Abstract
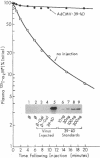
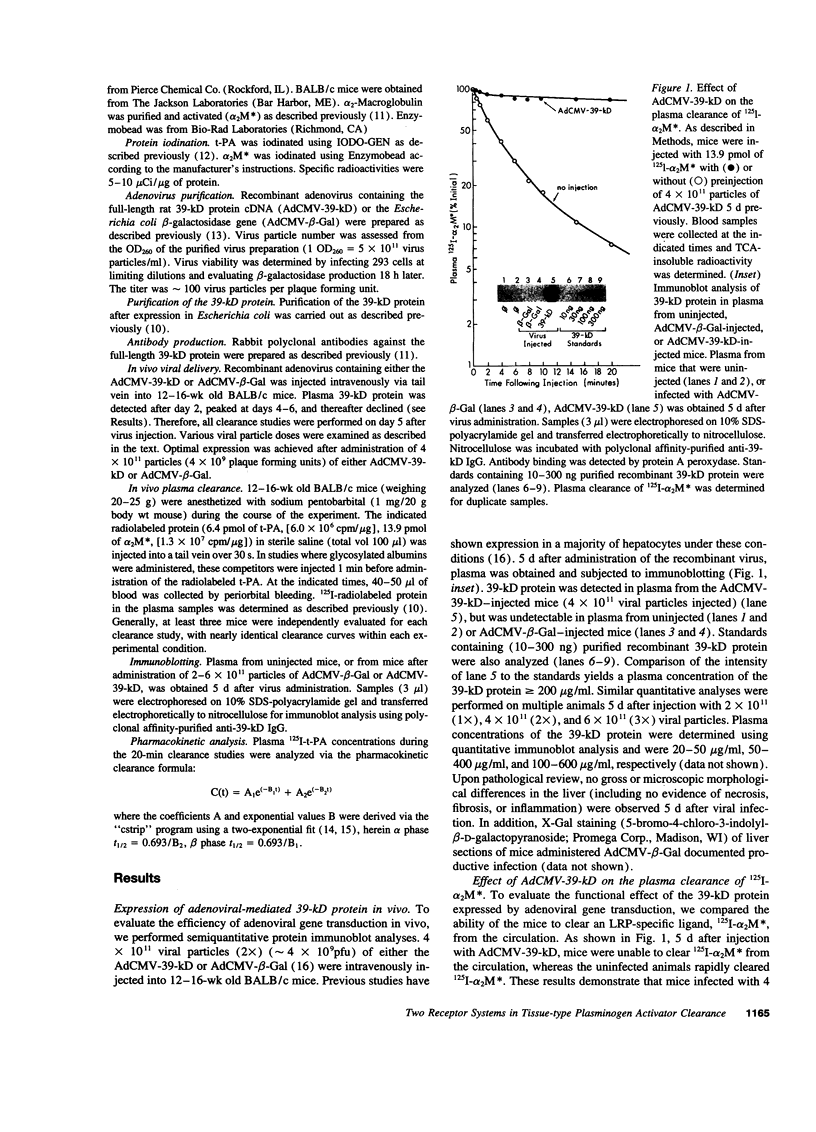
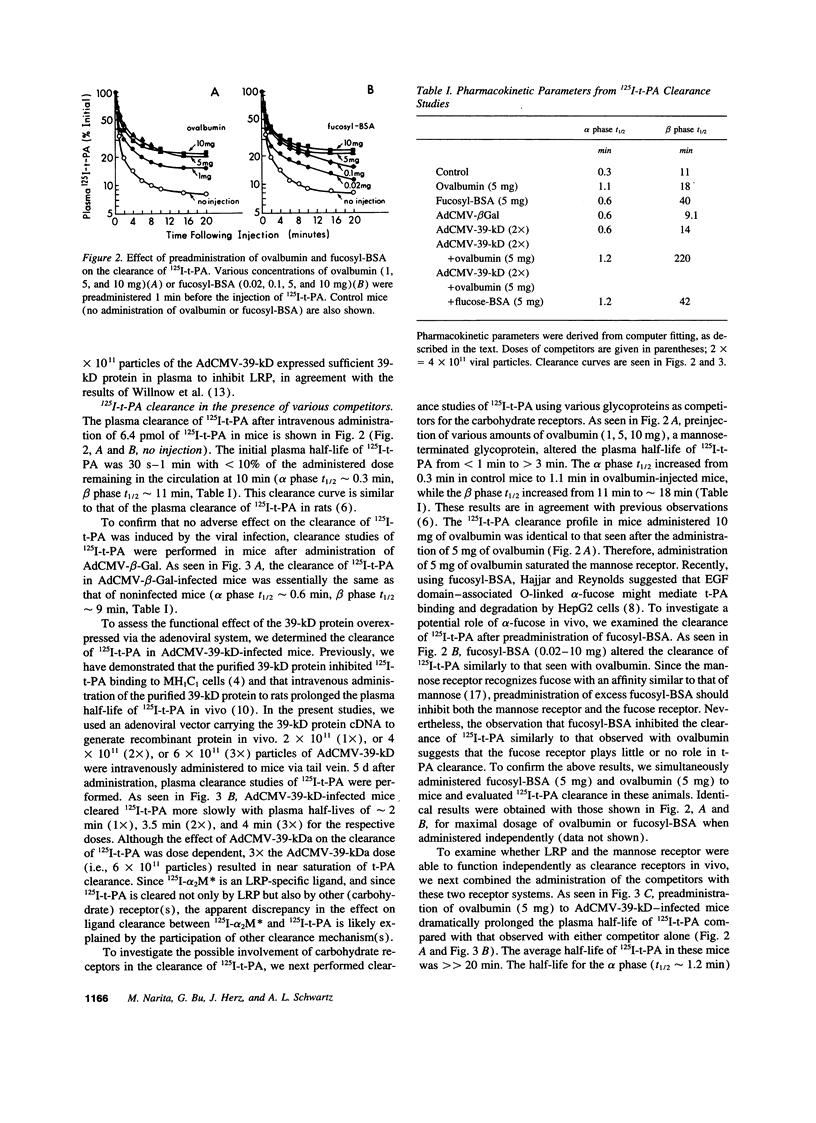
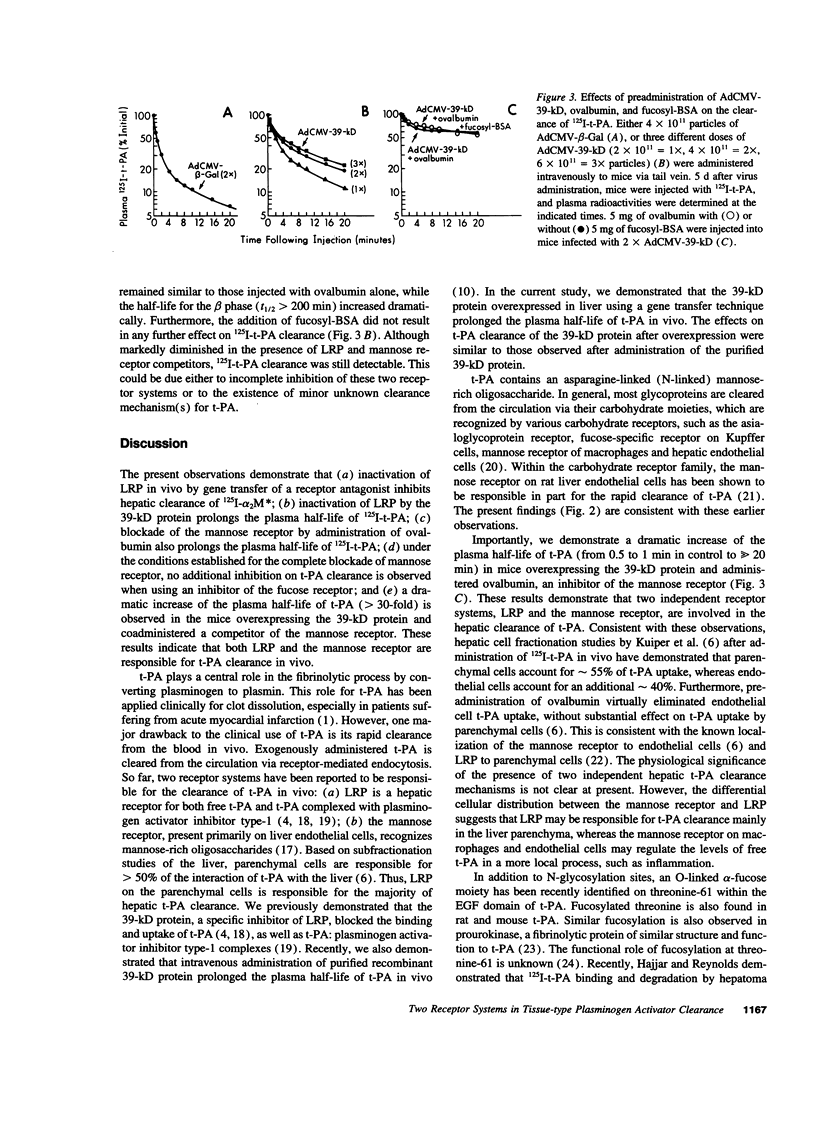
Tissue-type plasminogen activator (t-PA) is a serine protease, catalyzing the initial step in the fibrinolytic process. Intravenously administered t-PA is rapidly cleared from the circulation by the liver. Two distinct clearance mechanisms, which are mediated by the low density lipoprotein receptor-related protein (LRP) on liver parenchymal cells and by the mannose receptor on liver endothelial cells, have been described. Using competitors and inhibitors of the receptors, we investigated the role of LRP and carbohydrate receptors in t-PA clearance in vivo. To inhibit LRP, the 39-kD protein, which is a potent inhibitor of LRP activity, was overexpressed in the liver of mice using an adenoviral gene transfer technique. Expression of the 39-kD protein resulted in a sustained plasma concentration and an increase in the plasma half-life of 125I-t-PA from less than 1 min to 4-5 min. Blockade of the mannose receptor by intravenous administration of ovalbumin also prolonged the plasma half-life of 125I-t-PA to 3-4 min. The same degree of inhibition of t-PA clearance was also observed after administration of an inhibitor of the fucose receptor, fucosyl-BSA. However, under the conditions established for the complete blockade of the mannose receptor, no additional inhibition of t-PA clearance was observed using fucosyl-BSA, suggesting little or no role for the fucose receptor in the clearance of t-PA. Furthermore, a dramatic increase of the plasma half-life of 125I-t-PA (>> 20 min) was observed in mice overexpressing 39-kD protein and administered ovalbumin +/- fucosyl-BSA. Our results clearly demonstrate that two independent receptor systems, LRP and the mannose receptor, are involved in the hepatic clearance of t-PA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J. U. Tissue-type plasminogen activator: a role for O-linked fucose. J Clin Invest. 