Abstract
In the epileptic brain, hippocampal dentate granule cells become synaptically interconnected through the sprouting of mossy fibers. This new circuitry is expected to facilitate epileptiform discharge. Prolonged seizures induce the long-lasting neoexpression of neuropeptide Y (NPY) in mossy fibers. NPY is released spontaneously from recurrent mossy fiber terminals, reduces glutamate release from those terminals by activating presynaptic Y2 receptors, and depresses granule cell epileptiform activity dependent on the recurrent pathway. These effects are much greater in rats than in C57Bl/6 mice, despite apparently equivalent mossy fiber sprouting and neoexpression of NPY. This species difference can be explained by contrasting changes in the expression of mossy fiber Y2 receptors; seizures upregulate Y2 receptors in rats but downregulate them in mice. The recurrent mossy fiber pathway may synchronize granule cell discharge more effectively in humans and mice than in rats, due to its lower expression of either NPY (humans) or Y2 receptors (mice).
1. Introduction
Temporal lobe epilepsy is the most common form of epilepsy in the adult population. This condition afflicts at least 800,000 Americans. Unfortunately, pharmacotherapy usually fails to achieve long-term remission [31]. A rational approach to improved pharmacotherapy of temporal lobe epilepsy requires investigation of its unique pathology and pathophysiology. One unique feature of temporal lobe epilepsy is the anatomical reorganization of the dentate gyrus (Fig. 1) [1,14,20,42,51]. This phenomenon is replicated in several animal models of epilepsy, including pilocarpine-treated rats and mice [35]. Dentate granule cells become interconnected through the growth of recurrent mossy fibers. These mossy fiber collaterals mediate recurrent excitation [13,33,36,57], a type of innervation that is hardly present on dentate granule cells in normal brain. In addition, seizures increase the rate of granule cell replication, and some of these newly-born neurons migrate to ectopic locations, most notably the dentate hilus [39,44]. Finally, many granule cells in epileptic brain are found to have a basal dendrite [7,43,47], which provides a novel surface for innervation by recurrent mossy fibers [43]. Normal granule cells, granule cells with a basal dendrite, and hilar ectopic granule cells are synaptically interconnected by recurrent mossy fibers, forming a reverberating network unique to the epileptic brain. Formation of recurrent excitatory circuitry in the dentate gyrus is associated with a reduced threshold for granule cell synchronization in both human [16,27] and animal models [11,19,37,40,52]. It may thus contribute to progressively enhanced excitability [18,58], because in non-epileptic animals dentate granule cells have been shown to resist the propagation of seizures from the entorhinal cortex to the hipocampus [9,25,48]. Development of monosynaptic recurrent excitation is not the only mechanism that can synchronize granule cells. However, recurrent excitatory circuitry serves as the major substrate for synchronization of CA3 pyramidal cells [32], and it would be expected to play a similar role in the dentate gyrus. Thus we suggested that granule cell synaptic reorganization plays a significant role in epileptogenesis [34,35,52].
Fig. 1.
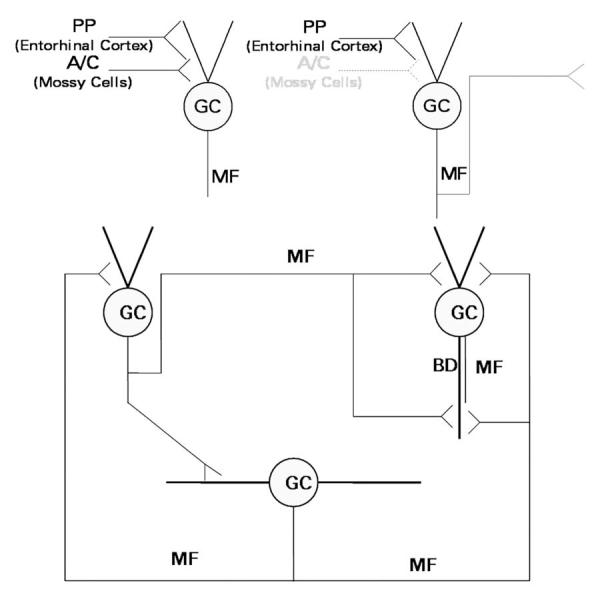
Schematic diagram of the excitatory innervation of dentate granule cells (GC). Upper left, In normal brain, granule cells receive excitatory innervation from the perforant path (PP), which originates in the entorhinal cortex, and the associational-commissural (A-C) fibers, which originate from mossy cells of the dentate hilus. Their axons, the mossy fibers (MF), innervate pyramidal cells of area CA3 and interneurons of area CA3 and the dentate gyrus, but only minimally innervate granule cells. Upper right, Seizures kill the hilar mossy cells, triggering the development of mossy fiber collaterals that grow into the synaptic territory abandoned by the degenerated associational-commissural fibers. Bottom, The granule cell network develops with time after brain-damaging seizures. Components of this network include normally-located granule cells of normal cellular morphology (upper left), normally-located granule cells with a basal dendrite (BD; upper right), and newly-generated hilar ectopic granule cells (bottom). These components are synaptically interconnected by mossy fibers. In animal models, these fibers express NPY de novo.
