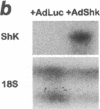
Abstract
Excitability is governed primarily by the complement of ion channels in the cell membrane that shape the contour of the action potential. To modify excitability by gene transfer, we created a recombinant adenovirus designed to overexpress a Drosophila Shaker potassium channel (AdShK). In vitro, a variety of mammalian cell types infected with AdShK demonstrated robust expression of the exogenous channel. Spontaneous action potentials recorded from cardiac myocytes in primary culture were abbreviated compared with noninfected myocytes. Intravascular infusion of AdShK in neonatal rats induced Shaker potassium channel mRNA expression in the liver, and large potassium currents could be recorded from explanted hepatocytes. Thus, recombinant adenovirus technology has been used for in vitro and in vivo gene transfer of ion channel genes designed to modify cellular action potentials. With appropriate targeting, such a strategy may be useful in gene therapy of arrhythmias, seizure disorders, and myotonic muscle diseases.
Full text
PDF

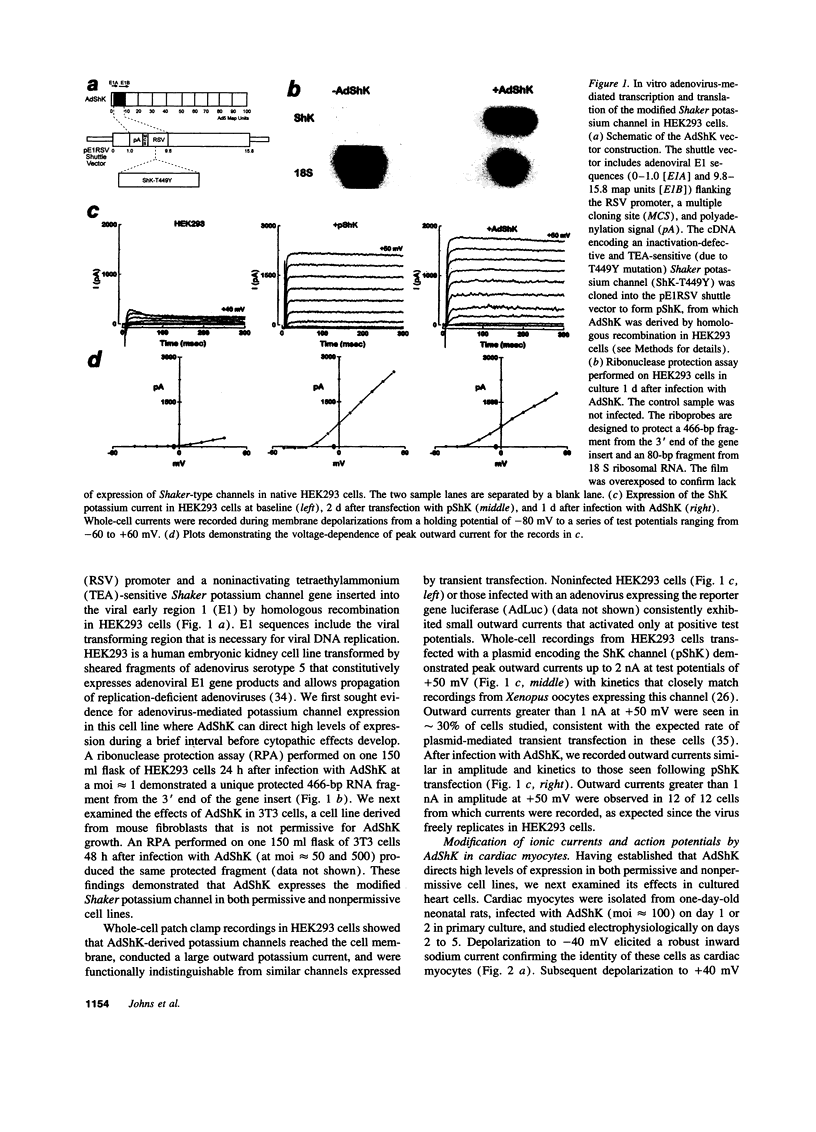
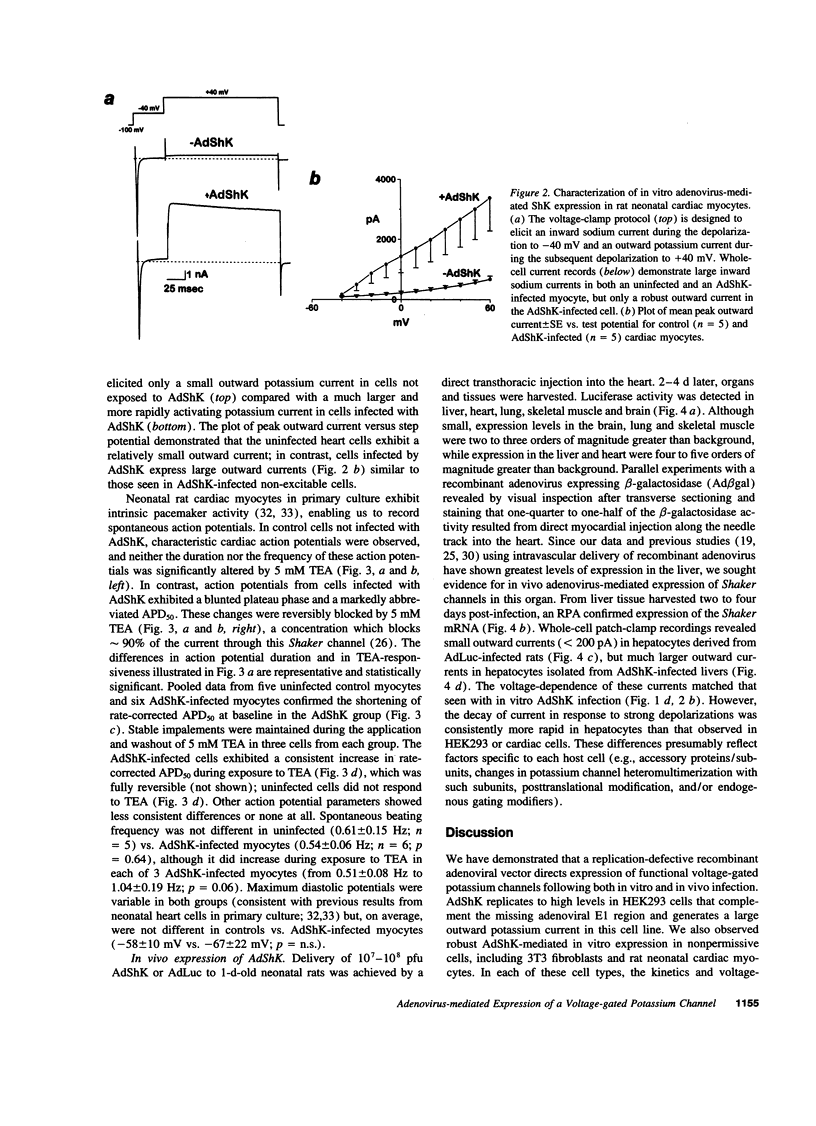
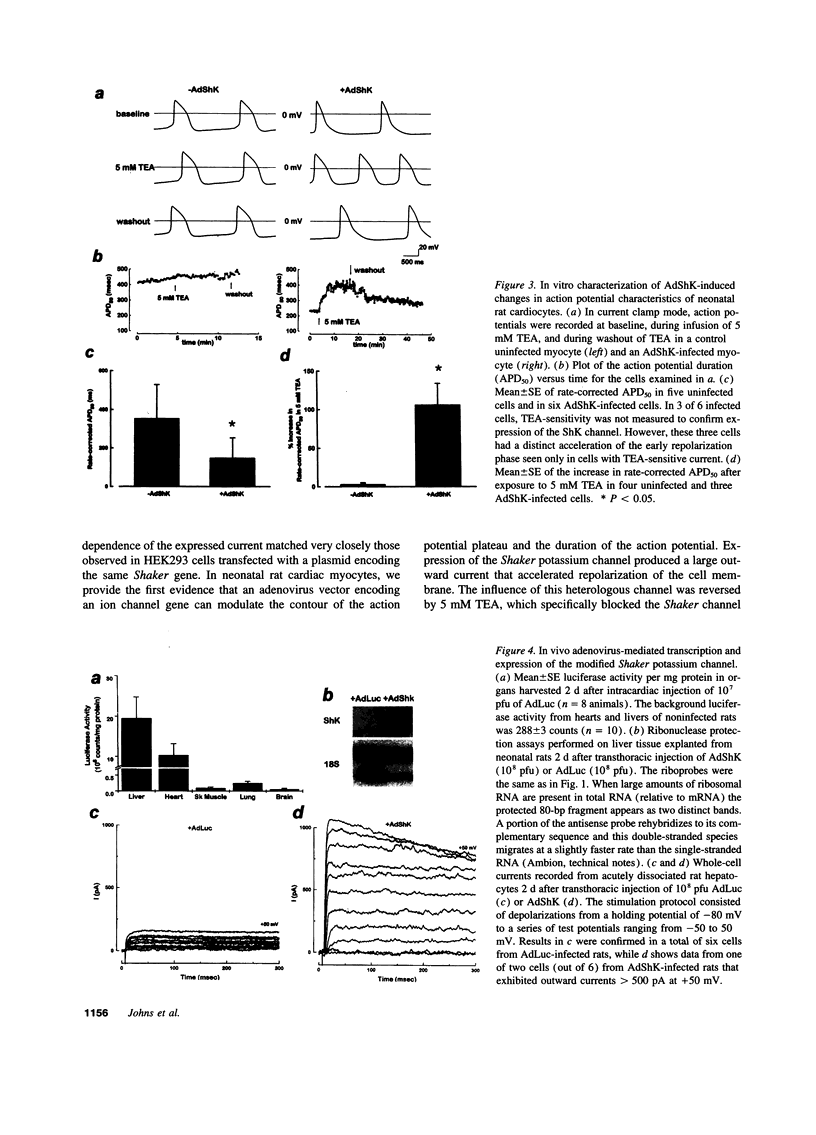


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backx P. H., Gao W. D., Azan-Backx M. D., Marban E. The relationship between contractile force and intracellular [Ca2+] in intact rat cardiac trabeculae. J Gen Physiol. 1995 Jan;105(1):1–19. doi: 10.1085/jgp.105.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bett A. J., Haddara W., Prevec L., Graham F. L. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bett A. J., Prevec L., Graham F. L. Packaging capacity and stability of human adenovirus type 5 vectors. J Virol. 1993 Oct;67(10):5911–5921. doi: 10.1128/jvi.67.10.5911-5921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckelmann D. J., Näbauer M., Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993 Aug;73(2):379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Näbauer M., Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 1992 Mar;85(3):1046–1055. doi: 10.1161/01.cir.85.3.1046. [DOI] [PubMed] [Google Scholar]
- Buttrick P. M., Kass A., Kitsis R. N., Kaplan M. L., Leinwand L. A. Behavior of genes directly injected into the rat heart in vivo. Circ Res. 1992 Jan;70(1):193–198. doi: 10.1161/01.res.70.1.193. [DOI] [PubMed] [Google Scholar]
