and hTERT signal was not 100% in vivo, and not all primary keratinocytes expressed detectable hTERT when proliferating under subconfluent conditions in vitro, suggesting possible inhibitory factors or rapid degradation of hTERT. Recently, telomerase catalytic subunit gene (hTERT) expression was reported to reflect the enzyme activity in cancer and immortalized cells. 9,10 RNase protection and RT-PCR assays in this study, however, showed that some of control tissues expressed similar levels of hTERT mRNA to those in cancer tissues, which may indicate that some portion of these tissues might contain dysplastic or hyperplastic epithelia in which hTERT expression is up-regulated. Nevertheless, high telomerase activity is specific in cancer tissues, and up-regulation of hTERT expression in the control tissues does not indicate a high level of enzyme activity. Increased signals of hTERT detected by the in situ hybridization method were focally distributed within carcinoma regions. An increase in the proportion of lymphocytes to epithelial cells could mask a difference in hTERT levels between subsets of carcinoma and normal epithelial cells, resulting in a discrepancy between the mRNA level and telomerase activity. However, it is difficult to explain the cultured cell result in which primary (mortal) keratinocytes have no or weak telomerase activity despite a similar level of hTERT expression as immortal keratinocytes. The intensity of the in situ hybridization signal was not obviously different between primary and immortal keratinocytes, although heterogeneity of the signal was present in both, because keratinocytes are difficult to synchronize (data not shown). Primary keratinocytes are highly proliferative in vitro until senescence or terminal differentiation, as are most dysplastic epidermal cells in vivo. Proliferation activity seems to be more tightly correlated with hTERT expression than is telomerase activity, at least in keratinocytes, although this enzyme activity was recently reported to be a proliferation biomarker. 16 These results suggest a second level of regulation in telomerase activity besides hTERT gene expression.
Among normal tissues, except germline tissues, colon and small intestine tissues express hTERT mRNA, as shown by Meyerson et al. 9 A recent study 21 has also shown that its expression is detected by RT-PCR in many kinds of normal tissues in addition to the above tissues. Telomerase is not activated in the majority of normal somatic tissues, 3 suggesting the possibility that the hTERT gene product may be modulated during the process of translation and posttranslation in these tissues. It is also possible that splicing of hTERT mRNA differs between normal and cancer tissues as evidenced by Kilian et al, 22 or a difference in minor nucleotide sequence exists between them, given that a mutation of hTERT abrogates its activity in vitro. 23 The Ro60 protein as recently reported by Ramakrishnan et al 24 possibly forms a complex with telomerase and modulates its function. Overexpression of the anti-apoptosis protein bcl-2 25 and phosphorylated status 26 have been shown to activate telomerase in cancer cell lines, factors that might contribute to the difference between cervical cancer and normal tissues. Epidermal cells in the suprabasal layers of normal cervical tissues are differentiated; therefore, the enzyme activity could be inhibited, even though hTERT mRNA is expressed, because differentiation has an inhibitory effect on telomerase activity. 27 The relation between telomerase activation and deregulation of cell cycle regulator proteins has been demonstrated in breast cancer. 28 Although direct interaction between the p53 pathway and telomerase activation has not been noted in HPV-16 E6/E7-immortalized cervical keratinocytes, 14 we have confirmed aberrant staining of other cell cycle regulator proteins in the cervical cancer tissues (data not shown).
We conclude that up-regulation of hTR and hTERT are early events during carcinogenesis and that additional regulation, such as modulation of hTERT gene product or coordination with other proteins that currently are not identified, may participate in the activation of telomerase in cervical cancer.
Acknowledgments
We thank Dr. Peggy Porter and Martha Shellenberger (Fred Hutchinson Cancer Research Center) for organizing the collection of cervical tissues, and Dr. Juichi Saito (Juntendo University School of Medicine) for providing normal cervical tissues. We are also grateful to Jennifer Ihle and Aloysius Klingelhutz for discussion and cell lines, and Marci Wright for preparing the manuscript.
Footnotes
Address reprint requests to James K. McDougall, Program in Cancer Biology, Fred Hutchinson Cancer Research Center, C1-105, 1105 Fairview Avenue North, P.O. Box 19024, Seattle, WA 19024. E-mail: jmcdougall@fhcrc.org.
Supported by National Institutes of Health Grant CA 42792 (to JKM). KN was partly supported by a fellowship of Sankyo Science Foundation, Japan.