2. Functions of NPY in the Normal and Reorganized Dentate Gyrus
The anticonvulsant/antiepileptogenic properties of NPY have drawn considerable interest [10,55]. Exogenous NPY attenuates epileptiform activity in vitro [3,23] and blocks seizures when infused into the CSF [56]. NPY−/− mice sometimes develop spontaneous seizures and exhibit severe, and often fatal, seizures upon the administration of kainic acid [2]. Conversely, overexpression of NPY raises the seizure threshold [54]. NPY is particularly abundant in the dentate gyrus. In this region, it is normally expressed mainly by a subset of hilar GABA neurons (HIPP cells) that also express somatostatin [24]. Somatostatin/NPY-immunoreactive neurons provide feedback (mossy fiber-evoked) inhibition to the granule cell dendrites. These neurons are killed readily by seizures [5,8], with a corresponding reduction in feedback inhibition to granule cells [36]. HIPP cells innervate granule cell dendrites in the outer part of the molecular layer (perforant path terminal zone). Although NPY is generally viewed as an endogenous anticonvulsant, microelectrode recordings detected no effect of applied NPY on granule cell membrane properties (except for depressed function of N-type calcium channels [30]), and NPY does not inhibit excitatory transmission to granule cells [22]. In fact, the only significant action of NPY found to date in the normal dentate gyrus is disinhibitory. NPY hyperpolarizes about half the “non-mossy” cells (presumed GABA interneurons) of the dentate hilus, probably the HIPP cells, by Y1 receptor-mediated activation of a Gi-regulated inwardly-rectifying K+ (GIRK) current [38]. Thus NPY acts on somatic/dendritic autoreceptors to reduce HIPP cell activity. It is not known whether or under what circumstances endogenous NPY might reach those receptors. Released NPY might also feed back on the terminal from which it is released to reduce the probability of subsequent GABA release [49]. Thus endogenous NPY might facilitate seizures in the normal dentate gyrus. Indeed NPY can have proconvulsant actions; intracerebroventricular administration of NPY reportedly increases the duration of pentylenetetrazole seizures [41].
Seizures dramatically alter NPY localization and function in the dentate gyrus. In the pilocarpine and/or kainic acid model of temporal lobe epilepsy, HIPP cells degenerate and granule cells and their mossy fibers express NPY de novo [26,46]. NPY is transported through the mossy fibers to their terminals, from which it can be released [29]. This is the only known instance in which NPY is released from a glutamate pathway. Applied NPY inhibits synaptic transmission at mossy fiber synapses on CA3 pyramidal cells [22]. Thus release of NPY at recurrent mossy fiber synapses might feed back onto synaptic terminals and inhibit the release of glutamate. Corelease of NPY may explain, in part, why activation of the mossy fibers usually does not evoke reverberating excitation in the dentate gyrus of epileptic brain, despite the presence of robust recurrent mossy fiber growth [6,11,19,40,52]. Moreover, studies in other systems indicated that NPY is released preferentially by stimulus trains, compared with stimuli presented at low frequency [21,50]. Thus release of NPY could have explained the “reversal of facilitation” we noted when the recurrent mossy fiber pathway is stimulated at a frequency of 10 Hz with pulses of increasing magnitude [13].
Our studies investigated the actions of mossy fiber NPY on synaptic transmission in the recurrent circuit and on granule cell epileptiform activity. They utilized the pilocarpine model of temporal lobe epilepsy. Pilocarpine administered to rats or mice induces 6-8 h of status epilepticus, followed by a hippocampal lesion similar to those found in persons with temporal lobe epilepsy, formation of recurrent excitatory circuitry in the dentate gyrus, and spontaneous recurrent seizures.
3. Long-lasting expression of NPY in mossy fibers of rats and mice after pilocarpine-induced status epilepticus
NPY immunostaining was performed simultaneously on brain sections from rats and mice that had and had not experienced pilocarpine-induced status epilepticus (Fig. 2). In the dentate gyrus of control rats and mice, somata immunoreactive for NPY were mainly confined to the hilus. These neurons were presumably HIPP cells. Immunostained fibers were present in the outer part of the molecular layer, where axons of the HIPP cells terminate. NPY immunoreactivity was significantly more intense in the outer molecular layer of rats (P <0.001 by densitometry, Student's t-test; n = 6 rats and 4 mice), in accordance with the apparently smaller number of NPY-immunoreactive HIPP cells. Three changes were evident in sections from pilocarpine-treated epileptic animals. First, NPY immunoreactivity appeared de novo in the mossy fiber pathway, particularly in the recurrent mossy fibers. Second, there were markedly fewer NPY-immunoreactive somata in the dentate hilus. Third, immunostaining in the outer part of the molecular layer was less distinct, consistent with a loss of HIPP cell axons and terminals. No species difference in the response to status epilepticus was evident when hippocampal sections from rats and mice were processed simultaneously. These findings replicate previous reports [4,44].
Fig. 2.