- Curran M. E., Splawski I., Timothy K. W., Vincent G. M., Green E. D., Keating M. T. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995 Mar 10;80(5):795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Guzman R. J., Lemarchand P., Crystal R. G., Epstein S. E., Finkel T. Efficient gene transfer into myocardium by direct injection of adenovirus vectors. Circ Res. 1993 Dec;73(6):1202–1207. doi: 10.1161/01.res.73.6.1202. [DOI] [PubMed] [Google Scholar]
- Gwathmey J. K., Copelas L., MacKinnon R., Schoen F. J., Feldman M. D., Grossman W., Morgan J. P. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987 Jul;61(1):70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- Herz J., Gerard R. D. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman W. M., Friday K. J., Anderson J. L., Aliot E. M., Clark M., Lazzara R. The long QT syndromes: a critical review, new clinical observations and a unifying hypothesis. Prog Cardiovasc Dis. 1988 Sep-Oct;31(2):115–172. doi: 10.1016/0033-0620(88)90014-x. [DOI] [PubMed] [Google Scholar]
- Jiang C., Atkinson D., Towbin J. A., Splawski I., Lehmann M. H., Li H., Timothy K., Taggart R. T., Schwartz P. J., Vincent G. M. Two long QT syndrome loci map to chromosomes 3 and 7 with evidence for further heterogeneity. Nat Genet. 1994 Oct;8(2):141–147. doi: 10.1038/ng1094-141. [DOI] [PubMed] [Google Scholar]
- Kass-Eisler A., Falck-Pedersen E., Alvira M., Rivera J., Buttrick P. M., Wittenberg B. A., Cipriani L., Leinwand L. A. Quantitative determination of adenovirus-mediated gene delivery to rat cardiac myocytes in vitro and in vivo. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11498–11502. doi: 10.1073/pnas.90.24.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating M., Atkinson D., Dunn C., Timothy K., Vincent G. M., Leppert M. Linkage of a cardiac arrhythmia, the long QT syndrome, and the Harvey ras-1 gene. Science. 1991 May 3;252(5006):704–706. doi: 10.1126/science.1673802. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum L. A., MacLellan W. R., Mazur W., French B. A., Schneider M. D. Highly efficient gene transfer into adult ventricular myocytes by recombinant adenovirus. J Clin Invest. 1993 Jul;92(1):381–387. doi: 10.1172/JCI116577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Parmacek M. S., Morle G., Bolling S., Leiden J. M. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation. 1990 Dec;82(6):2217–2221. doi: 10.1161/01.cir.82.6.2217. [DOI] [PubMed] [Google Scholar]
