Abstract
The vitamin D endocrine system plays a primary role in the maintenance of calcium homeostasis as well as exerting a wider range of biological activities including the regulation of cellular differentiation and proliferation, immunity, and reproduction. Most of these latter activities have been demonstrated using in vitro techniques. A major issue is to place such in vitro findings into their physiological context. Vitamin D exerts its genomic effects through a nuclear gene transcription factor, the vitamin D receptor (VDR), while metabolism of vitamin D both to its biologically active form, as well as to its excretory product, plays a major role in determining biological activity at the tissue level. Considerable information has become available recently concerning the metabolism of vitamin D both in the kidney and in non-renal tissues. These data confirm the endocrine action of vitamin D through renal metabolism which provides 1,25 dihydroxyvitamin D (1,25D) to the circulation. The major organ responding to the endocrine action of 1,25D is the intestine where it controls absorption of calcium and phosphate. Preliminary information regarding the contribution of tissue-specific production of 1,25D to its paracrine/autocrine activity is now becoming available. In bone cells, these data provide evidence for the modulation of cell proliferation and stimulation of bone cell maturation. The relevance of these concepts to the clinical laboratory is discussed in the context of vitamin D insufficiency and the increased risk of hip fracture amongst the elderly.
Introduction
The vitamin D endocrine system plays a primary role in the maintenance of calcium homeostasis. The stringent regulation of extracellular fluid (ECF) calcium concentration within narrow limits is essential to support normal nerve and muscle function. ECF calcium is also required to provide calcium for the maintenance of skeletal health. In this context, it appears that the most critical endocrine role of vitamin D is to enhance the intestinal absorption of dietary calcium and phosphate. Significant evidence indicates that renal metabolism of vitamin D plays a key role in regulating the vitamin D endocrine system.
Interestingly, however, vitamin D has also been demonstrated to exert a wider range of biological activities including regulation of cellular differentiation and proliferation, immune function, and reproduction. Current opinion is that the local metabolism of vitamin D to its active form, 1,25 dihydroxyvitamin D (1,25D) provides a mechanism to act in a paracrine or autocrine fashion. Non-renal metabolism of vitamin D has been described over many years but it has been difficult to integrate these concepts into the currently accepted paradigm of the vitamin D endocrine system. For example 1,25D has numerous actions on bone cells, although their physiological significance remains unclear.
Recent technical developments including the cloning of genes responsible for the synthesis and catabolism of 1,25D have provided the opportunity to investigate the role of vitamin D metabolism in regulating vitamin D activity at the tissue level. These latest findings are reviewed here and discussed within their clinical context, with a focus on vitamin D metabolism by bone cells.
Vitamin D Metabolism
Vitamin D3 is synthesised from 7-dehydrocholesterol in the skin by exposure to ultraviolet light (200nm–300nm) from the sun. Alternatively, vitamin D, in the form of vitamin D2 (from plants) or vitamin D3 (from animals), can be derived from dietary sources.1 Biological activation of vitamin D involves firstly 25-hydroxylation, followed by 1α-hydroxylation to synthesise 1,25D. We currently regard the synthesis of 25 hydroxyvitamin D (25D) by the liver as constitutive, and the synthesis of 1,25D by the renal 25-hydroxyvitamin D-1α-hydroxylase enzyme, (CYP27B1) to be tightly regulated.2 A third vitamin D metabolising enzyme, the 25-hydroxyvitamin D-24α-hydroxylase (CYP24) converts 25D to 24,25 dihydroxyvitamin D (24,25D) or 1,25D to 1,24,25 trihydroxyvitamin D (1,24,25D), which is the first step of the C-24 oxidation pathway.3 It catabolises vitamin D metabolites to the water soluble calcitroic acid for rapid excretion by the kidney. This enzyme appears to be co-expressed in tissues with VDR which elicits the action of 1,25D. Thus the biological activity of vitamin D is determined by the combination of the level of VDR expression and the activities of the CYP27B1 and CYP24 metabolising enzymes.
1. 25-Hydroxylation of Vitamin D
25-hydroxylation is the first step in the activation of vitamin D (Figure 1) and the liver is considered the principal site of this hydroxylation step. Two 25-hydroxylase enzymes have been identified in the liver; a mitochondrial hydroxylase, CYP27A, and a microsomal enzyme, CYP2D25.4 Liver microsomal CYP2D25 has been reported in rodents and pigs and a cDNA clone for the pig liver enzyme has been isolated.5 By contrast, in humans, the expression of the mitochondrial but not the microsomal 25-hydroxylase has been identified, implicating CYP27A as the only vitamin D-25 hydroxylase.6 Thus our knowledge of the physiology of 25D production in humans remains limited. In addition to liver, a recent report demonstrates that the expression of CYP27A is widespread in other tissues including kidney, intestine, bone, skin, lung, spleen and central nervous system in the rat.6
Figure 1.
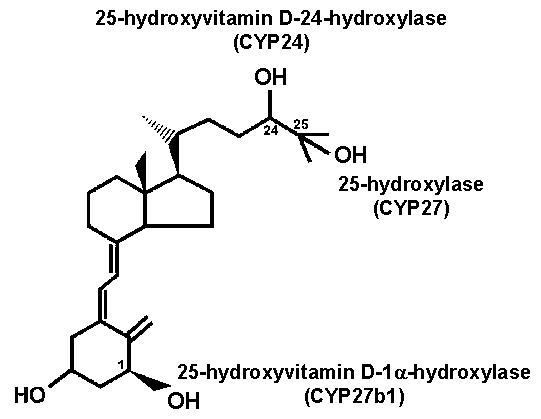
The structure of vitamin D and the addition of hydroxyl groups to either activate (carbon 1 and 25 positions) or deactivate (carbon 24 position) vitamin D. Specific enzymes are responsible for each of these hydroxylation steps.
25D is the major circulating metabolite of vitamin D due to its stability when bound to vitamin D-binding protein in blood and greater water solubility compared to vitamin D.7–9 When vitamin D metabolites are bound to vitamin D binding protein they are protected from photo-degradation and thus are stable in blood for significant periods even at room temperature.10 Serum 25D increases in proportion to vitamin D intake and sunlight exposure (Figure 2).11 Thus the measurement of serum 25D is the accepted indicator of vitamin D status. The metabolic fate of 25D is dependent on the calcium requirement although other factors may be discovered in the future. A need for calcium increases renal 1α-hydroxylation of 25D, whereas an abundance of calcium results in 24-hydroxylation of 25D as described in more detail later.
Figure 2.
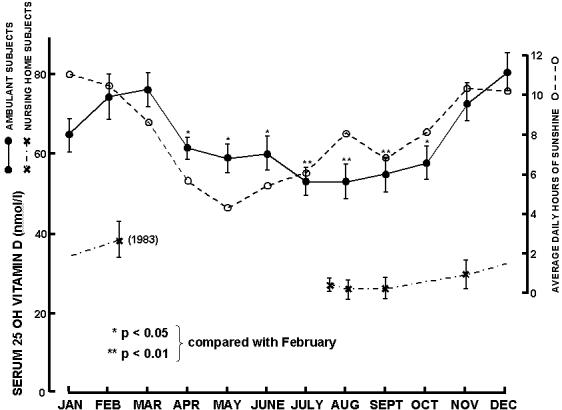
Seasonal variation (mean ± SEM) of serum 25D concentrations for ambulant subjects and daily hours of sunshine for Adelaide, South Australia during 1982. Values represented by the symbol (x) are for nursing home residents who showed no significant seasonal variation. (Reproduced with permission from 11.)