1994 Feb;93(2):459–459. doi: 10.1172/JCI116991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxenbaum H. G., Riegelman S., Elashoff R. M. Statistical estimations in pharmacokinetics. J Pharmacokinet Biopharm. 1974 Apr;2(2):123–148. doi: 10.1007/BF01061504. [DOI] [PubMed] [Google Scholar]
- Bu G., Maksymovitch E. A., Schwartz A. L. Receptor-mediated endocytosis of tissue-type plasminogen activator by low density lipoprotein receptor-related protein on human hepatoma HepG2 cells. J Biol Chem. 1993 Jun 15;268(17):13002–13009. [PubMed] [Google Scholar]
- Bu G., Morton P. A., Schwartz A. L. Identification and partial characterization by chemical cross-linking of a binding protein for tissue-type plasminogen activator (t-PA) on rat hepatoma cells. A plasminogen activator inhibitor type 1-independent t-PA receptor. J Biol Chem. 1992 Aug 5;267(22):15595–15602. [PubMed] [Google Scholar]
- Bu G., Warshawsky I., Schwartz A. L. Cellular receptors for the plasminogen activators. Blood. 1994 Jun 15;83(12):3427–3436. [PubMed] [Google Scholar]
- Bu G., Williams S., Strickland D. K., Schwartz A. L. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor is an hepatic receptor for tissue-type plasminogen activator. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7427–7431. doi: 10.1073/pnas.89.16.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer K. Clearing up glycoprotein hormones. Cell. 1991 Dec 20;67(6):1029–1032. doi: 10.1016/0092-8674(91)90278-7. [DOI] [PubMed] [Google Scholar]