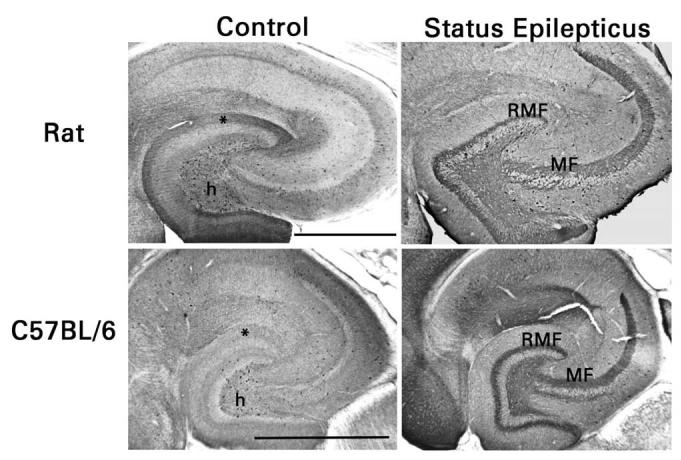
Similar expression of NPY in mossy fibers of rats and C57Bl/6 mice after pilocarpine-induced status epilepticus. Mossy fibers in area CA3 (MF) of control animals lack NPY immunoreactivity. NPY-immunoreactive HIPP cells appear less numerous in the dentate hilus (h) of mice than of rats and their terminal zone in the outer part of the dentate molecular layer (*) is less intensely immunostained. Mossy fibers in area CA3 and the dentate hilus and recurrent mossy fibers (RMF) in the dentate molecular layer develop NPY immunoreactivity after status epilepticus. There are many fewer NPY-immunoreactive HIPP cells, and their terminal zone in the dentate molecular layer is hardly discernable. Scale bars, 0.5 mm.
4. Spontaneous release of NPY tonically inhibits recurrent mossy fiber transmission in rats
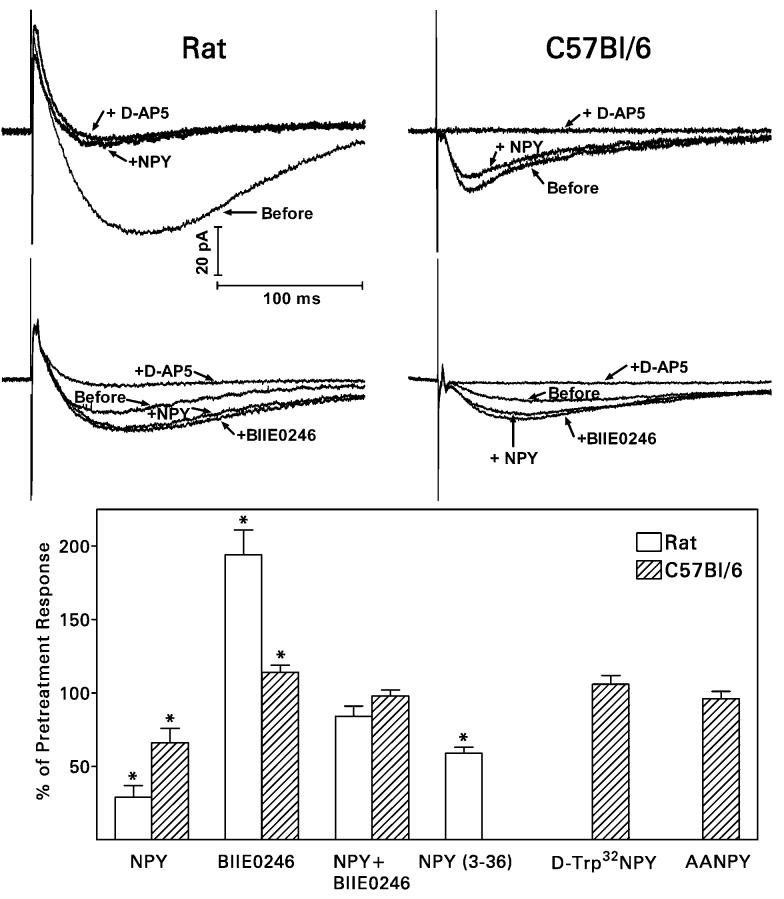
To study the actions of applied and endogenous NPY at recurrent mossy fiber synapses, whole cell patch clamp recordings were made in dentate granule cells of hippocampal slices prepared from pilocarpine-treated epileptic rats [53]. Some of these experiments, to be described later, were also performed with hippocaqmpal slices prepared from C57Bl/6 mice. First, we tested the effect of bath-applied NPY (1 μM) on synaptic responses evoked by stimulating each of the three excitatory projections to granule cells: mossy fibers, the perforant path, and the associational-commissural fibers. NPY reversibly inhibited synaptic transmission at recurrent mossy fiber synapses, but not at perforant path or associational-commissural synapses. It reduced the pharmacologically-isolated NMDA receptor-mediated component of the compound recurrent mossy fiber EPSC (recorded instead of the full EPSC to eliminate polysynaptic components) by 71% (Fig. 3) and increased the failure rate of minimal electrically-evoked EPSCs by 23%. Applied NPY also reduced the frequency (but did not affect the amplitude or kinetics) of miniature EPSCs (mEPSCs) in granule cells from pilocarpine-treated epileptic rats, but not from control rats. These actions of NPY were mediated by activation of presynaptc Y2 receptors. The Y2 receptor agonist NPY (3-36) (1 μM) replicated the actions of NPY and the selective Y2 receptor antagonist BIIE0246 (100 nM) blocked them. Y1 receptor ligands had no effect. Importantly, BIIE0246 produced effects opposite to those of NPY, when it was applied by itself. It increased the amplitude of compound NMDA receptor-mediated recurrent mossy fiber EPSC by 94%, reduced the failure rate of minimally-evoked EPSCs by 20%, and increased the frequency (but did not affect the amplitude or kinetics) of mEPSCs in granule cells from pilocarpine-treated epileptic rats. These effects were obtained in the absence of a prepulse. Several observations supported the selectivity of BIIE0246, including no effect on perforant path and associational-commissural synaptic transmission or on membrane properties of granule cells. An action of an antagonist when applied by itself can be interpreted to indicate that the endogenous agonist acts in the opposite way. Because BIIE0246 enhanced recurrent mossy fiber synaptic transmission without requiring any prior stimulation, we concluded from these results that NPY (or possibly an active metabolite, such as methionine sulfoxide NPY [29]) is released spontaneously from recurrent mossy fiber terminals and that the quantity released is sufficient to depress glutamate release from those terminals. This is in contrast to the need for high-frequency activity to release NPY from most terminals that normally express this peptide. Recurrent mossy fiber synapses are rather weak; 60-70% of minimal stimuli fail to evoke glutamate release in hippocampal slices maintained at room temperature [13,33]. Tonic release of NPY accounts for about one-third of these response failures.