2. 25 Hydroxyvitamin D-1α-Hydroxylation
The second step of vitamin D activation is the conversion of 25D to 1,25D (Figure 1). The enzyme CYP27B1 catalyses the introduction of a hydroxyl group into the carbon 1α position of the A ring of 25D to form 1,25D. CYP27B1 was so named due to its structural similarity to the liver CYP27A1 enzyme. The kidney was shown to be the major source for the synthesis of 1,25D in 1970 when Fraser and Kodicek used nephrectomised animals to demonstrate that CYP27B1 enzyme activity was predominantly in the kidney.12 Expression of CYP27B1 mRNA and protein are located in the renal proximal tubules and, to a lesser extent, in distal tubules and collecting ducts.13
CYP27B1 enzyme is present in a number of non-renal tissues and cells as well. The original description of extra-renal CYP27B1 enzyme activity was based on studies of the granulomatous disease sarcoidosis, which frequently presents with hypercalcaemia.14,15 1,25D was identified in macrophages within granulomatous deposits implicating the local expression and activity of CYP27B1. CYP27B1 has since been shown to be expressed in a number of other tissues including skin, intestine, brain, testis, macrophages, placenta and bone.16,17 Experiments investigating the regulation of CYP27B1 enzyme activity and transcriptional activity in vivo have proved difficult due to its low level of expression.
While the regulation of CYP27B1 activity in non-renal tissues is largely unknown, CYP27B1 mRNA expression and protein synthesis in the kidney is tightly regulated by a number of factors. Studies of the mechanisms involved in CYP27B1 gene regulation have been made possible with the cloning of mouse, rat and human cDNA for CYP27B1.18–22 Age is a major determinant of CYP27B1 activity. Conversion of 25D to 1,25D in the kidney is increased in 2-month-old rats compared to older animals and this activity decreases with age until 2 years of age.23 Parathyroid hormone (PTH) is considered to be an important stimulator of CYP27B1 transcription and activity in the kidney, with this response being mediated through a cAMP signal transduction mechanism.24 Reports have highlighted potential cAMP response elements (CREs) in the CYP27B1 promoter,25 although none has been shown to be functional. Most recent data suggest that high concentrations of PTH are required for up-regulation of CYP27B1 gene expression, with PTH targeting a CCAAT box protein bound to the promoter,26 and it has been proposed that PTH only acts in the setting of hypocalcaemia.27
While PTH acts to up-regulate CYP27B1 expression, 1,25D acts as a potent suppressor of this gene, down-regulating CYP27B1 gene activation whether it is PTH-stimulated or not. The inhibitory action of 1,25D is intriguing since no vitamin D responsive element (VDRE) is present in the CYP27B1 promoter.28,29 However, Murayama and co-workers have identified a region on the promoter of the CYP27B1 gene, which is negatively regulated by 1,25D and requires VDR for the inhibition to occur.29 VDR does not, however, directly bind to this region in the promoter and the mechanism of inhibition has not been fully elucidated yet.30
3. 25-hydroxyvitamin D-24α-hydroxylation
The high potency of 1,25D requires its activity to be tightly regulated and, as mentioned earlier, activity of 1,25D is attenuated by the addition of a hydroxyl group in the C-24 position, catalysed by CYP24 (Figure 1). CYP24 is a multicatalytic enzyme which, in addition to 24-hydroxylation of either 25D or 1,25D, is able to catalyse the side chain hydroxylations at the C23 and C26 positions.31 These hydroxylation steps of the C-24 oxidation pathway convert 25D and 1,25D to the water-soluble calcitroic acid and other carboxylated products which are excreted via the kidney.30–33 The catabolism of 1,25D limits the action of 1,25D in target cells once the initial wave of 1,25D-mediated gene expression has been initiated.
CYP24 enzyme activity was first reported in chicken kidney tissue.34 However, expression of CYP24 appears ubiquitous and has been found in a number of other tissues including intestine and bone.35 It is considered to be expressed in all vitamin D-responsive tissues.36–38 Under normal physiological circumstances, renal CYP24 activity was shown to be lowest in young female rats and to gradually increase with age. This is converse to the change with age in expression and activity of CYP27B1 discussed earlier.23
A cDNA clone for rat CYP24 was isolated in the early 1990s and the gene has been described for mouse, rat and humans.39–41 In each case, the CYP24 gene possesses two VDREs in its promoter region, which is unique for vitamin D responsive genes.42–45 These two VDREs allow for the direct 1,25D/VDR-mediated up-regulation of CYP24 and act synergistically to induce gene expression.46,47
Molecular Mechanisms of Vitamin D Activity
The biological activity of 1,25D is mediated by a high-affinity receptor, VDR, which acts as a ligand-activated transcription factor. VDR was found originally in the organs involved in calcium homeostasis, including the intestine, bone, kidney, and the parathyroid glands. The isolation of cDNA clones for the avian, human, mouse, and rat VDR has led to VDR being detected in many other non-classical tissues and cell types.48–51 The action of 1,25D in these tissues has been associated with a diverse range of biological systems such as modulation of immune function, inhibition of cell growth, and induction of cell differentiation.30
There is evidence that for many, if not all, vitamin D responsive genes, unliganded VDR/retinoid X receptor (RXR) heterodimer binds to VDRE of the promoter of the target gene, recruiting a co-repressor complex resulting in repression of basal gene transcription.30 Upon VDR binding of 1,25D, the co-repressor is displaced allowing recruitment of a co-activator complex of proteins that interacts with the RNA polymerase machinery and stimulates genetranscription (reviewed by Omdahl et al 2002).30 In short, the interaction of the 1,25D-bound VDR-RXR complex with nuclear proteins forms a "pre-initiation complex", regulating the rate of transcription of the target gene.
This so-called genomic action of 1,25D can be preceded by more rapid non-genomic actions, occurring in minutes. These involve cytoplasmic membrane-associated events such as activation by 1,25D of the mitogen-activated protein kinases (MAP kinases), protein kinase C and other cell signalling pathways.52,53 The non-genomic effects of 1,25D could be elicited by the classical nuclear VDR acting at the cytoplasmic membrane or by a separate membrane VDR. Recent evidence, in which the non-genomic responses to 1,25D were abrogated in VDR-null mice totally lacking 1,25D activity, strongly supports the contention that the classical VDR mediates this non-genomic action.54 These non-genomic activities play a critical role in nuclear transcriptional activity. For example in COS-1 cells, physiological concentrations of 1,25D rapidly activate protein kinase C and MAP kinase activities and inhibition of these pathways almost completely inhibits 1,25D-directed transcriptional activity on the CYP24 gene promoter.55,56
The Vitamin D Endocrine System
Maintenance of calcium homeostasis involves a coordinated mechanism largely from intestine, kidney, bone and parathyroid glands (Figure 3). The complex interactions of these tissues, in which circulating 1,25D plays an integral role, ensure adequate availability of calcium and phosphate for a number of biological functions, including nerve and muscle functions as well the maintenance of mineralised tissues.
Figure 3.
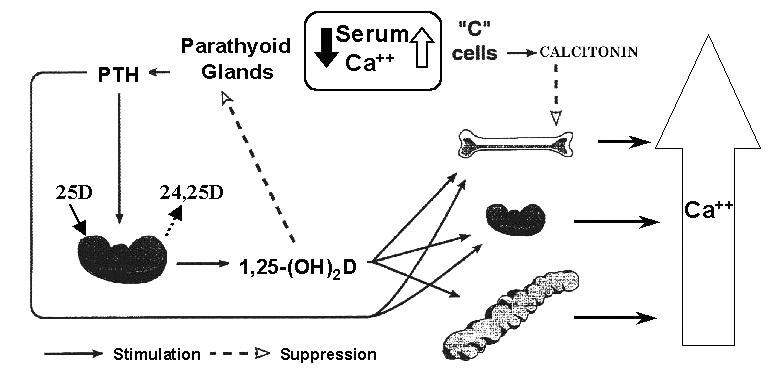
The calcium homeostatic system emphasising the effect of hypocalcaemia on parathyroid hormone (PTH) and 1,25 dihydroxyvitamin D (1,25D) synthesis and secretion and the concomitant actions of these calciotropic hormones to restore serum calcium. The effect of elevated serum calcium is also represented on calcitonin synthesis and secretion and its action to inhibit bone resorption.
