SUMMARY
This study applied improved DNA extraction and polymerase chain reaction strategies for screening and identification of Trypanosoma cruzi lineages directly from faeces of triatomines collected in a well-defined rural area in northwestern Argentina. Amplification of the variable regions of the kinetoplastid minicircle genome (kDNA-PCR) was performed in faecal lysates from 33 microscope (MO)-positive and 93 MO-negative Triatoma infestans, 2 MO-positive and 38 MO-negative Triatoma guasayana and 2 MO-positive and 73 MO-negative Triatoma garciabesi. kDNA-PCR detected T. cruzi in 91% MO-positive and 7.5% MO-negative T. infestans, which were confirmed by amplification of the minicircle conserved region. In contrast, kDNA-PCR was negative in all faecal samples from the other triatomine species. A panel of PCR-based genomic markers (intergenic region of spliced-leader DNA, 24Sα and 18S rRNA genes and A10 sequence) was implemented to identify the parasite lineages directly in DNA lysates from faeces and culture isolates from 28 infected specimens. Two were found to be infected with TCI, 24 with TCIIe, 1 with TCIId and 1 revealed a mixed TCI+TCII infection in the faecal sample whose corresponding culture only showed TCII, providing evidence of the advantages of direct typing of biological samples. This study provides an upgrade in the current diagnosis and lineage identification of T. cruzi in field-collected triatomines and shows T. cruzi II strains as predominant in the region.
Keywords: Trypanosoma cruzi, Triatoma infestans, Triatoma guasayana, Triatoma garciabesi, Chagas disease, PCR, lineage
INTRODUCTION
Chagas disease, a zoonosis caused by Trypanosoma cruzi and transmitted by triatomine bugs, is considered one of the most important vector-borne diseases in Latin America (World Bank, 1993). The risk of transmission of T. cruzi in endemic rural areas mainly depends on the density of triatomine bugs and the prevalence of T. cruzi infection in triatomine vectors, humans and animal reservoirs (Cohen and Gürtler, 2001). Triatoma infestans, the main vector of T. cruzi in South America, is currently the target of an elimination programme through residual spraying with insecticides (Schofield and Dias, 1999). During the surveillance phase after spraying, other sylvatic or peridomestic triatomine species have been reported to emerge as putative secondary vectors of T. cruzi (Schofield, Diotaiuti and Dujardin, 1999). In the Gran Chaco, Triatoma sordida, Triatoma guasayana and Triatoma garciabesi were considered candidates for domestication (Wisnivesky-Colli et al. 1993; Diotaiuti et al. 1995; Noireau et al. 1995, Noireau et al. 1999; Castañera et al. 1998). T. cruzi was recently isolated from peridomestic T. guasayana (Lauricella et al. 2005).
Control programs periodically monitor the prevalence of T. cruzi infection in target triatomine bugs through microscopical observation (MO) of diluted faeces in search for active trypanosomes. However, MO presents limited sensitivity in samples with low parasite numbers and may lack specificity due to infections with other trypanosomatids, such as Trypanosoma rangeli and Blastocrithidia triatomae (Cerisola et al. 1971; Chiurillo et al. 2003). A more sensitive and specific method to assess T. cruzi infection in triatomine bugs is the polymerase chain reaction (PCR), which has been applied directly to DNA preparations from faecal samples (Breniere et al. 1995; Russomando et al. 1996; Dorn et al. 2001).
T. cruzi has been classified into 2 major phylogenetic lineages, T. cruzi I (TCI) and T. cruzi II (TCII) (Anonymous, 1999); the latter includes 5 sublineages designated TCIIa to TCIIe (Brisse, Barnabé and Tibayrenc, 2000). T. cruzi lineages appear to be distributed differentially among triatomine species, hosts and habitats throughout the Americas. Epidemiological studies suggest that TCIIb, IId and IIe are more related with anthroponotic environments and chronic Chagas disease patients, lineages IIa and IIc with sylvatic environments and lineage I with both (Souto et al. 1996; Zingales et al. 1998; Fernandes et al. 1999; Barnabé et al. 2000; Brisse et al. 2000; Brisse, Verhoef and Tibayrenc, 2001; Yeo et al. 2005). T. cruzi lineages have usually been identified from cultured stocks, which may underestimate parasite diversity in natural infections due to possible strain selection during culture expansion (Macedo and Pena, 1998). As part of a wider eco-epidemiological study conducted in rural northwestern Argentina, the present study aimed to optimize and evaluate DNA extraction and PCR-based procedures for screening and identification of T. cruzi lineages directly from faecal samples of triatomine bugs.
MATERIALS AND METHODS
Triatomine collection
Field studies were carried out in Amamá and nearby rural villages (27° 12′ 33″ S, 63° 02′ 10″ W), Province of Santiago del Estero, Argentina, during October 2002. The study area has been described elsewhere (Gürtler et al. 1999; Cecere et al. 2004). Two areas were visited (i) the core area, which included Amamá, Trinidad, Mercedes, Pampa Pozo, San Pablo and Villa Matilde, where regular triatomine surveillance has been conducted since a residual application of insecticides in 1992, and (ii) the peripheral area, that included 30 villages clustered in 6 groups around the core area, and where insecticide sprays were conducted mostly in 1994-1996 and 2001, with no regular surveillance activities in the intervening period. Householders were informed of the purpose of the research project, anticipated benefits and potential ways in which they could participate.
Triatomine infestation was assessed in 300 houses and their peridomestic structures using timed manual collections for 30 min per house as described previously (Gürtler et al. 1995). Two teams of 3 experienced bug collectors each from the National Vector Control Program captured triatomine bugs with the aid of an irritant agent (0.2% tetramethrin, Icona, Buenos Aires). Peridomestic structures searched for bugs included goat, sheep or pig corrals, cow or horse corrals, chicken coops, trees where chicken roosted, storerooms, kitchens and other putative refuges for triatomines within the area of human activity. Some householders collected domestic bugs and kept them in plastic bags that they handed on to the research group. Triatomines were also collected in peridomestic and sylvatic sites with light traps as described by Vazquez-Prokopec et al. (2004). All captured bugs were placed in labelled plastic bags with folded filter paper inside and transported to the field laboratory at 10 °C, where they were identified to species, stage and collection site as described by Canale et al. (2000).