Fig. 3.
NPY (1 μM) and BIIE0246 (100 nM) have qualitatively similar, but quantitatively very different, effects on recurrent mossy fiber synaptic transmission in rats and C57Bl/6 mice. Top, Whole cell patch clamp recordings from representative experiments on hippocampal slices are averages of 10 traces. NMDA receptor-mediated recurrent mossy fiber EPSCs were isolated pharmacologically with use of 10 μM NBQX (to block AMPA/kainate receptors) and 30 μM bicuculline (to block GABAA receptors) and recorded at a holding potential of −20 or −30 mV. “Before” refers to responses recorded before exposure of the tissue to NPY or BIIE0246. Upper traces, NPY was added to the superfusion medium and then 50 μM D-AP5 was added in the continued presence of NPY. In slices from both rats and mice, NPY inhibited synaptic transmission and the subsequent addition of D-AP5 abolished the response. The latter result confirms that the response was mediated entirely by the synaptic activation of NMDA receptors. Lower traces, BIIE0246 was added to the superfusion medium and then NPY and NPY + D-AP5 were added in the continued presence of BIIE0246. BIIE0246 enhanced synaptic transmission in both species when it was applied by itself, and the subsequent addition of NPY to the medium had no effect. Bottom, The effects of NPY and BIIE0246 on the peak amplitude of the NMDA receptor-mediated EPSC were much greater in rats than in mice. *P <0.01, paired t-test.
We observed no effect of NPY or BIIE0246 that appeared to be postsynaptically-mediated. However, our experimental conditions precluded the detection of a GIRK current, such as applied NPY has been shown to produce in HIPP cells [38]. If present in granule cells, an NPY-mediated GIRK current would add an inhibitory postsynaptic action to the tonic inhibition of glutamate release we demonstrated.
5. Spontaneous release of NPY depresses granule cell epileptiform activity
Effects of NPY receptor ligands on granule cell epileptiform activity were studied during superfusion of hippocampal slices at 35°C with 30 μM bicuculline and 6 mM [K+]o [53]. Under these conditions, mossy fiber stimulation evokes epileptiform activity in the granule cell body layer that depends entirely on the formation of recurrent mossy fiber connections; no epileptiform activity is observed in slices from control rats [19,37,52]. The effects of NPY receptor ligands on mossy fiber-evoked granule cell epileptiform activity were entirely predictable from their effects on recurrent mossy fiber synaptic transmission. NPY substantially reduced the magnitude of short-latency epileptiform activity and markedly attenuated or abolished the delayed bursts evoked by mossy fiber stimulation. Delayed bursts are presumably generated by polysynaptic activation of recurrent mossy fibers. BIIE0246 blocked these actions and enhanced epileptiform activity when applied by itself. Stimulation of the perforant path under the same conditions evoked multiple granule cell population spikes superimposed on either a positively-directed or negatively-directed wave. The negative wave indicated the presence of a current sink close to the granule cell body layer, consistent with disynaptic activation of recurrent mossy fibers. Delayed bursts were often evoked as well. In these experiments, NPY reduced the magnitude of the short-latency activity and abolished the delayed bursts. NPY had no effect when the waveform was positively-directed. Perforant path-evoked epileptiform activity of the latter type can be evoked in disinhibited slices from control rats, which lack a significant recurrent mossy fiber pathway. Thus NPY receptor ligands only affected granule cell epileptiform activity dependent on the recurrent mossy fiber pathway. We concluded that tonic release of NPY impedes the ability of recurrent mossy fibers to synchronize granule cell discharge and may thus protect the hippocampus from seizures that involve the entorhinal cortex.
6. Lesser effects of endogenous and applied NPY on recurrent mossy fiber transmission in mice
We aimed to gain a more detailed understanding of the role of NPY in the normal and reorganized dentate gyrus with use of mutant mice genetically engineered to lack expression of NPY or Y2 receptors. In preparation for these studies, we repeated the experiments on the NMDA receptor-mediated component of the compound recurrent mossy fiber EPSC with pilocarpine-treated C57BI/6 mice. Experiments on mice were interleaved with some of those on rats, so that the results could be compared across species. The qualitative effects of NPY and BIIE0246 on recurrent mossy fiber synaptic transmission were the same in mice as in rats: applied NPY was inhibitory, BIIE0246 blocked its action, and BIIE0246 enhanced transmission when applied by itself (Fig. 3). The Y5 receptor agonists D-trp32NPY and [ala31,aib32]-NPY did not replicate the action of NPY. These results argue that Y2 and not Y5 receptors mediate the NPY-induced reduction in glutamate release from mossy fiber terminals in C57Bl/6 mice. Our most important finding, however, was that both NPY and BIIE0246 were much less effective in mice than in rats. NPY reduced the size of the NMDA receptor-mediated component of the compound EPSC by an average of only 34% (compared with 71% in rats) and BIIE0246 increased the size of this response by an average of only 14% (compared with 94% in rats). This finding implies that both endogenous and applied NPY are less efficacious in mice, despite apparently equivalent expression of NPY in the recurrent mossy fibers of both species.