1. Intestine
Arguably the most important role for 1,25D is to stimulate dietary calcium and phosphate absorption in the small intestine. This has been convincingly demonstrated by examination of VDR-null and CYP27B1-null mice. In the mutant mice all vitamin D biological activity was abolished and the mice developed marked hypocalcaemia as a result of calcium malabsorption.54,57,58 1,25D increases intestinal calcium absorption through a VDR-mediated increase in the transcription of specific genes involved in active calcium absorption. Serum 1,25D correlates positively with the intestinal active transport of radiocalcium (Figure 4).59 A number of proteins are essential for these processes including those involved in the entry of calcium through the basement membrane into the enterocyte, the movement of calcium through the cytoplasm, and the transfer of calcium across the basolateral membrane into the circulation.60 The cytosolic calcium-binding protein, calbindin-D9k, is thought to be particularly important in the translocation of calcium across the enterocyte. 1,25D is able to up-regulate the expression of calbindin-D9k through a VDRE in the proximal promoter of the gene,61 and mRNA and protein for calbindin-D9k are dramatically reduced in the intestine of VDR knockout mice.62
Figure 4.
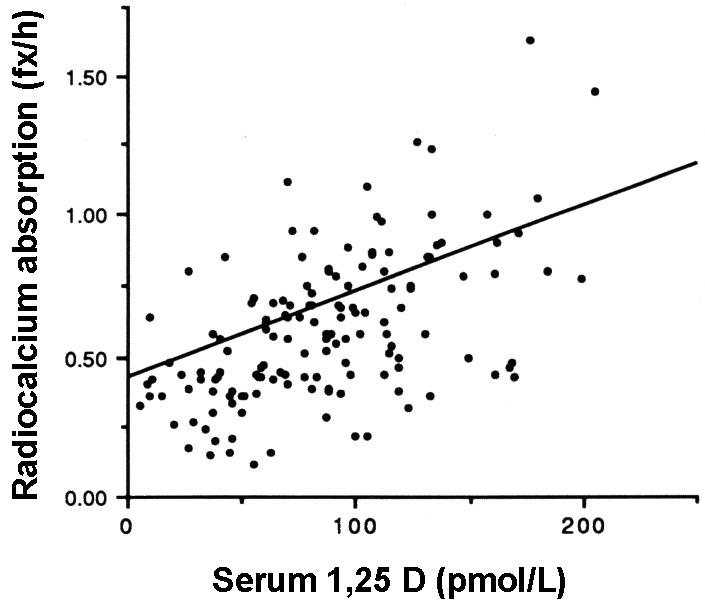
The relation between serum 1,25D and radiocalcium absorption in osteoporotic patients with the mean regression line for normal postmenopausal subjects. Note that these relationships are statistically significant (p<0.001) and that the majority of data points for the osteoporotic patients fall below the normal line. (Reproduced by permission from 59.)
2. Bone
Vitamin D deficiency in normal animals and humans as well as inactivation of 1,25D by genetic manipulation in mice, as discussed above, produce defects in bone mineralisation such as rickets and osteomalacia, characterised by an increase in osteoid (unmineralised bone matrix protein) and impaired calcium phosphate deposition.63 Interestingly, normal bone mineralisation occurred in VDR-null and CYP27B1-null mice when they were fed a high calcium and phosphate diet.54,57,58 Such data suggest that 1,25D may not be essential for bone mineralisation when high dietary calcium is available and that the basis of the mineralisation defect seen in vitamin D deficiency may be the decreased availability of calcium and phosphate through impaired intestinal absorption.
Nonetheless, in vitro studies have shown that 1,25D is capable of regulating osteoblast gene transcription, differentiation and mineralisation.64,65 Effects on matrix proteins such as collagen type I, osteopontin and osteocalcin are believed to be mediated via transcription as all possess VDRE within their promoter regions. 1,25D, in association with other factors including PTH, also induces osteoclastogenesis by stimulating the differentiation of bone marrow-derived pro-myelocytes and monocytes to active osteoclasts.66,67 This process occurs indirectly through increased expression of the osteoblast-derived factor, RANKL, which promotes osteoclastic differentiation.68 Osteoblasts from VDR knockout mice are unable to stimulate the differentiation of osteoclasts, clearly demonstrating the requirement for VDR in the osteoblast for this 1,25D-mediated effect.69
A current view is that 1,25D stimulation of bone resorption, by increasing osteoclastogenesis, is another mechanism by which 1,25D maintains normocalcaemia. If this is so, then it is interesting to note that in vitamin D deficiency or VDR-null mice, hypocalcaemia develops despite a high PTH,54,70 suggesting that PTH requires 1,25D in order to stimulate significant bone resorption and normalise extracellular fluid (ECF) calcium. However, evidence that 1,25D can also mediate the mineralisation process, suggests that 1,25D may be important for the initiation of bone re-modelling processes for the repair of microfractures.71 That is, the 1,25D-induced osteoclastic activity may provide calcium for the coupled bone mineralisation process, rather than only to maintain serum calcium.72
3. Parathyroid Glands
PTH plays a central role in calcium homeostasis. When ECF calcium decreases, the transient fall is detected by the calcium sensing receptor (CaR) in the parathyroid gland, leading to induction of PTH synthesis and secretion. PTH can restore ECF calcium to normal by stimulating renal tubular reabsorption of calcium and renal production of 1,25D, and by promoting osteoclastogenesis. 1,25D can exert a negative feedback signal on the parathyroid glands to suppress further synthesis and secretion of PTH and to control parathyroid cell growth.73–75 The PTH gene promoter contains a VDRE which acts in a negative fashion.76 The lack of 1,25D feedback on the PTH promoter is pronounced in vitamin D deficiency which, combined with hypocalcaemia, results in parathyroid hyperplasia and secondary hyperparathyroidism. The correction of parathyroid gland growth and serum PTH in the VDR-null mice through dietary calcium supplementation indicates, however, that the feedback of 1,25D on PTH production is not essential and that the ECF calcium concentration is the major regulator of PTH synthesis and parathyroid cell proliferation.62
4. Kidney
Perhaps the most important effect of 1,25D in the kidney is the negative feedback on circulating 1,25D concentration through the suppression of renal CYP27B1 activity and stimulation of renal CYP24 activity.29,41 1,25D is also involved in renal calcium reabsorption as demonstrated by studies in the VDR null mouse.54 1,25D increases the expression of the renal calcium-transport protein, calbindin-D28k77,78 and serum 1,25D correlates strongly with the expression of calbindin-28k in the kidney measured in rats over a wide range of age groups.79 Furthermore, 1,25D promotes the PTH-dependent calcium transport in the distal tubule where active calcium reabsorption occurs.88,81
Regulation of Renal Metabolism of Vitamin D
Considerable evidence indicates that renal metabolism of vitamin D determines serum 1,25D.30 In normal physiology, the factors that regulate vitamin D metabolism such as PTH, calcium, 1,25D itself and calcitonin (as described later) are inextricably linked, to ensure that extracellular fluid calcium concentration is tightly controlled within a narrow range (Figure 3). ECF ionised calcium is maintained within a narrow range for the individual, considerably less than the population variance and lower than the imprecision of current measurement technologies.82 Thus the response to even a slight fall in ionised calcium such as occurs with an overnight fast, results in a cascade of biological processes to return the calcium to normal for that individual. The effects of a number of factors on vitamin D metabolism may occur indirectly or in concert with other factors and are summarised in the accompanying table.
Table.