Diagnosis of T. cruzi infection
All live or moribund third, fourth and fifth instar nymphs and adult triatomines were examined for T. cruzi infection by microscopical observation (MO) of faecal samples, within 2 days of capture. Faecal drops obtained by abdominal compression from each bug were diluted with 1 drop of saline solution (approximately 50 μl) and thoroughly examined for active trypanosomes at 220-400X. When preparing the slides for MO inspection, triatomine faecal samples were also stored in sterile microtubes at 4-12 °C for PCR analysis. Samples for PCR were collected in order to represent nearly all capture sites. Forceps were rinsed in 10% bleach and 70% ethanol between extracting successive samples. Contamination controls of this procedure were obtained by systematically rinsing forceps in saline solution on a slide and storing the wet preparation in sterile microtubes.
DNA from 25 μl of each faecal sample was purified using DNAzol reagent (Gibco BRL, USA) as recommended by the manufacturer. A subset of samples was processed by boiling 25 μl of faecal samples for 15 min and centrifuging to discard the debris, as previously described (Breniere et al. 1995). To analyse culture isolates, parasites were pelleted by centrifugation at 3000 g for 5 min. The pellets were suspended in sterile water, boiled for 10 min and centrifuged at 13 000 g. The supernatant, diluted 1/3 with sterile water, was used for PCR.
The PCR assay used to detect T. cruzi was the amplification of a fragment of 330 bp from the variable regions of minicircles of the kinetoplastid genome (vkDNA), as described previously (Schijman et al. 2003). To validate vkDNA-PCR findings in MO-negative samples, PCR-positive cases were further tested applying another PCR procedure targeted to the conserved region of the minicircle DNA, using primer pairs 34 5′ TATATTACA-CCAACCCCAATCGAACC 3′ and 67 5′ TG-GTTTTGGGAGG-GGSSKTCAAM TTT 3′ (ckDNA-PCR) under the following cycling conditions: 3 min at 94 °C; 2 cycles at 58 °C-45 sec, 72 °C-1 min, 94 °C-45 sec; 2 cycles at 56 °C-45 sec, 72 °C-1 min, 94 °C-45 sec; 2 cycles at 54 °C-45 sec, 72 °C-1 min, 94 °C-45 sec and 36 cycles at 52 °C-45 sec, 72 °C-1 min, 94 °C-45 sec with 1 final extension step at 72 °C for 10 min. The detection limits of both kDNA-based PCR procedures ranged from 1/40 to 1/20 parasite genomes per reaction tube.
Detection of PCR inhibition
The presence of PCR inhibitors was examined in PCR-negative faeces by spiking the corresponding DNAzol lysates with 100 fg of a recombinant plasmid containing an internal vkDNA-PCR standard to evaluate its amplification, as previously described (Schijman et al. 2003).
Parasite culture
All MO positive bugs were shipped to the National Institute of Parasitology Fatala Chabén for T. cruzi isolation and culture in biphasic medium (Nutrient agar defibrinated rabbit blood /Brain Heart Infusion). Cultures were kept at 28 °C and 50% relative humidity and microscopically monitored for parasite growth bimonthly for 4 months. Cultures were then stored in liquid nitrogen and defrosted for genotyping as previously described (Lauricella et al. 2005).
T. cruzi molecular typing
To identify TCI and TCIIa to TCIIe lineages, a panel of PCR procedures targeted to 4 different parasite genomic sequences was applied, namely the intergenic spacer of the spliced leader genes (SL-DNA-PCR), the D7 domain of the 24Sα ribosomal RNA genes, the 18S ribosomal RNA genes and the A10 fragment, as recently proposed by Brisse and coworkers (2001). The amplification conditions were modified to enhance PCR sensitivity and specificity to allow lineage identification directly from DNA lysates of triatomine faeces (Table 1).
Table 1.
PCR-based identification of Trypanosoma cruzi lineages in faeces and culture isolates from triatomine bugs: DNA targets, application, primer sets and amplicon expected sizes in base pairs
| DNA Target | Application | Primer sets | TCl | TClla | TCllb | TCllc | TClld | TClle | |
|---|---|---|---|---|---|---|---|---|---|
| I | Spliced leader intergenic region | Distinction of TCl from TClla, llc & TCllb, lld, lle | TCC-TC1-TC2a | 350 | n/a | 300 | n/a | 300 | 300 |
| TCac-UTCCb | 157 | 200 | 150 | 200 | 150 | 150 | |||
| II | 24S alpha rDNA | Distinction between TClla & TCllc or between TClld & TCllb, lle | D71-D76b | 125 | 135 | 140 | 125 | 125+140 | 140 |
| III | 18S rDNA A10 fragment | Distinction between TCllb & TClle | V1-V2c | 175 | 155 | 165 | 165 | 165 | n/ac,d |
| P3-P6c | n/a | 657 | n/a | 657 | 657 | 657 |
I to III: Work flow for lineage identification. Boxed amplicon sizes denote the PCR findings of this survey.
n/a: no amplification.
Heminested PCR (Burgos et al. 2005).
Most TClle isolates from northwestern Argentina amplify a 165bp 18S rDNA fragment.
SL-DNA PCR
A hot-start multiplex PCR using TC1-TC2-TCC primer set was carried out (Souto et al. 1996). The 50 μl volume PCR reaction contained 1·25 units (U) of Taq DNA polymerase bound to proprietary antibody (Platinum Taq polymerase, Invitrogen, Life Technologies, USA), 1·5 μM of each primer, 250 μM dNTPs, 3 mM MgCl2, in buffer 1 ×. Cycling conditions: 3 min at 94 °C; a 5-step-touch-down PCR, from 60 °C to 52 °C, was undertaken with 4 rounds of 3 cycles each, consisting of 1 min at 94 °C, 1 min annealing at 60 °C, 58 °C, 56 °C and 54 °C from the first to the fourth round respectively and 1 min elongation at 72 °C; 35 cycles of 1 min at 94 °C, 1 min at 52 °C and 1 min at 72 °C; and final step at 72 °C for 10 min. SL-DNA PCR rendered a 350 bp amplicon for TCI or 300 bp for TCIIb, TCIId or TCIIe (Table 1).