References
- 1.Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR: Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J 1995, 14:4240-4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Lange T: Activation of telomerase in a human tumor. Proc Natl Acad Sci USA 1994, 91:2882-2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PLC, Coviello GM, Wright WE, Weinrich SL, Shay JW: Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266:2011-2015 [DOI] [PubMed] [Google Scholar]
- 4.Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, Le S, West MD, Harley CB, Andrews WH, Greider CW, Villeponteau B: The RNA component of human telomerase. Science 1995, 269:1236-1241 [DOI] [PubMed] [Google Scholar]
- 5.Soder AI, Hoare SF, Muir S, Going JJ, Parkinson EK, Keith WN: Amplification, increased dosage and in situ expression of the telomerase RNA gene in human cancer. Oncogene 1997, 14:1013-1021 [DOI] [PubMed] [Google Scholar]
- 6.Yashima K, Litzky LA, Kaiser L, Rogers T, Lam S, Wistuba II, Milchgrub S, Srivastava S, Piatyszek MA, Shay JW, Gazdar AF: Telomerase expression in respiratory epithelium during the multistage pathogenesis of lung carcinomas. Cancer Res 1997, 57:2373-2377 [PubMed] [Google Scholar]
- 7.Avilion AA, Piatyszek MA, Gupta J, Shay JW, Bacchetti S, Greider CW: Human telomerase RNA and telomerase activity in immortal cell lines and tumor tissues. Cancer Res 1996, 56:645-650 [PubMed] [Google Scholar]
- 8.Blasco MA, Rizen M, Greider CW, Hanahan D: Differential regulation of telomerase activity and telomerase RNA during multi-stage tumorigenesis. Nat Genet 1996, 12:200-204 [DOI] [PubMed] [Google Scholar]
- 9.Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, Bacchetti S, Haber DA, Weinberg RA: hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 1997, 90:785-795 [DOI] [PubMed] [Google Scholar]
- 10.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR: Telomerase catalytic subunit homologs from fission yeast and human. Science 1997, 277:955-959 [DOI] [PubMed] [Google Scholar]
- 11.Hiyama E, Gollahon L, Kataoka T, Kuroi K, Yokoyama T, Gazdar AF, Hiyama K, Piatyszek MA, Shay JW: Telomerase activity in human breast tumors. J Natl Cancer Inst 1996, 88:116-122 [DOI] [PubMed] [Google Scholar]
- 12.Tahara H, Nakanishi T, Kitamoto M, Nakashio R, Shay JW, Tahara L, Kajiyama G, Ide T: Telomerase activity in human liver tissues: comparison between chronic liver disease and hepatocellular carcinomas. Cancer Res 1995, 55:2734-2736 [PubMed] [Google Scholar]
- 13.Kuniyasu H, Domen T, Hamamoto T, Yokozaki H, Yasui W, Tahara H, Tahara E: Expression of human telomerase RNA is an early event of stomach carcinogenesis. Jpn J Cancer Res 1997, 88:103-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klingelhutz AJ, Foster SA, McDougall JK: Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 1996, 380:79-82 [DOI] [PubMed] [Google Scholar]
- 15.Anderson S, Shera K, Ihle J, Billman L, Goff B, Greer B, Tamimi H, McDougall JK, Klingelhutz AJ: Telomerase activation in cervical cancer. Am J Pathol 1997, 151:25-31 [PMC free article] [PubMed] [Google Scholar]
- 16.Belair CD, Yeager TR, Lopez PM, Reznikoff CA: Telomerase activity: a biomarker of cell proliferation, not malignant transformation. Proc Natl Acad Sci USA 1997, 94:13677-13682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaur P, McDougall JK: Characterization of primary human keratinocytes transformed by human papillomavirus type 18. J Virol 1988, 62:1917-1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laborda J: 36β4 cDNA used as an estradiol-independent mRNA control is the cDNA for acidic ribosomal phosphoprotein PO. Nucleic Acid Res 1991, 19:3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallinen P, Miettinen H, Sallinen S-L, Haapasalo H, Helin H, Kononen J: Increased expression of telomerase RNA component is associated with increased cell proliferation in human astrocytomas. Am J Pathol 1997, 150:1159-1164 [PMC free article] [PubMed] [Google Scholar]
- 20.Rushing EJ, Yashima K, Brown DF, White CL, Shay JW, Risser RC, Gazdar AF: Expression of telomerase RNA component correlates with the MIB-1 proliferation index in ependymomas. J Neuropathol Exp Neurol 1997, 56:1142-1146 [DOI] [PubMed] [Google Scholar]
- 21.Ramakrishnan S, Eppenberger U, Mueller H, Shinkai Y, Narayanan R: Expression profile of the putative catalytic subunit of the telomerase gene. Cancer Res 1998, 58:622-625 [PubMed] [Google Scholar]
- 22.Kilian A, Bowtell DDL, Abud HE, Hime GR, Venter DJ, Keese PK, Duncan EL, Reddel RR, Jefferson RA: Isolation of a candidate human telomerase catalytic subunit gene, which reveals complex splicing patterns in different cell types. Hum Mol Genet 1997, 6:2011-2019 [DOI] [PubMed] [Google Scholar]
- 23.Harrington L, Zhou W, McPhail T, Oulton R, Yeung DSK, Mar V, Bass MB, Robinson MO: Human telomerase contains evolutionarily conserved catalytic and structural subunits. Genes Dev 1997, 11:3109-3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramakrishnan S, Sharma HW, Farris AD, Kaufman KM, Harley JB, Collins K, Pruijn GJM, van Venrooij WJ, Martin ML, Narayanan R: Characterization of human telomerase complex. Proc Natl Acad Sci USA 1997, 94:10075-10079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandal M, Kumar R: Bcl-2 modulates telomerase activity. J Biol Chem 1997, 272:14183-14187 [DOI] [PubMed] [Google Scholar]
- 26.Li H, Zhao L-L, Funder JW, Liu J-P: Protein phosphatase 2A inhibits nuclear telomerase activity in human breast cancer cells. J Biol Chem 1997, 272:16729-16732 [DOI] [PubMed] [Google Scholar]
- 27.Sharma HW, Sokoloski JA, Perez JR, Maltese JY, Sartorelli AC, Stein CA, Nichols G, Khaled Z, Telang NT, Narayanan R: Differentiation of immortal human cells inhibits telomerase activity. Proc Natl Acad Sci USA 1995, 92:12343-12346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landberg G, Nielsen NH, Nilsson P, Emdin SO, Cajander J, Roos G: Telomerase activity is associated with cell cycle deregulation in human breast cancer. Cancer Res 1997, 57:549-554 [PubMed] [Google Scholar]