Regulation of Renal Metabolism of Vitamin D
| Factor | CYP27B1 | CYP24 |
|---|---|---|
| PTH | Increased (with hypocalcaemia) | Decreased |
| 1,25D | Decreased | Increased |
| Calcium | Decreased | Unknown |
| Calcitonin | ?Increased | ?Increased |
| Age | Decreased | Increased |
1. Parathyroid Hormone
As indicated earlier, the release of PTH during hypocalcaemia is a potent stimulator of renal 1,25D production, with PTH up-regulating CYP27B1 mRNA expression and enzyme activity predominantly in proximal tubular cells of the kidney.21 In parathyroidectomised animals, no stimulation of renal CYP27B1 activity was detected in response to hypocalcaemia,83 and PTH administration restored induction of CYP27B1 enzyme activity.84 While there is evidence that the transfected CYP27B1 promoter can be induced in kidney cells, in these studies high concentrations of PTH were required for stimulation.26,85 Moreover, it has been suggested that PTH can only exert this effect in the presence of hypocalcaemia.27 The molecular mechanism behind the PTH-mediated regulation of CYP27B1 activity remains to be fully elucidated.
Besides stimulating the expression of the renal CYP27B1 enzyme, PTH markedly suppresses the 1,25D-induced up-regulation of the renal CYP24 enzyme activity.86 The mechanism involved here is at the level of post-transcription, with PTH, in some way, decreasing the half-life of the CYP24 mRNA.87 The suppressive effect of PTH on CYP24 in the kidney does not necessarily occur in other tissues.86 Thus it is clear that PTH-mediated stimulation of CYP27B1 activity and inhibition of renal CYP24 activity results in a marked elevation of circulating plasma 1,25D.88
2. Calcium
Calcium primarily affects the renal production of 1,25D by interacting with CaR located in the parathyroid gland, inhibiting the secretion of PTH which can modulate renal vitamin D metabolism as discussed above. Calcium may also be able to directly regulate the metabolism of vitamin D in the kidney. Studies using parathyroidectomised, PTH-replete rats have shown that an elevated serum calcium is able to reduce circulating 1,25D.89 Bland et al. found a 5-fold increase in the 1,25D production in the human proximal tubule cell line, HKC-8, when treated with a medium containing a low concentration of calcium.90 When these cells were treated with a high calcium medium the 1,25D production was significantly reduced. The direct effect of calcium was suggested to be mediated by the CaR, which is present in all segments of the nephron but the exact mechanism of such inhibition is unknown.
3. Calcitonin
High serum calcium such as occurs directly following a calcium-rich meal, inhibits secretion of PTH by the parathyroid gland and also stimulates the secretion of calcitonin by the parafollicular cells of the thyroid gland in a process that involves the CaR. Calcitonin acts directly on osteoclasts, reducing bone resorption and calcium mobilisation from the skeleton, thus lowering serum calcium.91
Although the role of calcitonin in vitamin D metabolism is suggested by some to be secondary to the role of PTH,2 a direct role for calcitonin in the metabolism of vitamin D has been demonstrated.92 Of interest are recent studies in our laboratory which have demonstrated that calcitonin can markedly up-regulate CYP24 promoter activity in the human kidney cell line AOK-B50.93 These data indicate that calcitonin could, therefore, lower circulating 1,25D. On the other hand, it has been reported that calcitonin can stimulate the expression of CYP27B1;27 the possible physiological significance of this finding is not clear. However, we do find a massive synergy between 1,25D and calcitonin in the induction of the CYP24 promoter, raising the possibility that this action would ensure a low 1,25D in the face of high serum calcium. Overall, the precise role of calcitonin in mediating vitamin D metabolism remains to be clarified.
4. 1,25 Dihydroxyvitamin D
The most striking effect of 1,25D on the metabolism of vitamin D is the up-regulation of CYP24 activity. This up-regulation was first shown in the kidney and intestine, and since then in other tissues.3 The induction of CYP24, and catabolism of 1,25D by 1,25D itself, is an important feedback loop which modulates 1,25D signalling in target tissues. When 1,25D was administered to vitamin D-deficient rats, CYP24 activity in the kidney was markedly induced with a reciprocal disappearance of renal CYP27B1 activity.94
The Vitamin D Paracrine/Autocrine System: Evidence for Bone Metabolism of Vitamin D
More recently, 1,25D has been shown to be associated with biological functions in other tissues, including keratinocytes, T cells and macrophages of the immune system, islet cells of the pancreas, ovarian cells of the female and certain neuronal tissue. In most cases, the precise roles for 1,25D in these tissues are still to be determined. However, it is clear the vitamin D physiology has a variety of biological effects beyond calcium homeostasis.
1. Bone 25-hydroxyvitamin D-1α-hydroxylation
It has been some two decades since Turner et al. demonstrated that bone can synthesise 1,25D, although little is still known about the regulation of CYP27B1 activity in these cells or about the importance of this locally produced 1,25D.95 They found that primary cultures of human bone cells, taken from iliac crest biopsies and incubated with 25D for 4 hours, synthesised both 1,25D and 24,25D, with specific activities similar in magnitude to those of the enzymes found in kidney cells. The suppression of CYP27B1 activity and stimulation of CYP24 activity by 1,25D itself in these bone cells supported their proposition that the bone cell production of 1,25D could be regulated in order to mediate bone mineral metabolism. Similar studies since that time have confirmed the original observations.30
Bone marrow macrophages also convert 25D to both 1,25D and 24,25D. Reichel et al. demonstrated that on exposure to recombinant human interferon-gamma (IFN-γ), bone marrow-derived macrophages initially synthesised 1,25D from 25D.96 A delay in the appearance of 24,25D suggested that the production of 24,25D was stimulated by the initial production of 1,25D rather than by IFN-γ. The 1,25D synthesised by bone marrow macrophages promotes the differentiation of pro-myelomonocytic HL-60 cells into macrophage-like cells. Since a number of myelomonocytic cell lines express VDR, it is suggested that locally produced 1,25D may be involved in autocrine signalling associated with regulation of haematopoietic cell differentiation.97
More recently, CYP27B1 mRNA has been identified in bone tissue, using highly sensitive molecular techniques. Panda and co-workers have shown by qualitative RT-PCR that the expression of CYP27B1 mRNA in rats was significantly higher in foetal bone than in adult bone.98 They identified CYP27B1 mRNA expression in growth plate chondrocytes and osteoblasts by in situ hybridisation but were unable, however, to detect CYP27B1 mRNA in osteoclasts or in bone marrow using this method. Mice with a specific deletion of the CYP27B1 gene in chondrocytes have been bred.99 Their serum biochemistry revealed normal calcium homeostasis, and preliminary analysis of the growth plate did not indicate any gross abnormalities. Once again the role of tissue-specific synthesis of 1,25D remains unclear.
Although it is clear that specific bone cells contain the CYP27B1 enzyme and are able to convert 25D to 1,25D, the functional relevance of this locally produced 1,25D is still unknown and such activities have to be placed in their physiological context. Whether some or all of these activities are specific for 1,25D derived from the circulation or for 1,25D derived from local bone cell-specific synthesis is of considerable interest. Such bone cell specific synthesis may best explain the apparent contradiction between low circulating 25D, normal circulating 1,25D and low bone 1,25D associated with increased risk of hip fracture.
Sagiv et al. found that, although serum 1,25D was similar in mature women, 1,25D in bone was higher in women of 45 years of age and less, when compared to that found in older age groups (46–60 years and 61+ years of age).100 They also discovered that women of approximately 80 years of age who had had a subcapital fracture of the femur, had 5-times lower 1,25D in the bone when compared to aged matched women who had not had a fracture.101 This finding was despite the fact that serum 1,25D was only slightly lower in the women with fractures than in the non-fracture women. The association between low 1,25D in bone, aging and subcapital fracture may be significant in understanding why normal concentrations of circulating 1,25D in hip fracture patients are unable to normalise bone turnover. Moreover, the findings from these clinical studies suggest that 1,25D production in bone may indeed be important for maintaining a healthy skeleton.