A novel PCR to detect TCIIa and TCIIc sub-lineages, which are not recognized by TC1 or TC2 primer sequences was developed using TCac 5′ CTCCCCAGTGTGGCCTGGG 3′ as sense primer and UTCC 5′ CGTACCAATATAGTACAGAAACTG 3′ as antisense (Burgos et al. 2005). A 50 μl total volume PCR reaction containing 1 U of Taq Platinum, 150 pmol of each primer, 250 μM dNTPs, 3 mM MgCl2, in buffer 1×. Cycling conditions were the same as SL-DNA PCR but annealing temperatures ranged from 68 to 60 °C during touch-down PCR cycles.
24Sα ribosomal DNA-heminested PCR
The first PCR-round was done with D75-D76 primers as reported (Briones et al. 1999) in a 50 μl volume reaction containing 4 μM of each primer, 250 μM dNTPs, 3μM MgCl and 1.25 U of Taq Platinum. The second round was carried out using 1 μl (faeces) or 1 μl of a 1: 50 dilution (cultures) of the first-round PCR products in a 30 μl volume reaction using 5 μM of D71-D76 primers (Burgos et al. 2005) 250 μM dNTPs, 2 μM MgCl and 0.75 U of Taq Platinum. PCR conditions were 3 min at 94 °C, 3 cycles of 94 °C for 1 min, 60 °C for 1 min and 72 °C for 1 min; 3 cycles of 57 °C for the annealing step; 35 cycles of 55 °C as annealing temperature, and elongation at 72 °C for 7 min.
To differentiate between TCIIb and TCIIe sub-lineages 2 PCR assays were applied (Table 1). (a) 18S rDNA-PCR amplified a 165 bp fragment from TCIIb but not from TCIIe DNA (Brisse et al. 2001). It was carried out in a 50 μl volume reaction with 100 pmol of each primer V1 and V2 (Clark and Pung, 1994), 250 μM dNTPs, 2 mM MgCl2 and 1·25 U of Taq Platinum. Cycling conditions were 3 min at 94 °C, 3 cycles at 94 °C for 1 min, 70 °C for 1 min and 72 °C for 1 min; 3 cycles using 68 °C for the annealing step, 33 cycles using 65 °C for the annealing step and a final step at 72 °C for 7 min. (b) A10-PCR (Brisse et al. 2000) was used to distinguish between TCIIb (A10 negative) from TCIIe (A10 positive, 657 bp product). This PCR used primers p3 and p6 in a 50 μl volume reaction containing 250 μM dNTPs, 2 mM MgCl2 and 0·75 U of Taq Platinum, under the following cycling conditions: 3 min at 94 °C, 40 cycles of 94 °C for 1 min, 55 °C for 1 min and 72 °C for 1 min and a final step at 72 °C for 7 min.
Amplified products were analysed in 3% agarose gels (agarose 1000, GibcoBRL/Life Technologies, USA) and ultraviolet light (UV) visualization after ethidium bromide staining. Distinction between 150 and 157 bp TCac-UTCC SL-DNA products, 155, 165 and 175 bp 18S rDNA products and 135 and 140 bp 24sα rDNA products was established by 10% polyacrylamide gel electrophoresis and UV visualization after Sybr green dye staining, as described (Burgos et al. 2005).
The detection limits of the different PCR assays were estimated by serial dilution experiments with DNA from reference strains of different lineages spiked into faeces from laboratory-reared T. infestans bugs. The detection limits of SL-DNA and 18s rDNA based PCR were approximately 2·5 parasite genomes in the reaction tube. The detection limit of first-round 24Sα rDNA-PCR was between 5 parasite genomes, increasing to ½ parasite genome after D71-D76 heminested-PCR. The A10 based PCR showed a detection limit of 25 parasite genomes.
T. cruzi strains of different lineages were used as controls, namely: TCI (X-10, G, TCC, HA, GAL 61, MG10, SN3); TCIIa (Can III); TCIIb (Tu 18, AF1, Y); TCIIc (M5631); TCIId (Mn Cl2); TCIIe (Tul II, CL-Brener, Tep 7). Some reference strains were kindly provided by Patricio Diosque and Miguel Angel Basombrio (Instituto de Patología Experimental, Universidad Nacional de Salta, Argentina), Michel Tibayrenc (UR62 “Genetics of Infectious Diseases”, IRD Centre, Montpellier, France) and Omar Triana Chavez (University of Antioquía, Medellin, Colombia).
RESULTS
Of 2238 triatomines collected from domestic, peridomestic or sylvatic habitats using various methods, 80% were T. infestans, 14% T. garciabesi and 6% T. guasayana. Using timed manual collections, most of the T. infestans (86%) were captured from peridomestic sites, and >95% of the domestic T. infestans were from the area under no regular surveillance. Only 5 adult T. guasayana were collected from domestic sites; the remainder and all T. garciabesi were collected from peridomestic structures or in sylvatic habitats with light traps. T. cruzi infection was detected by MO in 41 (3.2%) of 1264 T. infestans, 2(1.8%) of 110 T. guasayana, and 3 (1.1%) of 283 T. garciabesi. Twenty five (61%) infected T. infestans had been captured in domiciles or human habitations. Only 3 (7.3%) of the total infected T. infestans were from the core area under regular entomological surveillance.