7. Species difference explained by plasticity of Y2 receptor expression
The lesser effect of both endogenous and applied NPY on the recurrent mossy fiber pathway of mice suggested a paucity of functional Y2 receptors. We tested this hypothesis by performing immunohistochemistry that utilized an antibody to the Y2 receptor (Neuromics, Edina, MN). This polyclonal antibody was raised in rabbits by immunization with a 10 amino acid polypeptide that corresponded to a portion of the Y2 receptor identical in rat and mouse. Thus equivalent immunoreactivity was expected in hippocampal sections from the two species. Indeed densitometry confirmed that the mean intensity of immunostaining differed by only about 10%.
Hippocampal sections from control mice and rats and from pilocarpine-treated epileptic mice and rats, five animals per group, were processed simultaneously. In control mice and rats, the terminal zones of Schaffer collateral-commissural-ipsilateral associational fibers in areas CA1 and CA3 and the mossy fiber terminal zones in area CA3 and the dentate hilus were immunoreactive (Fig. 4). In contrast, terminal zones of entorhinal cortical afferent fibers were not immunostained. These findings correlated well with the ability or inability of NPY to block synaptic transmission in those pathways. Notably, the immunostaining of the mossy fiber terminal zone in area CA3 (stratum lucidum) was considerably more intense in mice than in rats (P <0.02 by densitometry, Student's t-test). Immunoreactivity was also present in the inner third of the dentate molecular layer. There is no obvious explanation for this finding. The immunostaining was probably not associated with the associational-commissural pathway, the major excitatory pathway that projects to this part of the molecular layer, because NPY has no effect on transmission in the associational-commissural pathway and degeneration of this pathway after pilocarpine-induced status epilepticus did not reduce immunostaining. Further studies are needed to determine which cellular element in the inner molecular layer expresses Y2 receptors. The lack of neuropil immunostaining in the hippocampus of Y2−/− mice confirmed the specificity of the antibody.
Fig. 4.
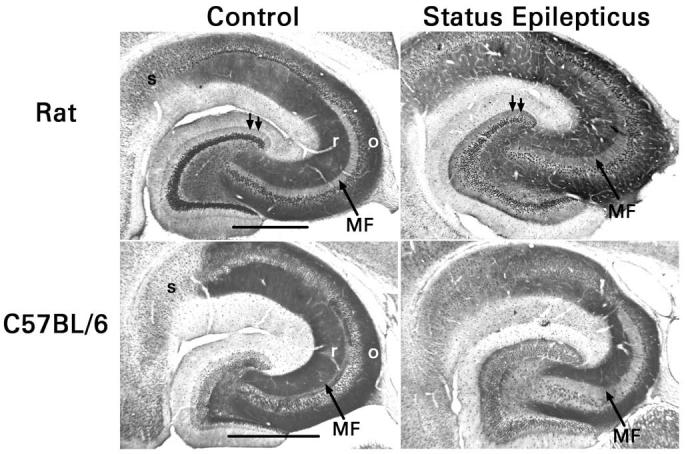
Opposite changes in mossy fiber Y2 receptor expression in rats and C57Bl/6 mice after pilocarpine-induced status epilepticus. In control animals, stratum radiatum (r) and stratum oriens (o) of areas CA1 and CA3 (loci of the Schaffer collateral-commissural projections) are strongly immunoreactive. The mossy fiber terminal zone of area CA3 (MF) is far more immunoreactive in mice than in rats, whereas the subiculum (s) is more immunoreactive in rats. Status epilepticus reduces Y2 receptor immunostaining in the mossy fiber terminal zone of mice, but increases it in rats. In rats, but not in mice, growth of recurrent mossy fibers into the inner third of the dentate molecular layer was associated consistently with increased Y2 receptor immunoreactivity (double arrows). Scale bars, 0.5 mm.
Examination of animals that had survived pilocarpine-induced status epilepticus indicated a profound down regulation of Y2 receptors in the mossy fibers of pilocarpine-treated mice (P <0.01 in stratum lucidum by densitometry, paired t-test). Importantly, the growth of recurrent mossy fibers into the inner molecular layer was not accompanied by any significant increase in Y2 receptor immunostaining (P = 0.8 by densitometry, paired t-test). This finding confirms that low expression of presynaptic Y2 receptors can explain the small effects of NPY and BIIE0246 on synaptic transmission in this pathway. In contrast, Y2 receptor immunoreactivity increased in stratum lucidum after pilocarpine-induced status epilepticus in rats (P <0.02 by densitometry, paired t-test). In addition, the growth of recurrent mossy fibers into the inner molecular layer was accompanied by a significant increase in the Y2 receptor immunoreactivity of that layer (P = 0.05 by densitometry, paired t-test). Our findings in rats agree with the seizure-induced up regulation of mossy fiber Y2 receptor binding reported by previous investigators, although quantitation in those studies was limited to the dentate hilus [51,59,60]. These contrasting receptor changes can explain the quantitatively different effects of NPY and BIIE0246 on recurrent mossy fiber transmission in mice and rats. Other possible forms of seizure-induced plasticity, such as changes in receptor function caused by altered post-translational modifications, are not excluded by our results, however, and merit investigation.