2. Bone 25 hydroxyvitamin D-24α-hydroxylation
In early studies, isolated rat calvarial cells produced 24,25D following incubation with 25D.95 Thus 1,25D activity in bone tissue is regulated by expression of CYP27B1 and CYP24 genes as well as the level of VDR. The regulation of the CYP24 mRNA levels in osteoblasts and other bone cells has, however, only been extensively studied since the isolation of a cDNA clone for CYP24.43,102 A key role for CYP24 in bone development has been suggested from studies of a CYP24-null mouse model.103 These mutant mice exhibited abnormal bone histology characterised by excessive under-mineralised bone matrix. It was suggested that this phenotype was the result of toxic 1,25D concentrations due to the loss of CYP24 activity. This proposal was supported by studies of a double gene knockout mouse in which the VDR-null mouse was crossed with the CYP24-null mouse.103 The bones of the double-knockout mouse were phenotypically normal, provided sufficient calcium was available in the diet. In the absence of VDR, high concentrations of 1,25D were unable to promulgate toxic effects on the bone. The finding that these animals had normal bones, despite the absence of CYP24 activity, also disproved the theory that 24,25D, produced by the 24-hydroxylation of 25D, was an important vitamin D metabolite in the process of bone development.
Nishimura et al. were amongst the first to show that the administration of 1,25D to rats resulted in an increase in the expression of CYP24 mRNA in the bone.104 They also found that the 1,25D induction of CYP24 mRNA expression in the rat immature osteoblastic cell line, C-26, was greater than the induction in the more mature osteoblast cell line, C-11. PTH did not down-regulate the 1,25D-mediated expression of CYP24 mRNA in either the C-26 or the C-11 cell line, which is in contrast to the finding that PTH suppresses the expression of CYP24 mRNA in the kidney. These findings suggest that the control of the expression of CYP24 mRNA by PTH is tissue specific and that 1,25D-modulated expression of CYP24 is regulated during osteoblast development. While the stimulation of CYP24 activity by 1,25D in bone clearly shows that it is involved in the modulation of 1,25D-mediated processes, it is less clear whether the induction of CYP24 activity occurs predominantly in response to the concentration of circulating 1,25D or to the local bone-cell synthesis of 1,25D.
Implications for Laboratory Testing for Vitamin D Status
It is well established, for reasons discussed above, that vitamin D nutritional status is best indicated by serum 25D. Radioimmunoassays for 25D are available from commercial sources105 and an automated chemiluminescence protein-binding assay is also now available.106 All of these assays are suitable for a routine clinical laboratory service. The major clinical requirement for the 25D assay is to detect low vitamin D status which is a common cause of secondary hyperparathyroidism and strongly associated with increased risk of hip fracture in the elderly.107,108 A meta-analysis of serum 25D in elderly hip-fracture patients from 28 studies indicates that serum 25D in hip fracture patients was on average 0.66 standard deviation below the control group.109 Vitamin D toxicity is also a clinical issue but this is a relatively rare finding, with serum 25D needing to be greater than 700 nmol/L to generate hypercalcaemia.110
There has been some discussion about the inter-laboratory (in reality inter-assay) variation of 25D measurements, particularly with relation to the ability to measure the ergocalciferol (D2) metabolite compared with the cholecalciferol (D3) metabolite.105 Ergocalciferol is used widely in Australia as the pharmaceutical preparation for the treatment of vitamin D deficiency. An international comparison study of assays found significant variation between assay values for 25D3 analyses but that the ranking order of each assay for the specimens was equivalent.111 Similar results have been demonstrated by the end-of-cycle reports for external quality assurance programmes (RCPA-AACB Chemical Pathology Group, Australia, 25 Hydroxyvitamin D EQAS, UK). Whether all commercial assays can adequately measure the 25D2 metabolite remains controversial.105 However a marked variation between RIAs was not supported by End-of-Cycle Reports for an external quality assurance programme (25 Hydroxyvitamin D EQAS, UK).
Perhaps the critical issue for clinical laboratories performing routine clinical 25D assays is the provision of suitable interpretation for patient results. Generation of reference intervals using standard methods simply provides a range of 25D values due to the amount of sunlight exposure of the reference population. Such ranges vary as a result of the latitude at which the reference population is living. Objective evidence exist for clinically relevant 25D decision limits for deficiency (undetectable or with more sensitive assays <5 nmol/L) and toxicity (>700 nmol/L). However there is considerable evidence to suggest that a status of vitamin D "insufficiency" is a clinically useful concept. This is a concentration of 25D which is insufficient to maintain serum PTH or bone turnover markers within their respective reference intervals.112 The 25D cut-off value for defining vitamin D insufficiency is controversial although values between 50 and 70 nmol/L have been suggested, using serum PTH and bone turnover markers as surrogate markers of calcium homeostasis and skeletal health (Figure 5).112,113
Figure 5.
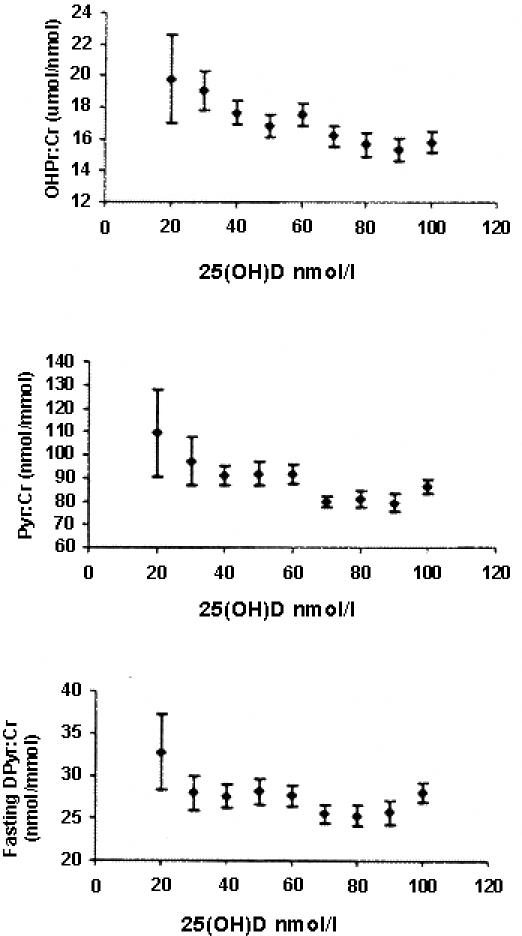
Relationships between three fasting urine bone resorption markers and serum 25D in 486 ambulant postmenopausal white women. (OHPr/Cr, hydroxproline/creatinine; PYD/Cr, pyridinoline/creatinine; DPD/Cr, deoxypyridinoline/creatinine). Note that each bone resorption marker begins to rise at serum 25D below 60 nmol/L. (Reproduced with permission from 113.)
Assays for serum 1,25D are also available from commercial sources, mainly in the form of (125)I-based RIA following extraction from serum. Serum 1,25D correlates with radiocalcium active transport by the intestine59 but its clinical role is essentially limited to the investigation of renal disease in which the reduction of serum 1,25D and impairment of intestinal calcium absorption are early developments.114
Conclusions
Over the last decade there has been considerable evolution of our understanding of vitamin D metabolism and its resulting biological activity. The earlier work of the twentieth century established that vitamin D plays an essential role in maintaining calcium homeostasis and skeletal health. It was recognised that these effects are the result of steroid hormone endocrine activity following the metabolism of vitamin D to the 1,25D metabolite. During the latter years of the twentieth century, however, information has accrued that 1,25D has a number of biological activities outside of its role in calcium homeostasis and maintenance of skeletal health. 1,25D can be synthesised in a variety of tissues, which apparently does not contribute to its endocrine activity. Currently it is presumed that the locally produced 1,25D exerts an autocrine or paracrine action. The action of locally produced 1,25D in bone provides a plausible hypothesis to explain the clinical consequences of vitamin D depletion in the elderly and the marked increase in risk for hip fracture. These concepts reinforce the clinical importance of serum 25D, providing information not only of the availability of substrate for the renal synthesis of circulating 1,25D metabolite but also for its local synthesis in a variety of tissues. The concentration of circulating 25D required to provide sufficient substrate for both the renal and non-renal synthesis of 1,25D is an important issue that requires further investigation. At this time, limited data suggest that serum 25D at least greater than 50 nmol/l is necessary to provide sufficient vitamin D activity to normalise serum PTH and bone metabolism in the elderly who are at the greatest risk of fractures.