Amplification of the variable region of minicircle DNA (vkDNA-PCR) was performed in 38 MO-positive samples that included 33 T. infestans, 2 T. guasayana and 2 T. garciabesi (Table 2). Variable kDNA-based PCR detected T. cruzi in 30 (91%) T. infestans. Three T. infestans samples and all samples from the other triatomine species were kDNA-PCR negative. However, the DNA extracts from 2 T. infestans, 1 T. guasayana and 1 T. garciabesi that had been both MO-positive and PCR-negative did not amplify the internal kDNA standard used to test inhibition, and thus gave invalid results (Table 2). Of 204 MO-negative faecal samples screened by vkDNA-PCR, 7 (3.4%) T. infestans were PCR-positive (Table 2). In these cases, PCR positivity was further confirmed by means of amplification of the 120 bp conserved region of the minicircle DNA (ckDNA-PCR). PCR inhibition was tested in 110 DNAzol lysates that were both MO and PCR-negative. Inhibitors were detected in 20%, 16% and 42% of the DNAzol lysates from T. infestans, T. guasayana and T. garciabesi, respectively. A Chi-square showed that the difference in inhibition varied according to the tested species (X2=7.73; P=0.025).
Table 2.
Comparison of MO and kDNA-PCR screening of Trypanosoma cruzi infection in faeces of field-captured triatomines
| MO-positive faeces |
MO-negative faeces |
|||||||
|---|---|---|---|---|---|---|---|---|
| Species | No. tested | PCR+ | PCR- | Inhibited/testeda | No. tested | PCR+ | PCR- | Inhibited/testeda |
| T. infestans | 33 | 30 | 3b | 2/3 | 93 | 7 | 86 | 7/35 |
| T. guasayana | 2 | 0 | 2b | 1/2 | 38 | 0 | 38 | 5/32 |
| T. garciabesi | 2 | 0 | 2b | 1/2 | 73 | 0 | 73 | 18/43 |
| Total | 37 | 30 | 7 | 5/8 | 204 | 7 | 197 | 30/110 |
inhibition was tested in PCR negative samples;
only 1 true negative.
A subset of 32 MO- and PCR-negative T. infestans faecal samples was also tested by the boiling method (Breniere et al. 1995), but 19 cases (59.3%) were shown to carry PCR inhibitors (not shown). PCR inhibition was deactivated by diluting in sterile water 10 times the 7 DNAzol lysates or 120 times the 19 boiled lysates with inhibitors, but no additional PCR positive samples were detected.
T. cruzi molecular typing
Parasite lineages were identified directly from faecal samples and/or culture isolates obtained from faeces in a total of 28 kDNA-PCR positive T. infestans (Table 3). TCII was the predominant lineage in both domestic and peridomestic sites, whereas TCI was detected in only 2 adult bugs (7.1%) captured in 2 different villages. Examples of PCR-based identification of T. cruzi lineages are shown in Fig. 1. In 8 specimens, the parasite lineages were identified from both faecal and culture samples. The concordance between the types of tested samples was 87.5%, because in 1 specimen the culture sample revealed only TCII (DG-100-10 in Fig. 1A) whereas the faecal sample revealed a mixed infection with TCI+TCII (DG-100-F in Fig. 1A) (Table 3). In 4 specimens, only faeces were typed and all were TCII (P-15, Fig. 1A); among 16 specimens in which only cultures were characterized, 14 were TCII and 2 were TCI (IG-41, Fig. 1A).
Table 3.
Trypanosoma cruzi lineages identified by PCR in faeces and/or culture isolates from faeces of 28 field-collected T. infestans
|
Trypanosoma cruzi lineage |
||||||
|---|---|---|---|---|---|---|
| Type of sample | Number | l | lla or llc | lle or llb+lle | lld | l+ll |
| Faeces | 12 | 0 | 0 | 11 | 0 | 1 |
| Culture isolates | 24 | 2 | 0 | 21 | 1 | 0 |
Fig. 1.
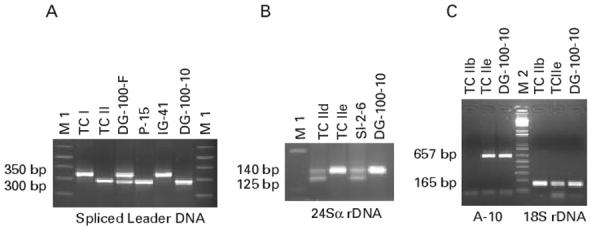
PCR-based identification of Trypanosoma cruzi lineages in faeces and culture isolates of naturally infected T. infestans. (A) Identification of TCI and TCII by SL-DNA PCR. TCI is identified from a culture isolate of specimen IG-41 and TCII from faeces of specimen P-15. DG-100-F and DG-100-10 are faecal and culture samples from a same triatomine, which revealed a dual TCI+TCII infection in faeces (DG-100 F) but only TCII in culture (DG-100-10). (B) Distinction between TCIId and TCIIb or TCIIe by 24Sα rDNA-PCR. TCIId is identified in a culture isolate from specimen SI-2-6 (125 bp + 140 bp amplicons), whereas TCIIb or TCIIe are detected in the culture isolate of DG-100-10 (140 bp product alone). (C) Differential amplification of TCIIb and TCIIe by A10 and 18S rDNA-PCR. TCIIb control strain shows only amplification of 18S rDNA genes. In contrast, TCIIe control strain and culture isolate from specimen DG-100-10 show amplification of both markers. T. cruzi control strains: TCI, T. cruzi I (X-10); TCllb, T. cruzi IIb (Tu 18); TC IId, T. cruzi IId (Mn CL2); TCIIe, T. cruzi IIe (CL-Brener). M1: 100 bp ladder DNA molecular weight marker, M2, 50 bp ladder DNA molecular weight marker; 3% agarose gel electrophoresis stained with ethidium bromide.