- Hajjar K. A., Reynolds C. M. alpha-Fucose-mediated binding and degradation of tissue-type plasminogen activator by HepG2 cells. J Clin Invest. 1994 Feb;93(2):703–710. doi: 10.1172/JCI117023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltiwanger R. S., Lehrman M. A., Eckhardt A. E., Hill R. L. The distribution and localization of the fucose-binding lectin in rat tissues and the identification of a high affinity form of the mannose/N-acetylglucosamine-binding lectin in rat liver. J Biol Chem. 1986 Jun 5;261(16):7433–7439. [PubMed] [Google Scholar]
- Harris R. J., Leonard C. K., Guzzetta A. W., Spellman M. W. Tissue plasminogen activator has an O-linked fucose attached to threonine-61 in the epidermal growth factor domain. Biochemistry. 1991 Mar 5;30(9):2311–2314. doi: 10.1021/bi00223a004. [DOI] [PubMed] [Google Scholar]
- Herz J., Gerard R. D. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J., Goldstein J. L., Strickland D. K., Ho Y. K., Brown M. S. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J Biol Chem. 1991 Nov 5;266(31):21232–21238. [PubMed] [Google Scholar]
- Kuiper J., Otter M., Rijken D. C., van Berkel T. J. Characterization of the interaction in vivo of tissue-type plasminogen activator with liver cells. J Biol Chem. 1988 Dec 5;263(34):18220–18224. [PubMed] [Google Scholar]
- Lijnen H. R., Collen D. Strategies for the improvement of thrombolytic agents. Thromb Haemost. 1991 Jul 12;66(1):88–110. [PubMed] [Google Scholar]
- Orth K., Madison E. L., Gething M. J., Sambrook J. F., Herz J. Complexes of tissue-type plasminogen activator and its serpin inhibitor plasminogen-activator inhibitor type 1 are internalized by means of the low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7422–7426. doi: 10.1073/pnas.89.16.