References
- 1.Holick MF. Photobiology of vitamin D. In: Feldman D, Glorieux FH, Pike JW. (eds) Vitamin D. Academic Press: San Diego. 1997; pp 33–39.
- 2.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 3.Omdahl JL, May BK. The 25-hydroxyvitamin D 24-hydroxylase. In Feldman D, Glorieux FH, Pike JW (eds) Vitamin D. Academic Press: San Diego. 1997; pp 69–85
- 4.Gascon-Barré M. The vitamin D 25 hydroxylase. In: Feldman D, Glorieux F, Pike JW (eds) Vitamin D. Academic Press: San Diego. 1997; pp 41–55
- 5.Hosseinpour F, Wikvall K. Porcine microsomal vitamin D(3) 25-hydroxylase (CYP2D25). Catalytic properties, tissue distribution, and comparison with human CYP2D6. J Biol Chem. 2000;275:34650–34655. doi: 10.1074/jbc.M004185200. [DOI] [PubMed] [Google Scholar]
- 6.Theodoropoulos C, Demers C, Petit JL, Gascon-Barré M. High sensitivity of rat hepatic vitamin D3-25 hydroxylase CYP27A to 1,25-dihydroxyvitamin D3 administrations. Am J Physiol Endocrinol Metab. 2003;284:E138–E147. doi: 10.1152/ajpendo.00303.2002. [DOI] [PubMed] [Google Scholar]
- 7.Hollis BW, Pittard WB, Reinhardt TA. Relationships among vitamin D, 25-hydroxyvitamin D, and vitamin D-binding protein concentrations in the plasma and milk of human subjects. J Clin Endocrinol Metab. 1986;62:41–44. doi: 10.1210/jcem-62-1-41. [DOI] [PubMed] [Google Scholar]
- 8.Cooke NE, Haddad JG. Vitamin D binding protein (Gc-globulin) Endocr Rev. 1989;10:294–307. doi: 10.1210/edrv-10-3-294. [DOI] [PubMed] [Google Scholar]
- 9.Cooke NE, McLeod JF, Wang XK, Ray K. Vitamin D binding protein: genomic structure, functional domains, and mRNA expression in tissues. J Steroid Biochem Mol Biol. 1991;40:787–793. doi: 10.1016/0960-0760(91)90304-n. [DOI] [PubMed] [Google Scholar]
- 10.Mason RS, Posen S. Some problems associated with assay of 25-hydroxycalciferol in human serum. Clin Chem. 1977;23:806–810. [PubMed] [Google Scholar]
- 11.Morris HA, Morrison GW, Burr M, Thomas DW, Nordin BE. Vitamin D and femoral neck fracture in elderly South Australian women. Med J Aust. 1984;140:519–521. doi: 10.5694/j.1326-5377.1984.tb108222.x. [DOI] [PubMed] [Google Scholar]
- 12.Fraser DR, Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970;228:764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- 13.Zehnder D, Bland R, Walker EA, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in the human kidney. J Am Soc Nephrol. 1999;10:2465–24. doi: 10.1681/ASN.V10122465. [DOI] [PubMed] [Google Scholar]
- 14.Papapoulos SE, Clemens TL, Fraher LJ, Lewin IG, Sandler LM, O'Riordan JL. 1, 25-dihydroxycholecalciferol in the pathogenesis of the hypercalcaemia of sarcoidosis. Lancet. 1979;1(8117):627–630. doi: 10.1016/s0140-6736(79)91076-6. [DOI] [PubMed] [Google Scholar]
- 15.Barbour GL, Coburn JW, Slatopolsky E, Norman AW, Horst RL. Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N Engl J Med. 1981;305:440–443. doi: 10.1056/NEJM198108203050807. [DOI] [PubMed] [Google Scholar]
- 16.Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25-hydroxyvitamin D(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 17.Hendrix I, Morris HA, May BK. Tissue-specific distribution and regulation of CYP1. Bone. 2000;27(Supplement 4):38. (abstract). [Google Scholar]
- 18.Fu GK, Portale AA, Miller WL. Complete structure of the human gene for the vitamin D 1alpha-hydroxylase, P450c1alpha. DNA Cell Biol. 1997;16:1499–1507. doi: 10.1089/dna.1997.16.1499. [DOI] [PubMed] [Google Scholar]
- 19.Monkawa T, Yoshida T, Wakino S, et al. Molecular cloning of cDNA and genomic DNA for human 25-hydroxyvitamin D3 1 alpha-hydroxylase. Biochem Biophys Res Commun. 1997;239:527–533. doi: 10.1006/bbrc.1997.7508. [DOI] [PubMed] [Google Scholar]
- 20.Shinki T, Shimada H, Wakino S, et al. Cloning and expression of rat 25-hydroxyvitamin D3-1alpha-hydroxylase cDNA. Proc Natl Acad Sci USA. 1997;94:12920–12925. doi: 10.1073/pnas.94.24.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.St-Arnuad R, Messerlian S, Moir JM, Omdahl JL, Glorieux FH. The 25-hydroxyvitamin D 1-alpha-hydroxylase gene maps to the pseudovitamin D-deficiency rickets (PDDR) disease locus. J Bone Miner Res. 1997;12:1552–1559. doi: 10.1359/jbmr.1997.12.10.1552. [DOI] [PubMed] [Google Scholar]
- 22.Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science. 1997;277:1827–1830. doi: 10.1126/science.277.5333.1827. [DOI] [PubMed] [Google Scholar]
- 23.Ishida M, Bulos B, Takamoto S, Sacktor B. Hydroxylation of 25-hydroxyvitamin D3 by renal mitochondria from rats of different ages. Endocrinology. 1987;121:443–448. doi: 10.1210/endo-121-2-443. [DOI] [PubMed] [Google Scholar]
- 24.Henry HL, Luntao EM. Interactions between intracellular signals involved in the regulation of 25-hydrox-yvitamin D3 metabolism. Endocrinology. 1989;124:2228–2234. doi: 10.1210/endo-124-5-2228. [DOI] [PubMed] [Google Scholar]
- 25.Brenza HL, DeLuca HF. Regulation of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression by parathyroid hormone and 1,25-dihydroxyvitamin D3. Arch Biochem Biophys. 2000;381:143–152. doi: 10.1006/abbi.2000.1970. [DOI] [PubMed] [Google Scholar]
- 26.Gao X, Dwivedi PP, Choe S, et al. Basal and parathyroid hormone-induced expression of human 25-hydroxyvitamin D 1alpha-hydroxylase gene promoter in kidney AOK-B50 cells: role of SP 1, Ets and CCAAT box protein binding sites. Int J Biochem Cell Biol. 2002;34:921–930. doi: 10.1016/s1357-2725(01)00165-0. [DOI] [PubMed] [Google Scholar]
- 27.Shinki T, Ueno Y, DeLuca HF, Suda T. Calcitonin is a major regulator for the expression of renal 25-hydroxyvitamin D3-1alpha-hydroxylase gene in normocalcemic rats. Proc Natl Acad Sci USA. 1999;96:8253–8258. doi: 10.1073/pnas.96.14.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portale AA, Miller WL. Human 25-hydroxyvitamin D-1alpha-hydroxylase: cloning, mutations, and gene expression. Pediatr Nephrol. 2000;14:620–625. doi: 10.1007/pl00009639. [DOI] [PubMed] [Google Scholar]
- 29.Murayama A, Takeyama K, Kitanaka S, Kodera Y, Hosoya T, Kato S. The promoter of the human 25-hydroxyvitamin D3 1 alpha-hydroxylase gene confers positive and negative responsiveness to PTH, calcitonin, and 1 alpha,25(OH)2D3. Biochem Biophys Res Commun. 1998;249:11–16. doi: 10.1006/bbrc.1998.9098. [DOI] [PubMed] [Google Scholar]