Lineages TCIIa or TCIIc were not detected in these samples, since none amplified the expected 200 bp TCac-UTCC based SL-DNA product (Table 1). TCIId was detected in the culture isolate from 1 domiciliary male T. infestans faecal sample, but no direct PCR analysis from the corresponding faeces was performed, due to lack of material. The identification of TCIId was based on the amplification of both 125 bp and 140 bp products by heminested PCR of the 24Sα rDNA genes (Table 1 and Fig. 1B, specimen SI-2-6). The remaining tested samples showed TCIIb or TCIIe strains, because they amplified only the major 140 bp 24Sα rDNA product. To distinguish between TCIIb and TCIIe sublineages, 18S rDNA- and A10-based PCR procedures were assayed (Table 1). Surprisingly, most cases amplified both sequences (DG-100-10, Fig. 1C); those that did not amplify the A10 fragment were not informative because they were also negative at the first round 24Sα rDNA-PCR, revealing low parasite numbers or partial PCR inhibition. This type of strain predominated in the tested triatomine population (85.7%). It has been described that a proportion of TCIIe strains may amplify a 165 bp 18S rDNA sequence (Brisse et al. 2001). Indeed, Tulahuen II and CL-Brener reference TCIIe strains did amplify both A10 and 18S rDNA sequences (Fig. 1C), whereas Tep 7 only amplified the A10 fragment (not shown). In this context, the biological samples under study would be most likely infected by TCIIe strains that carry the 165 bp 18S rDNA sequence, although mixed infections of TCIIe+TCIIb strains can not be excluded.
Lineage types were not significantly associated with capture site (Fisher exact test, P=1) or triatomine developmental stage (Fisher exact test, P=0.96).
DISCUSSION
PCR-based screening of T. cruzi infection from faecal samples of field-captured triatomines
Minicircle DNA-based PCR screening of T. infestans specimens confirmed most T. cruzi infections detected by MO and detected new ones. Following a massive residual spraying with insecticides of Amamá and nearby villages in 1992 and regular surveillance, the prevalence of T. cruzi in T. infestans was 2.4% based on MO findings during 1993-1997 (Cecere et al. 1999). The present study in the same villages in 2002 detected a prevalence of 1.1% by MO and PCR, which was not significantly different from the 1993-1997 estimates.
PCR was shown to be more sensitive than MO for detection of T. cruzi in different biological specimens and settings (Junqueira, Chiari and Wincker, 1996; Kirchoff et al. 1996; Schijman et al. 2000, Schijman et al. 2003, Schijman et al. 2004; Burgos et al. 2005). In the present study, PCR procedures targeted to the 330 bp variable or the 120 bp conserved regions of the minicircle genome were 100% concordant in the detection of T. cruzi infection in the tested MO negative samples. Both kDNA-PCR tests allowed identification of 7.5% positive faecal samples that had been MO-negative, but failed to detect 1 sample that was both MO and culture-positive.
PCR-based bug infection rates may be underestimated because of PCR inhibitors. The extent of inhibition varied between triatomine species for unknown reasons. DNA extraction methods from faecal samples need to be improved in order to increase PCR sensitivity. Indeed, PCR performed from DNAzol extracts showed a lower degree of inhibition than amplification performed directly from boiled faeces. The effect of inhibitors could be neutralized after diluting DNAzol lysates 10 times or boiled samples 120 times. However, high dilutions may decrease the sensitivity of the test to detect samples with low parasite numbers.
Previous MO-based studies reported that T. guasayana faeces had “T. cruzi-like” trypanosomes (Wisnivesky-Colli et al. 1993), “flagellates” (Noireau et al. 1999) or T. cruzi as determined by morphology (Gajate et al. 1996; Cecere et al. 1999). Application of PCR confirmed the frequent occurrence of T. cruzi in T. guasayana in the Bolivian Chaco (Noireau et al. 1999) but not in the Paraguayan (Yeo et al. 2005) and Argentinean Chaco. We corroborated the negative kDNA-PCR results obtained in the MO-positive T. guasayana and T. garciabesi faecal specimens without inhibition, by applying another PCR assay targeted to the nuclear 195 bp satellite sequence of T. cruzi (Schijman et al. 2000) which did not show amplification. However, positive amplification of a 24Sα rDNA conserved fragment revealed infection with another trypanosomatid (Schijman, A. G., unpublished data). This suggests that caution should be taken when diagnosing T. cruzi infection based only on MO of unstained fresh preparations of bug faeces. ‘False positives’ may lead to invalid incrimination of a vector species and invalid inferences on the relationships between sylvatic and domestic transmission cycles. Nevertheless, as some MO positive faecal samples from these triatomine species presented PCR inhibitors, we could not exclude T. cruzi infections in them.
PCR-based identification of T. cruzi lineages from faecal samples of T. infestans
The identification of lineages has required the isolation of parasites by culture expansion at the possible expense of selecting certain strains (De Luca d’Oro et al. 1993; Montamat et al. 1987, Montamat et al. 1992; Diosque et al. 2003). In the present study, the detection of a faecal sample infected both by TCI and TCII, which exhibited only TCII in the corresponding culture, provides evidence of the advantages of direct lineage identification of parasites from biological samples.
We detected only 1 domestic T. infestans with TCIId outside the core area, which represented the 3.6% of the tested infected specimens. Interestingly, this sublineage is the one most frequently detected in peripheral blood of chronic Chagas disease patients from endemic areas of Argentina (Diosque et al. 2003; Burgos et al. 2004).
Two molecular markers have been proposed to discriminate TCIIe from TCIIb strains: 18S rDNA-PCR was reported to detect TCIIb and not TCIIe, whereas A10-PCR was reported to detect TCIIe and not TCIIb (Brisse et al.2001).However, our PCR study showed that 85.7% of the tested specimens were infected with TCII populations that amplified both 18S rDNA and A10 sequences. These findings could be interpreted as TCIIe+TCIIb mixed populations or TCIIe strains that carry both A10 and 18S rDNA sequences. TCIIe reference strains Tulahuen II and CL-Brener also amplify both genomic markers (not shown), in agreement with recent data from culture isolates from domestic mammal reservoirs of the same endemic region (Cardinal, unpublished results) and strains of the Paraguayan Chaco region (Yeo et al. 2005). Thus, it appears that in our study area TCIIe strains carrying the 165 bp 18S rDNA sequence prevail, unlike TCIIe strains from other regions of endemicity (Brisse et al. 2001). Nevertheless, as none of these culture isolates have been cloned, mixed TCIIb+TCIIe populations cannot be excluded. Further improvement of PCR-based strategies and development of new markers are still required for precise discrimination between these sublineages. In addition, molecular typing with highly polymorphic markers, such as microsatellite or minicircle sequences (Vago et al.1996; Macedo et al.2001; Burgos et al. 2005) may allow direct characterization of the T. cruzi IIe intra-lineage diversity in biological samples collected in the region. As mentioned above, most domestic animal reservoirs from the rural area under study appear to be infected by TCIIe strains that also amplify the 18S rDNA fragment, whereas most blood samples collected from chronic Chagas disease patients of different endemic regions of Argentina appear infected with TCIId strains (Burgos, unpublished results). A possible explanation would be that T. infestans bugs collected in this survey were more likely infected with parasite strains from domestic animal reservoirs than from human hosts. The future characterization of parasite lineages infecting patients and animals from the houses where bugs were collected may elucidate this issue. This work applied improved laboratory procedures for screening and molecular identification of T. cruzi lineages directly from faeces of field-collected triatomine bugs. The application of the PCR-based procedures herein proposed may be extended to samples from patients and animal reservoirs, providing a valuable tool for eco-epidemiological studies of T. cruzi.