- McClatchey A. I., Van den Bergh P., Pericak-Vance M. A., Raskind W., Verellen C., McKenna-Yasek D., Rao K., Haines J. L., Bird T., Brown R. H., Jr Temperature-sensitive mutations in the III-IV cytoplasmic loop region of the skeletal muscle sodium channel gene in paramyotonia congenita. Cell. 1992 Feb 21;68(4):769–774. doi: 10.1016/0092-8674(92)90151-2. [DOI] [PubMed] [Google Scholar]
- McGrory W. J., Bautista D. S., Graham F. L. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988 Apr;163(2):614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- Mohamed S. N., Holmes R., Hartzell C. R. A serum-free, chemically-defined medium for function and growth of primary neonatal rat heart cell cultures. In Vitro. 1983 Jun;19(6):471–478. doi: 10.1007/BF02619594. [DOI] [PubMed] [Google Scholar]
- Moule S. K., McGivan J. D. Regulation of the plasma membrane potential in hepatocytes--mechanism and physiological significance. Biochim Biophys Acta. 1990 Oct 8;1031(3):383–397. doi: 10.1016/0304-4157(90)90016-6. [DOI] [PubMed] [Google Scholar]
- Näbauer M., Beuckelmann D. J., Erdmann E. Characteristics of transient outward current in human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993 Aug;73(2):386–394. doi: 10.1161/01.res.73.2.386. [DOI] [PubMed] [Google Scholar]
- Ptáek L. J., Tawil R., Griggs R. C., Meola G., McManis P., Barohn R. J., Mendell J. R., Harris C., Spitzer R., Santiago F. Sodium channel mutations in acetazolamide-responsive myotonia congenita, paramyotonia congenita, and hyperkalemic periodic paralysis. Neurology. 1994 Aug;44(8):1500–1503. doi: 10.1212/wnl.44.8.1500. [DOI] [PubMed] [Google Scholar]
- Quantin B., Perricaudet L. D., Tajbakhsh S., Mandel J. L. Adenovirus as an expression vector in muscle cells in vivo. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2581–2584. doi: 10.1073/pnas.89.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragot T., Vincent N., Chafey P., Vigne E., Gilgenkrantz H., Couton D., Cartaud J., Briand P., Kaplan J. C., Perricaudet M. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature. 1993 Feb 18;361(6413):647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- Ricker K., Moxley R. T., 3rd, Heine R., Lehmann-Horn F. Myotonia fluctuans. A third type of muscle sodium channel disease. Arch Neurol. 1994 Nov;51(11):1095–1102. doi: 10.1001/archneur.1994.00540230033009. [DOI] [PubMed] [Google Scholar]