8. Application to human epilepsy
Immunohistochemical studies have not detected in human material the robust expression of mossy fiber NPY reported in animal models. There is an increased density of NPY-immunoreactive fibers in the terminal lamina of the recurrent mossy fiber projection [12,15,45]. It has not been determined whether this finding signifies expression of NPY by some recurrent mossy fibers or the growth of NPY-immunoreactive interneuron processes. On this basis, one would expect a modest effect of endogenous NPY on recurrent mossy fiber synaptic transmission, similar to that observed in mice. We predict that this pathway synchronizes granule cell discharge more effectively in humans and mice than in rat, due to its lower expression of either NPY or Y2 receptors. Accordingly, mice may offer a better model than rats for studying this aspect of temporal lobe epilepsy.
Acknowledgments
These studies were supported by NIH grants NS 17771 and NS 38108.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–363. doi: 10.1016/0306-4522(91)90380-7. [DOI] [PubMed] [Google Scholar]
- 2.Baraban SC, Hollopeter G, Erickson JC, Schwartzkroin PA, Palmiter RD. Knock-out mice reveal a critical antiepileptic role for neuropeptide Y. J Neurosci. 1997;17:8927–8936. doi: 10.1523/JNEUROSCI.17-23-08927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bijak M. Neuropeptide Y reduces epileptiform discharges and excitatory synaptic transmission in rat frontal cortex in vitro. Neuroscience. 2000;96:487–494. doi: 10.1016/s0306-4522(99)00594-1. [DOI] [PubMed] [Google Scholar]
- 4.Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, et al. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003;182:21–34. doi: 10.1016/s0014-4886(03)00086-4. [DOI] [PubMed] [Google Scholar]
- 5.Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- 6.Buckmaster PS, Dudek FE. Network properties of the dentate gyrus in epileptic rats with hilar neuron loss and granule cell axon reorganization. J Neurophysiol. 1997;77:2685–2696. doi: 10.1152/jn.1997.77.5.2685. [DOI] [PubMed] [Google Scholar]
- 7.Buckmaster PS, Dudek FE. In vivo intracellular analysis of granule cell axon reorganization in epileptic rats. J Neurophysiol. 1999;81:712–721. doi: 10.1152/jn.1999.81.2.712. [DOI] [PubMed] [Google Scholar]
- 8.Buckmaster PS, Jongen-Rêlo AL. Highly specific neuron loss preserves lateral inhibitory circuits in the dentate gyrus of kainate-induced epileptic rats. J Neurosci. 1999;19:9519–9529. doi: 10.1523/JNEUROSCI.19-21-09519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins RC, Tearse RG, Lothman EW. Functional anatomy of limbic seizures: focal discharges from medial entorhinal cortex in rat. Brain Res. 1983;280:25–40. doi: 10.1016/0006-8993(83)91170-8. [DOI] [PubMed] [Google Scholar]
- 10.Colmers WF, El Bahh B. Neuropeptide Y and epilepsy. Epilepsy Currents. 2003;3:53–58. doi: 10.1046/j.1535-7597.2003.03208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cronin J, Obenaus A, Houser CR, Dudek FE. Electrophysiology of dentate granule cells after kainate-induced synaptic reorganization of the mossy fibers. Brain Res. 1992;573:305–310. doi: 10.1016/0006-8993(92)90777-7. [DOI] [PubMed] [Google Scholar]
- 12.de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 13.Feng L, Molnár P, Nadler JV. Short-term frequency-dependent plasticity at recurrent mossy fiber synapses of the epileptic brain. J Neurosci. 2003;23:5381–5390. doi: 10.1523/JNEUROSCI.23-12-05381.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franck JE, Pokorny J, Kunkel DD, Schwartzkroin PA. Physiologic and morphologic characteristics of granule cell circuitry in human epileptic hippocampus. Epilepsia. 1995;36:543–558. doi: 10.1111/j.1528-1157.1995.tb02566.x. [DOI] [PubMed] [Google Scholar]
- 15.Furtinger S, Pirker S, Czech T, Baumgartner C, Ransmayr G, Sperk G. Plasticity of Y1 and Y2 receptors and neuropeptide Y fibers in patients with temporal lobe epilepsy. J Neurosci. 2001;21:5804–5812. doi: 10.1523/JNEUROSCI.21-15-05804.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabriel S, Njunting M, Pomper JK, Merschhemke M, Sanabria ERG, Eilers A, et al. Stimulus and potassium-induced epileptiform activity in the human dentate gyrus from patients with and without hippocampal sclerosis. J Neurosci. 2004;24:10416–10430. doi: 10.1523/JNEUROSCI.2074-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gobbi M, Gariboldi M, Piwko C, Hoyer D, Sperk G, Vezzani A. Distinct changes in peptide YY binding to, and mRNA levels of, Y1 and Y2 receptors in the rat hippocampus associated with kindling epileptogenesis. J Neurochem. 1998;70:1615–1622. doi: 10.1046/j.1471-4159.1998.70041615.x. [DOI] [PubMed] [Google Scholar]