Acknowledgments
We are grateful to Delmi Canale, Raúl Stariolo, María Carla Cecere and Cristina Maidana for assistance in the field and parasite cultures. We thank the collaboration of Margarita Bisio in the PCR laboratory. We are grateful to Miguel Angel Basombrío and Patricio Diosque (Instituto de Patología Experimental, Universidad Nacional de Salta, Argentina), to Michel Tibayrenc (UR62 “Genetics of Infectious Diseases”, IRD Centre, Montpellier, France) and to Omar Triana Chavez and Ana Mejía (University of Antioquía, Medellin, Colombia) for providing DNA from T. cruzi reference strains of different lineages. This project received major support by NIH Research Grant # R01 TW05836 funded by the Fogarty International Center and the National Institute of Environmental Health Sciences (NIEHS) to U.K. and R.E.G. Additional funding from Agencia Nacional de Promoción Científica y Técnológica de Argentina and UBA (to R.E.G.), WHO-TDR ID 20285 and Private Fundation Bunge & Born (to A.G.S.) and CONICET. A.G.S., M.J.L. and R.E.G. are members of CONICET Researcher’s Career.
REFERENCES
- Anonymous Recommendations from a Satellite Meeting. Memorias do Instituto Oswaldo Cruz. 1999;94:429–432. doi: 10.1590/s0074-02761999000700085. [DOI] [PubMed] [Google Scholar]
- Barnabé C, Brisse S, Tibayrenc M. Population structure and genetic epidemiology of Trypanosoma cruzi, the agent of Chagas’ disease: a multilocus enzyme electrophoresis approach. Parasitology. 2000;120:513–526. doi: 10.1017/s0031182099005661. [DOI] [PubMed] [Google Scholar]
- Breniere SF, Bosseno MF, Telleria J, Carrasco R, Vargas F, Yaksic N, Noireau F. Field application of polymerase chain reaction diagnosis and strain typing of Trypanosoma cruzi in Bolivian triatomines. American Journal of Tropical Medicine and Hygiene. 1995;53:179–184. doi: 10.4269/ajtmh.1995.53.179. [DOI] [PubMed] [Google Scholar]
- Briones MR, Souto RP, Stolf BS, Zingales B. The evolution of two Trypanosoma cruzi subgroups inferred from rRNA genes can be correlated with the interchange of American mammalian faunas in the Cenozoic and has implications to pathogenicity and host specificity. Molecular and Biochemical Parasitology. 1999;104:219–232. doi: 10.1016/s0166-6851(99)00155-3. [DOI] [PubMed] [Google Scholar]
- Brisse S, Barnabé C, Tibayrenc M. Identification of six Trypanosoma cruzi phylogenetic lineages by random amplified polymorphic DNA and multilocus enzyme electrophoresis. International Journal for Parasitology. 2000;30:35–44. doi: 10.1016/s0020-7519(99)00168-x. [DOI] [PubMed] [Google Scholar]
- Brisse S, Verhoef J, Tibayrenc M. Characterisation of large and small subunit rRNA and mini-exon genes further supports the distinction of six Trypanosoma cruzi lineages. International Journal for Parasitology. 2001;31:1218–1226. doi: 10.1016/s0020-7519(01)00238-7. [DOI] [PubMed] [Google Scholar]
- Burgos JM, Bisio M, Seidenstein ME, Altcheh J, Talarico N, Pontoriero R, Marcellac M, Matzkin R, Freilij H, Macchi L, Levin MJ, Schijman AG. Congenital Chagas disease: detection and molecular typing of natural populations of T. cruzi involved in vertical transmission. BIOCELL. 2004;28:330. [Google Scholar]
- Burgos JM, Begher S, Freitas JM, Bisio M, Duffy T, Altcheh J, Teijeiro R, Lopez Alcoba H, Deccarlini F, Freilij H, Levin MJ, Levalle J, Macedo A, Schijman AG. Molecular diagnosis and typing of Trypanosoma cruzi populations and lineages in cerebral Chagas disease in a patient with AIDS. American Journal of Tropical Medicine and Hygiene. 2005 (in the Press). [PubMed] [Google Scholar]
- Canale DM, Cecere MC, Chuit R, Gürtler RE. Peridomestic distribution of Triatoma garciabesi and Triatoma guasayana in north-west Argentina. Medical and Veterinary Entomology. 2000;14:383–390. doi: 10.1046/j.1365-2915.2000.00254.x. [DOI] [PubMed] [Google Scholar]
- Castañera MB, Lauricella MA, Chuit R, Gürtler RE. Evaluation of dogs as sentinels of the transmission of Trypanosoma cruzi in a rural area of north-western Argentina. Annals of Tropical Medicine and Parasitology. 1998;92:671–683. doi: 10.1080/00034983.1998.11813327. [DOI] [PubMed] [Google Scholar]
- Cecere MC, Castañera MB, Canale DM, Chuit R, Gürtler RE. Trypanosoma cruzi infection in Triatoma infestans and other triatomines: long-term effects of a control program in rural northwestern Argentina. Pan American Journal of Public Health. 1999;5:392–399. doi: 10.1590/s1020-49891999000500003. [DOI] [PubMed] [Google Scholar]
- Cecere MC, Vazquez-Prokopec GM, Gürtler RE, Kitron U. Spatio-temporal analysis of reinfestation by Triatoma infestans (Hemiptera: Reduviidae) following insecticide spraying in a rural community in Northwestern Argentina. American Journal of Tropical Medicine and Hygiene. 2004;71:803–810. [PMC free article] [PubMed] [Google Scholar]