- 30.Omdahl JL, Morris HA, May BK. Hydroxylase enzymes of the vitamin D pathway: expression, function and regulation. Ann Rev Nutr. 2002;22:139–166. doi: 10.1146/annurev.nutr.22.120501.150216. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto Y, Shinki T, Yamamoto K, et al. 1alpha,25-dihydroxyvitamin D3-24-hydroxylase (CYP24) hydroxylates the carbon at the end of the side chain (C-26) of the C-24-fluorinated analog of 1alpha,25-dihydroxyvitamin D3. J Biol Chem. 1997;272:14115–14119. doi: 10.1074/jbc.272.22.14115. [DOI] [PubMed] [Google Scholar]
- 32.Makin G, Lohnes D, Byford V, Ray R, Jones G. Target cell metabolism of 1,25-dihydroxyvitamin D3 to calcitroic acid. Evidence for a pathway in kidney and bone involving 24-oxidation. Biochem J. 1989;262:173–180. doi: 10.1042/bj2620173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy GS, Tserng KY. Calcitroic acid, end product of renal metabolism of 1,25- dihydroxyvitamin D3 through C-24 oxidation pathway. Biochemistry. 1989;28:1763–1769. doi: 10.1021/bi00430a051. [DOI] [PubMed] [Google Scholar]
- 34.Knutson JC, DeLuca HF. 25-Hydroxyvitamin D3-24-hydroxylase. Subcellular location and properties. Biochemistry. 1974;13:1543–1548. doi: 10.1021/bi00704a034. [DOI] [PubMed] [Google Scholar]
- 35.Kumar R. Metabolism of 1,25-dihydroxyvitamin D3. Physiol Rev. 1984;64:478–504. doi: 10.1152/physrev.1984.64.2.478. [DOI] [PubMed] [Google Scholar]
- 36.Reinhardt TA, Horst RL. Self-induction of 1,25-dihydroxyvitamin D3 metabolism limits receptor occupancy and target tissue responsiveness. J Biol Chem. 1989;264:15917–15921. [PubMed] [Google Scholar]
- 37.Tomon M, Tenenhouse HS, Jones G. Expression of 25-hydroxyvitamin D3-24-hydroxylase activity in Caco-2 cells. An in vitro model of intestinal vitamin D catabolism. Endocrinology. 1990;126:2868–2875. doi: 10.1210/endo-126-6-2868. [DOI] [PubMed] [Google Scholar]
- 38.Masuda S, Strugnell S, Calverley MJ, Makin HL, Kremer R, Jones G. In vitro metabolism of the anti-psoriatic vitamin D analog, calcipotriol, in two cultured human keratinocyte models. J Biol Chem. 1994;269:4794–4803. [PubMed] [Google Scholar]
- 39.Ohyama Y, Noshiro M, Okuda K. Cloning and expression of cDNA encoding 25-hydroxyvitamin D3 24-hydroxylase. FEBS Lett. 1991;278:195–198. doi: 10.1016/0014-5793(91)80115-j. [DOI] [PubMed] [Google Scholar]
- 40.Chen ML, Boltz MA, Armbrecht HJ. Effects of 1,25-dihydroxyvitamin D3 and phorbol ester on 25-hydroxyvitamin D3 24-hydroxylase cytochrome P450 messenger ribonucleic acid levels in primary cultures of rat renal cells. Endocrinology. 1993;132:1782–1788. doi: 10.1210/endo.132.4.7681765. [DOI] [PubMed] [Google Scholar]
- 41.Itoh S, Yoshimura T, Iemura O, et al. Molecular cloning of 25-hydroxyvitamin D-3 24-hydroxylase (Cyp-24) from mouse kidney: its inducibility by vitamin D-3. Biochim Biophys Acta. 1995;1264:26–28. doi: 10.1016/0167-4781(95)00147-9. [DOI] [PubMed] [Google Scholar]
- 42.Ohyama Y, Ozono K, Uchida M, et al. Identification of a vitamin D-responsive element in the 5'-flanking region of the rat 25-hydroxyvitamin D3 24-hydroxylase gene. J Biol Chem. 1994;269:10545–10550. [PubMed] [Google Scholar]
- 43.Chen KS, DeLuca HF. Cloning of the human 1 alpha,25-dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochim Biophys Acta. 1995;1263:1–9. doi: 10.1016/0167-4781(95)00060-t. [DOI] [PubMed] [Google Scholar]
- 44.Zhou A, Elgort MG, Allegretto EA. Retinoid X receptor (RXR) ligands activate the human 25-hydroxyvitamin D3-24-hydroxylase promoter via RXR heterodimer binding to two vitamin D- responsive elements and elicit additive effects with 1,25- dihydroxy vitamin D3. J Biol Chem. 1997;272:19027–19034. doi: 10.1074/jbc.272.30.19027. [DOI] [PubMed] [Google Scholar]
- 45.Zierold C, Darwish HM, DeLuca HF. Two vitamin D response elements function in the rat 1,25- dihydroxyvitamin D 24-hydroxylase promoter. J Biol Chem. 1995;270:1675–1678. doi: 10.1074/jbc.270.4.1675. [DOI] [PubMed] [Google Scholar]
- 46.Hahn CN, Kerry DM, Omdahl JL, May BK. Identification of a vitamin D responsive element in the promoter of the rat cytochrome P450(24) gene. Nucleic Acids Res. 1994;22:2410–2416. doi: 10.1093/nar/22.12.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerry DM, Dwivedi PP, Hahn CN, Morris HA, Omdahl JL, May BK. Transcriptional synergism between vitamin D-responsive elements in the rat 25-hydroxyvitamin D3 24-hydroxylase (CYP24) promoter. J Biol Chem. 1996;271:29715–29721. doi: 10.1074/jbc.271.47.29715. [DOI] [PubMed] [Google Scholar]
- 48.McDonnell DP, Mangelsdorf DJ, Pike JW, Haussler MR, O'Malley BW. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987;235:1214–1217. doi: 10.1126/science.3029866. [DOI] [PubMed] [Google Scholar]
- 49.Baker AR, McDonnell DP, Hughes M, et al. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci USA. 1988;85:3294–3298. doi: 10.1073/pnas.85.10.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burmester JK, Maeda N, DeLuca HF. Isolation and expression of rat 1,25-dihydroxyvitamin D3 receptor cDNA. Proc Natl Acad Sci USA. 1988;85:1005–1009. doi: 10.1073/pnas.85.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamei Y, Kawada T, Fukuwatari T, Ono T, Kato S, Sugimoto E. Cloning and sequencing of the gene encoding the mouse vitamin D receptor. Gene. 1995;152:281–282. doi: 10.1016/0378-1119(94)00735-b. [DOI] [PubMed] [Google Scholar]
- 52.Nemere I, Schwarz Z, Pedrozo H, Sylvia VL, Dean DD, Boyan BD. Identification of a membrane receptor for 1,25-dihydroxyvitamin D3 which mediates rapid activation of protein kinase C. J Bone Miner Res. 1998;13:1353–1359. doi: 10.1359/jbmr.1998.13.9.1353. [DOI] [PubMed] [Google Scholar]
- 53.Song X, Bishop JE, Okamura WH, Norman AW. Stimulation of phosphorylation of mitogen-activated protein kinase by 1a, 25-dihydroxyvitamin D3 in promyelocytic NB4 leukemia cells: a structure -function study. Endocrinology. 1998;139:457–465. doi: 10.1210/endo.139.2.5747. [DOI] [PubMed] [Google Scholar]