- 18.Gorter JA, van Vliet EA, Aronica E, Lopes da Silva FH. Progression of spontaneous seizures after status epilepticus is associated with mossy fibre sprouting and extensive bilateral loss of hilar parvalbumin and somatostatin-immunoreactive neurons. Eur J Neurosci. 2001;13:657–669. doi: 10.1046/j.1460-9568.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- 19.Hardison JL, Okazaki MM, Nadler JV. Modest increase in extracellular potassium unmasks effect of recurrent mossy fiber growth. J Neurophysiol. 2000;84:2380–2389. doi: 10.1152/jn.2000.84.5.2380. [DOI] [PubMed] [Google Scholar]
- 20.Houser CR. Morphological changes in the dentate gyrus in human temporal lobe epilepsy. Epilepsy Res Suppl. 1992;7:223–234. [PubMed] [Google Scholar]
- 21.Kennedy B, Shen GH, Ziegler MG. Neuropeptide Y-mediated pressor responses following high-frequency stimulation of the rat sympathetic nervous system. J Pharmacol Exp Ther. 1997;281:291–296. [PubMed] [Google Scholar]
- 22.Klapstein GJ, Colmers WF. On the sites of presynaptic inhibition by neuropeptide Y in rat hippocampus in vitro. Hippocampus. 1993;3:103–112. doi: 10.1002/hipo.450030111. [DOI] [PubMed] [Google Scholar]
- 23.Klapstein GJ, Colmers WF. Neuropeptide Y suppresses epileptiform activity in rat hippocampus in vitro. J Neurophysiol. 1997;78:1651–1661. doi: 10.1152/jn.1997.78.3.1651. [DOI] [PubMed] [Google Scholar]
- 24.Köhler C, Eriksson LG, Davies S, Chan-Palay V. Co-localization of neuropeptide tyrosine and somatostatin immunoreactivity in neurons of individual subfields of the rat hippocampal region. Neurosci Lett. 1987;78:1–6. doi: 10.1016/0304-3940(87)90551-9. [DOI] [PubMed] [Google Scholar]
- 25.Lothman EW, Stringer JL, Bertram EH. The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res Suppl. 1992;7:301–313. [PubMed] [Google Scholar]
- 26.Lurton D, Cavalheiro EA. Neuropeptide-Y immunoreactivity in the pilocarpine model of temporal lobe epilepsy. Exp Brain Res. 1997;116:186–190. doi: 10.1007/pl00005739. [DOI] [PubMed] [Google Scholar]
- 27.Masukawa LM, Uruno K, Sperling M, O'Connor MJ, Burdette LJ. The functional relationship between antidromically evoked field responses of the dentate gyrus and mossy fiber reorganization in temporal lobe epileptic patients. Brain Res. 1992;579:119–127. doi: 10.1016/0006-8993(92)90750-4. [DOI] [PubMed] [Google Scholar]
- 28.Mathern GW, Babb TL, Pretorius JK, Leite JP. Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci. 1995;15:3990–4004. doi: 10.1523/JNEUROSCI.15-05-03990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarthy JB, Walker M, Pierce J, Camp P, White JD. Biosynthesis and metabolism of native and oxidized neuropeptide Y in the hippocampal mossy fiber system. J Neurochem. 1998;70:1950–1963. doi: 10.1046/j.1471-4159.1998.70051950.x. [DOI] [PubMed] [Google Scholar]
- 30.McQuiston AR, Petrozzino JJ, Connor JA, Colmers WF. Neuropeptide Y1 receptors inhibit N-type calcium currents and reduce transient calcium increases in rat dentate granule cells. J Neurosci. 1996;16:1422–1429. doi: 10.1523/JNEUROSCI.16-04-01422.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikati M, Holmes G. Temporal Lobe Epilepsy. In: Wyllie E, editor. The Treatment of Epilepsy: Principles and Practice. 2nd ed Williams & Wilkins; Baltimore: 1996. pp. 401–414. [Google Scholar]
- 32.Miles R, Wong RKS, Traub RD. Synchronized afterdischarges in the hippocampus: contribution of local circuit interaction. Neuroscience. 1984;12:1016–1022. doi: 10.1016/0306-4522(84)90012-5. [DOI] [PubMed] [Google Scholar]
- 33.Molnár P, Nadler JV. Mossy fiber-granule cell synapses in the normal and epileptic rat dentate gyrus studied with minimal laser photostimulation. J Neurophysiol. 1999;82:1883–1894. doi: 10.1152/jn.1999.82.4.1883. [DOI] [PubMed] [Google Scholar]
- 34.Nadler JV. Seizures and neuronal cell death in the hippocampus. In: Chan-Palay V, Köhler C, editors. The Hippocampus – New Vistas. Liss; New York: 1989. pp. 463–481. [Google Scholar]
- 35.Nadler JV. The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res. 2003;28:1649–1658. doi: 10.1023/a:1026004904199. [DOI] [PubMed] [Google Scholar]
- 36.Okazaki MM, Molnár P, Nadler JV. Recurrent mossy fiber pathway in rat dentate gyrus: synaptic currents evoked in presence and absence of seizure-induced growth. J Neurophysiol. 1999;81:1645–1660. doi: 10.1152/jn.1999.81.4.1645. [DOI] [PubMed] [Google Scholar]
- 37.Okazaki MM, Nadler JV. Glutamate receptor involvement in dentate granule cell epileptiform activity evoked by mossy fiber stimulation. Brain Res. 2001;915:58–69. doi: 10.1016/s0006-8993(01)02824-4. [DOI] [PubMed] [Google Scholar]