- Cerisola JA, Rohwedder R, Bozzini JP, Del Prado CE. Blastocrithidia triatomae n. sp. found in Triatoma infestans from Argentina. The Journal of Protozoology. 1971;18:503–506. doi: 10.1111/j.1550-7408.1971.tb03362.x. [DOI] [PubMed] [Google Scholar]
- Chiurillo MA, Crisante G, Rojas A, Peralta A, Dias M, Guevara P, Anez N, Ramirez JL. Detection of Trypanosoma cruzi and Trypanosoma rangeli infection by duplex PCR assay based on telomeric sequences. Clinical and Diagnostic Laboratory Inmunology. 2003;10:775–779. doi: 10.1128/CDLI.10.5.775-779.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CG, Pung OJ. Host specificity of ribosomal DNA variation in sylvatic Trypanosoma cruzi from North America. Molecular and Biochemical Parasitology. 1994;10:175–179. doi: 10.1016/0166-6851(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Cohen JE, Gürtler RE. Modeling household transmission of American Trypanosomiasis. Science. 2001;293:694–698. doi: 10.1126/science.1060638. [DOI] [PubMed] [Google Scholar]
- De Luca D’oro GM, Gardenal CN, Perret B, Crisci JV, Montamat EE. Genetic structure of Trypanosoma cruzi populations from Argentina estimated from enzyme polymorphism. Parasitology. 1993;107:405–410. doi: 10.1017/s0031182000067755. [DOI] [PubMed] [Google Scholar]
- Diotaiuti L, Pereira AS, Loiola CF, Fernandes AJ, Schofield JC, Dujardin JP, Dias JC, Chiari E. Inter-relation of sylvatic and domestic transmission of Trypanosoma cruzi in areas with and without domestic vectorial transmission in Minas Gerais, Brazil. Memorias do Instituto Oswaldo Cruz. 1995;90:443–448. doi: 10.1590/s0074-02761995000400002. [DOI] [PubMed] [Google Scholar]
- Diosque P, Barnabe C, Padilla AM, Marco JD, Cardozo RM, Cimino RO, Nasser JR, Tibayrenc M, Basombrio MA. Multilocus enzyme electrophoresis analysis of Trypanosoma cruzi isolates from a geographically restricted endemic area for Chagas’ disease in Argentina. International Journal for Parasitology. 2003;33:997–1003. doi: 10.1016/s0020-7519(03)00139-5. [DOI] [PubMed] [Google Scholar]
- Dorn PL, Flores J, Brahney B, Gutierrez A, Rosales R, Rodas A, Monroy C. Comparison of polymerase chain reaction on fresh tissue samples and faecal drops on filter paper for detection of Trypanosoma cruzi in Rhodnius prolixus. Memorias do Instituto Oswaldo Cruz. 2001;96:503–505. doi: 10.1590/s0074-02762001000400010. [DOI] [PubMed] [Google Scholar]
- Fernandes O, Santos S, Junqueira A, Cansen A, Cupolillo E, Campbell D, Zingales B, Coura JR. Populational heterogeneity of Brazilian Trypanosoma cruzi isolates revealed by the mini-exon and ribosomal spacers. Memorias do Instituto Oswaldo Cruz. 1999;94:195–197. doi: 10.1590/s0074-02761999000700028. [DOI] [PubMed] [Google Scholar]
- Gajate PP, Bottazzi MV, Pietrokovsky SM, Wisnivesky-Colli C. Potential colonization of the peridomicile by Triatoma guasayana (Hemiptera: Reduviidae) in Santiago del Estero, Argentina. Journal of Medical Entomology. 1996;33:635–639. doi: 10.1093/jmedent/33.4.635. [DOI] [PubMed] [Google Scholar]
- Gürtler RE, Cecere MC, Castañera MB, Chuit R. Detecting domestic vectors of Chagas disease in northwest Argentina: a comparison of five sampling methods. World Health Organization. 1995;73:487–494. [PMC free article] [PubMed] [Google Scholar]
- Gürtler RE, Cecere MC, Canale DM, Castañera MB, Chuit R, Cohen JE. Monitoring house reinfestation by vectors of chagas disease: a comparative trial of detection methods during a 4-year follow-up. Acta Tropica. 1999;72:213–234. doi: 10.1016/s0001-706x(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Junqueira AC, Chiari E, Wincker P. Comparison of the polymerase chain reaction with two classical parasitological methods for the diagnosis of Chagas disease in an endemic region of north-eastern Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1996;90:129–132. doi: 10.1016/s0035-9203(96)90111-x. [DOI] [PubMed] [Google Scholar]
- Kirchhoff LV, Votava JR, Ochs DE, Moser DR. Comparison of PCR and microscopic methods for detecting Trypanosoma cruzi. Journal of Clinical Microbiology. 1996;34:1171–1175. doi: 10.1128/jcm.34.5.1171-1175.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauricella MA, Stariolo RL, Riarte AR, Segura EL, Gürtler RE. Distribution and pathogenicity of Trypanosoma cruzi isolated from peridomestic populations of Triatoma infestans and Triatoma guasayana from rural western Argentina. Memorias do Instituto Oswaldo Cruz. 2005;100:123–129. doi: 10.1590/s0074-02762005000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo AM, Pena SDJ. Genetic variability of Trypanosoma cruzi: implications for the pathogenesis of Chagas disease. Parasitology Today. 1998;14:119–123. doi: 10.1016/s0169-4758(97)01179-4. [DOI] [PubMed] [Google Scholar]
- Macedo AM, Pimenta JR, Aguiar RS, Melo AIR, Chiari E, Zingales B, Pena SDJ, Oliveira RP. Usefulness of microsatellite typing in population genetic studies of T. cruzi. Memorias do Instituto Oswaldo Cruz. 2001;96:407–413. doi: 10.1590/s0074-02762001000300023. [DOI] [PubMed] [Google Scholar]