- Schanne O. F., Ruiz-Ceretti E., Rivard C., Chartier D. Determinants of electrical activity in clusters of cultured cardiac cells from neonatal rats. J Mol Cell Cardiol. 1977 Apr;9(4):269–283. doi: 10.1016/s0022-2828(77)80034-5. [DOI] [PubMed] [Google Scholar]
- Simpson P., Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ Res. 1982 Jan;50(1):101–116. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Makeh I., Perricaudet M., Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992 Aug;90(2):626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. At age 2, gene therapy enters a growth phase. Science. 1992 Oct 30;258(5083):744–746. doi: 10.1126/science.1472258. [DOI] [PubMed] [Google Scholar]
- Tomaselli G. F., Beuckelmann D. J., Calkins H. G., Berger R. D., Kessler P. D., Lawrence J. H., Kass D., Feldman A. M., Marban E. Sudden cardiac death in heart failure. The role of abnormal repolarization. Circulation. 1994 Nov;90(5):2534–2539. doi: 10.1161/01.cir.90.5.2534. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. Effect of current flow on the membrane potential of cardiac muscle. J Physiol. 1951 Oct 29;115(2):227–236. doi: 10.1113/jphysiol.1951.sp004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Shen J., Splawski I., Atkinson D., Li Z., Robinson J. L., Moss A. J., Towbin J. A., Keating M. T. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995 Mar 10;80(5):805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- Wolff J. A., Malone R. W., Williams P., Chong W., Acsadi G., Jani A., Felgner P. L. Direct gene transfer into mouse muscle in vivo. Science. 1990 Mar 23;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Gönczöl E., Engelhardt J. F., Wilson J. M. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat Genet. 1994 Jul;7(3):362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- Yellen G., Jurman M. E., Abramson T., MacKinnon R. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 1991 Feb 22;251(4996):939–942. doi: 10.1126/science.2000494. [DOI] [PubMed] [Google Scholar]