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth K., Willnow T., Herz J., Gething M. J., Sambrook J. Low density lipoprotein receptor-related protein is necessary for the internalization of both tissue-type plasminogen activator-inhibitor complexes and free tissue-type plasminogen activator. J Biol Chem. 1994 Aug 19;269(33):21117–21122. [PubMed] [Google Scholar]
- Otter M., Barrett-Bergshoeff M. M., Rijken D. C. Binding of tissue-type plasminogen activator by the mannose receptor. J Biol Chem. 1991 Jul 25;266(21):13931–13935. [PubMed] [Google Scholar]
- Smedsrød B., Einarsson M. Clearance of tissue plasminogen activator by mannose and galactose receptors in the liver. Thromb Haemost. 1990 Feb 19;63(1):60–66. [PubMed] [Google Scholar]
- Stahl P. D. The macrophage mannose receptor: current status. Am J Respir Cell Mol Biol. 1990 Apr;2(4):317–318. doi: 10.1165/ajrcmb/2.4.317. [DOI] [PubMed] [Google Scholar]
- Warshawsky I., Bu G., Schwartz A. L. 39-kD protein inhibits tissue-type plasminogen activator clearance in vivo. J Clin Invest. 1993 Aug;92(2):937–944. doi: 10.1172/JCI116669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshawsky I., Bu G., Schwartz A. L. Identification of domains on the 39-kDa protein that inhibit the binding of ligands to the low density lipoprotein receptor-related protein. J Biol Chem. 1993 Oct 15;268(29):22046–22054. [PubMed] [Google Scholar]
- Willnow T. E., Sheng Z., Ishibashi S., Herz J. Inhibition of hepatic chylomicron remnant uptake by gene transfer of a receptor antagonist. Science. 1994 Jun 3;264(5164):1471–1474. doi: 10.1126/science.7515194. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Nakagawa T., Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978 Apr;6(2):165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]