- Montamat EE, Arauzo S, Cazzulo JJ, Subias E. Characterization by electrophoretic zymograms of 19 Trypanosoma cruzi clones derived from two chronic chagasic patients. Comparative Biochemistry and Physiology. B, Comparative Biochemistry. 1987;87:417–422. doi: 10.1016/0305-0491(87)90161-1. [DOI] [PubMed] [Google Scholar]
- Montamat EE, De Luca D’oro GM, Perret B, Rivas C. Characterization of Trypanosoma cruzi from Argentina by electrophoretic zymograms. Acta Tropica. 1992;50:125–133. doi: 10.1016/0001-706x(91)90005-5. [DOI] [PubMed] [Google Scholar]
- Noireau F, Bosseno MF, Carrasco R, Telleria J, Vargas F, Camacho C, Yaksic N, Breniere SF. Sylvatic triatomines (Hemiptera: Reduviidae) in Bolivia: trends toward domesticity and possible infection with Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae) Journal of Medical Entomology. 1995;32:594–598. doi: 10.1093/jmedent/32.5.594. [DOI] [PubMed] [Google Scholar]
- Noireau F, Gutierrez T, Flores R, Breniere F, Bosseno MF, Wisnivesky-Colli C. Ecogenetics of Triatoma sordida and Triatoma guasayana (Hemiptera: Reduviidae) in the Bolivian chaco. Memorias do Instituto Oswaldo Cruz. 1999;94:451–457. doi: 10.1590/S0074-02761999000400004. [DOI] [PubMed] [Google Scholar]
- Russomando G, Rojas De Arias A, Almiron M, Figueredo A, Ferreira ME, Morita K. Trypanosoma cruzi: polymerase chain reaction-based detection in dried faeces of Triatoma infestans. Experimental Parasitology. 1996;83:62–66. doi: 10.1006/expr.1996.0049. DOI:10.1006/expr.1996.0049. [DOI] [PubMed] [Google Scholar]
- Schijman A, Vigliano C, Burgos JM, Favaloro R, Perrone S, Laguens R, Levin MJ. Early diagnosis of recurrence of Trypanosoma cruzi infection by polymerase chain reaction after heart transplantation of a chronic Chagas’ heart disease patient. The Journal of Heart and Lung Transplantation. 2000;19:1114–1117. doi: 10.1016/s1053-2498(00)00168-6. [DOI] [PubMed] [Google Scholar]
- Schijman AG, Altcheh J, Burgos JM, Biancardi M, Bisio M, Levin M, Freilij H. Aetiological treatment of Congenital Chagas disease diagnosed and monitored by the polymerase chain reaction. Journal of Antimicrobial Chemotherapy. 2003;52:441–449. doi: 10.1093/jac/dkg338. [DOI] [PubMed] [Google Scholar]
- Schijman AG, Vigliano CA, Viotti RJ, Burgos JM, Brandariz S, Lococo BE, Leze MI, Armenti HA, Levin MJ. Trypanosoma cruzi DNA in cardiac lesions of Argentinean patients with end-stage chronic Chagas heart disease. American Journal of Tropical Medicine and Hygiene. 2004;70:210–220. [PubMed] [Google Scholar]
- Schofield CJ, Dias JC. The Southern Cone Initiative against Chagas disease. Advances in Parasitology. 1999;42:1–27. doi: 10.1016/s0065-308x(08)60147-5. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Diotaiuti L, Dujardin JP. The process of domestication in Triatominae. Memórias do Instituto Oswaldo Cruz. 1999;94:375–378. doi: 10.1590/s0074-02761999000700073. [DOI] [PubMed] [Google Scholar]
- Souto RP, Fernandes O, Macedo AM, Campbell DA, Zingales B. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Molecular and Biochemical Parasitology. 1996;83:141–152. doi: 10.1016/s0166-6851(96)02755-7. [DOI] [PubMed] [Google Scholar]
- Vago AR, Macedo AM, Oliveira RP, Andrade LO, Chiari E, Galvao LM, Reis D, Pereira ME, Simpson AJ, Tostes S, Pena SD. Kinetoplast DNA signatures of Trypanosoma cruzi strains obtained directly from infected tissues. American Journal of Pathology. 1996;149:2153–2159. [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Prokopec GM, Ceballos LA, Kitron U, Gürtler RE. Active dispersal of natural populations of Triatoma infestans (Hemiptera: Reduviidae) in rural northwestern Argentina. Journal of Medical Entomology. 2004;41:614–621. doi: 10.1603/0022-2585-41.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank . World Development Report 1993 Investing in Health. Oxford University Press; New York: 1993. The global burden of the diseases; pp. 216–218. [Google Scholar]
- Wisnivesky-Colli C, Gürtler RE, Solarz ND, Schweigmann NJ, Pietrokovsky SM, Alberti A, Flo J. Dispersive flight and house invasion by Triatoma guasayana and Triatoma sordida in Argentina. Memorias do Instituto Oswaldo Cruz. 1993;88:27–32. doi: 10.1590/s0074-02761993000100006. [DOI] [PubMed] [Google Scholar]
- Yeo M, Acosta N, Llewellyn M, Sanchez H, Adamson S, Miles GA, Lopez E, Gonzalez N, Patterson JS, Gaunt MW, de Arias AR, Miles MA. Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. International Journal for Parasitology. 2005;35:225–233. doi: 10.1016/j.ijpara.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Zingales B, Souto RP, Mangia RH, Lisboa CV, Campbell DA, Coura JR, Jansen A, Fernandes O. Molecular epidemiology of American trypanosomiasis in Brazil based on dimorphisms of rRNA and mini-exon gene sequences. International Journal for Parasitology. 1998;28:105–112. doi: 10.1016/s0020-7519(97)00178-1. [DOI] [PubMed] [Google Scholar]