- 38.Paredes MF, Greenwood J, Baraban SC. Neuropeptide Y modulates a G protein-coupled inwardly rectifying potassium current in the mouse hippocampus. Neurosci Lett. 2003;340:9–12. doi: 10.1016/s0304-3940(03)00036-3. [DOI] [PubMed] [Google Scholar]
- 39.Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patrylo PR, Dudek FE. Physiological unmasking of new glutamatergic pathways in the dentate gyrus of hippocampal slices from kainate-induced epileptic rats. J Neurophysiol. 1998;79:418–429. doi: 10.1152/jn.1998.79.1.418. [DOI] [PubMed] [Google Scholar]
- 41.Reibel S, Nadi S, Benmaamar R, Larmet Y, Carnahan J, Marescaux C, et al. Neuropeptide Y and epilepsy: varying effects according to seizure type and receptor activation. Peptides. 2001;22:529–539. doi: 10.1016/s0196-9781(01)00347-3. [DOI] [PubMed] [Google Scholar]
- 42.Represa A, Robain O, Tremblay E, Ben-Ari Y. Hippocampal plasticity in childhood epilepsy. Neurosci Lett. 1989;99:351–355. doi: 10.1016/0304-3940(89)90472-2. [DOI] [PubMed] [Google Scholar]
- 43.Ribak CE, Tran PH, Spigelman I, Okazaki MM, Nadler JV. Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J Comp Neurol. 2000;428:240–253. doi: 10.1002/1096-9861(20001211)428:2<240::aid-cne4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 44.Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20:6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwarzer C, Kofler N, Sperk G. Up-regulation of neuropeptide Y-Y2 receptors in an animal model of temporal lobe epilepsy. Mol Pharmacol. 1998;53:6–13. doi: 10.1124/mol.53.1.6. [DOI] [PubMed] [Google Scholar]
- 46.Schwarzer C, Williamson JM, Lothman EW, Vezzani A, Sperk G. Somatostatin, neuropeptide Y, neurokinin B and cholecystokinin immunoreactivity in two chronic models of temporal lobe epilepsy. Neuroscience. 1995;69:831–845. doi: 10.1016/0306-4522(95)00268-n. [DOI] [PubMed] [Google Scholar]
- 47.Spigelman I, Yan X-X, Obenaus A, Lee EY-S, Wasterlain CG, Ribak CE. Dentate granule cells form novel basal dendrites in a rat model of temporal lobe epilepsy. Neuroscience. 1998;86:109–120. doi: 10.1016/s0306-4522(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 48.Stringer JL, Williamson JM, Lothman EW. Induction of paroxysmal discharges in the dentate gyrus: frequency dependence and relationship to afterdischarge production. J Neurophysiol. 1989;62:126–135. doi: 10.1152/jn.1989.62.1.126. [DOI] [PubMed] [Google Scholar]
- 49.Sun Q-Q, Akk G, Huguenard JR, Prince DA. Differential regulation of GABA release and neuronal excitability mediated by neuropeptide Y1 and Y2 receptors in rat thalamic neurons. J Physiol. 2001;531:81–94. doi: 10.1111/j.1469-7793.2001.0081j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Q-Q, Baraban SC, Prince DA, Huguenard JR. Target-specific neuropeptide Y-ergic synaptic inhibition and its network consequences within the mammalian thalamus. J Neurosci. 2003;23:9639–9649. doi: 10.1523/JNEUROSCI.23-29-09639.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- 52.Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in the hippocampal formation of kainic acid-treated rats. J Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tu B, Timofeeva O, Jiao Y, Nadler JV. Spontaneous release of neuropeptide Y tonically inhibits recurrent mossy fiber synaptic transmission in epileptic brain. J Neurosci. 2005;25:1718–1729. doi: 10.1523/JNEUROSCI.4835-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vezzani A, Michalkiewicz M, Michalkiewicz T, Moneta D, Ravizza T, Richichi C, et al. Seizure susceptibility and epileptogenesis are decreased in transgenic rats overexpressing neuropeptide Y. Neuroscience. 2002;110:237–243. doi: 10.1016/s0306-4522(01)00581-4. [DOI] [PubMed] [Google Scholar]
- 55.Vezzani A, Sperk G. Overexpression of NPY and Y2 receptors in epileptic brain tissue: an endogenous neuroprotective mechanism in temporal lobe epilepsy? Neuropeptides. 2004;38:245–252. doi: 10.1016/j.npep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Woldbye DP, Larsen PJ, Mikkelsen JD, Klemp K, Madsen TM, Bolwig TG. Powerful inhibition of kainic acid seizures by neuropeptide Y via Y5-like receptors. Nature Med. 1997;3:761–764. doi: 10.1038/nm0797-761. [DOI] [PubMed] [Google Scholar]
- 57.Wuarin J-P, Dudek FE. Electrographic seizures and new recurrent excitatory circuits in the dentate gyrus of hippocampal slices from kainate-treated epileptic rat. J Neurosci. 1996;16:4438–4448. doi: 10.1523/JNEUROSCI.16-14-04438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang X, Cui S-S, Wallace AE, Hannesson DK, Schmued LC, Saucier DM, et al. Relations between brain pathology and temporal lobe epilepsy. J Neurosci. 2002;22:6052–6061. doi: 10.1523/JNEUROSCI.22-14-06052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]