- 54.Erben RG, Soegiarto DW, Weber K, et al. Deletion of deoxyribonucleic acid binding domain of the vitamin D receptor abrogates genomic and nongenomic functions of vitamin D. Mol Endocrinol. 2002;16:1524–1537. doi: 10.1210/mend.16.7.0866. [DOI] [PubMed] [Google Scholar]
- 55.Dwivedi PP, Omdahl JL, Kola I, Hume DA, May BK. Regulation of rat cytochrome P450C24 (CYP24) gene expression. Evidence for functional cooperation of Ras-activated Ets transcription factors with the vitamin D receptor in 1,25-dihydroxyvitamin D(3)-mediated induction. J Biol Chem. 2000;275:47–55. doi: 10.1074/jbc.275.1.47. [DOI] [PubMed] [Google Scholar]
- 56.Dwivedi PP, Hii CS, Ferrante A, et al. Role of MAP kinases in the 1,25-dihydroxyvitamin D3-induced transactivation of the rat cytochrome P450C24 (CYP24) promoter. Specific functions for ERK1/ERK2 and ERK5. J Biol Chem. 2002;277:29643–29653. doi: 10.1074/jbc.M204561200. [DOI] [PubMed] [Google Scholar]
- 57.Li YC, Pirro AE, Demay MB. Analysis of vitamin D-dependent calcium-binding protein messenger ribonucleic acid expression in mice lacking the vitamin D receptor. Endocrinology. 1998;139:847–851. doi: 10.1210/endo.139.3.5803. [DOI] [PubMed] [Google Scholar]
- 58.Dardenne O, Prud'homme J, Arabian A, Glorieux FH, St-Arnaud R. Targeted inactivation of the 25 hydroxyvitamin D(3)-1 (alpha)-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology. 2001;142:3135–3141. doi: 10.1210/endo.142.7.8281. [DOI] [PubMed] [Google Scholar]
- 59.Morris HA, Need AG, Horowitz M, O'Loughlin PD, Nordin BE. Calcium absorption in normal and osteoporotic postmenopausal women. Calcif Tissue Int. 1991;49:240–243. doi: 10.1007/BF02556211. [DOI] [PubMed] [Google Scholar]
- 60.Hoenderop JG, Hartog A, Stuiver M, Doucet A, Willems PH, Bindels RJ. Localization of the epithelial Ca(21) channel in rabbit kidney and intestine. J A Soc Nephrol. 2000;11:1171–1178. doi: 10.1681/ASN.V1171171. [DOI] [PubMed] [Google Scholar]
- 61.Darwish HM, DeLuca HF. Analysis of binding of the 1,25-dihydroxyvitamin D3 receptor to positive and negative vitamin D response elements. Arch Biochem Biophys. 1996;334:223–234. doi: 10.1006/abbi.1996.0450. [DOI] [PubMed] [Google Scholar]
- 62.Li YC, Amling M, Pirro AE, et al. Normalization of mineral ion homoestasis by dietary means prevents hyperparathyroidism, rickets and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology. 1998;139:4391–4396. doi: 10.1210/endo.139.10.6262. [DOI] [PubMed] [Google Scholar]
- 63.Parfitt AM. Vitamin D and the pathogenesis of rickets and osteomalacia. In Feldman D, Glorieux FH, Pike JW (eds) Vitamin D. Academic Press: San Diego. 1997; Pp 645–662.
- 64.Matsumoto T, Igarashi C, Takeuchi Y, et al. Stimulation by 1,25-dihydroxyvitamin D3 of in vitro mineralization induced by osteoblast-like MC3T3-E1 cells. Bone. 1991;12:27–32. doi: 10.1016/8756-3282(91)90051-j. [DOI] [PubMed] [Google Scholar]
- 65.Rickard DJ, Kazhdan I, Leboy PS. Importance of 1,25-dihydroxyvitamin D3 and the nonadherent cells of marrow for osteoblast differentiation from rat marrow stromal cells. Bone. 1995;16:671–678. doi: 10.1016/8756-3282(95)00099-y. [DOI] [PubMed] [Google Scholar]
- 66.Abe E, Miyaura C, Sakagami H, et al. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1981;78:4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanaka H, Abe E, Miyaura C, et al. 1 alpha, 25-Dihydroxycholecalciferol and a human myeloid leukaemia cell line (HL-60) Biochem J. 1982;204:713–719. doi: 10.1042/bj2040713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suda T, Udagawa N, Nakamura I, Miyaura C, Takahashi N. Modulation of osteoclast differentiation by local factors. Bone. 1995;17:87S–91S. doi: 10.1016/8756-3282(95)00185-g. [DOI] [PubMed] [Google Scholar]
- 69.Takeda S, Yoshizawa T, Nagai Y, et al. Stimulation of osteoclast formation by 1,25-dihydroxyvitamin D requires its binding to vitamin D receptor (VDR) in osteoblastic cells: studies using VDR knockout mice. Endocrinology. 1999;140:1005–1008. doi: 10.1210/endo.140.2.6673. [DOI] [PubMed] [Google Scholar]
- 70.Peacock M. Osteomalacia and rickets. In: Nordin BEC, Need AG, Morris HA. (eds) Metabolic Bone and Stone Disease, 3rd edition. Churchill Livingstone, Edinburgh. 1993; 83–118.
- 71.Langdahl BL, Mortensen L, Vesterby A, Eriksen EF, Charles P. Bone histomorphometry in hypoparathyroid patients treated with vitamin D. Bone. 1996;18:103–108. doi: 10.1016/8756-3282(95)00443-2. [DOI] [PubMed] [Google Scholar]
- 72.Eriksen EF, Steiniche T, Mosekilde L, Melsen F. Histomorphometric analysis of bone in metabolic bone disease. Endocrinol Metab Clin North Am. 1989;18:919–954. [PubMed] [Google Scholar]
- 73.Cantley LK, Russell J, Lettieri D, Sherwood LM. 1,25-Dihydroxyvitamin D3 suppresses parathyroid hormone secretion from bovine parathyroid cells in tissue culture. Endocrinology. 1985;117:2114–2119. doi: 10.1210/endo-117-5-2114. [DOI] [PubMed] [Google Scholar]
- 74.Chan YL, McKay C, Dye E, Slatopolsky E. The effect of 1,25 dihydroxycholecalciferol on parathyroid hormone secretion by monolayer cultures of bovine parathyroid cells. Calcif Tissue Int. 1986;38:27–32. doi: 10.1007/BF02556591. [DOI] [PubMed] [Google Scholar]
- 75.Szabo A, Merke J, Beier E, Mall G, Ritz E. 1,25(OH)2 vitamin D3 inhibits parathyroid cell proliferation in experimental uremia. Kidney Int. 1989;35:1049–1056. doi: 10.1038/ki.1989.89. [DOI] [PubMed] [Google Scholar]
- 76.Liu SM, Koszewski N, Lupez M, Malluche HH, Olivera A, Russell J. Characterization of a response element in the 5'-flanking region of the avian (chicken) PTH gene that mediates negative regulation of gene transcription by 1,25 dihydroxyvitamin D3 and binds the vitamin D3 receptor. Mol Endocrinol. 1996;10:206–215. doi: 10.1210/mend.10.2.8825560. [DOI] [PubMed] [Google Scholar]
- 77.Varghese S, Lee S, Huang YC, Christakos S. Analysis of rat vitamin D-dependent calbindin-D28k gene expression. J Biol Chem. 1988;263:9776–9784. [PubMed] [Google Scholar]
- 78.Bar A, Shani M, Fullmer CS, Brindak ME, Striem S. Modulation of chick intestinal and renal calbindin gene expression by dietary vitamin D3, 1,25-dihydroxyvitamin D3, calcium and phosphorus. Mol Cell Endocrinol. 1990;72:23–31. doi: 10.1016/0303-7207(90)90236-2. [DOI] [PubMed] [Google Scholar]
- 79.Armbrecht HJ, Boltz M, Strong R, Richardson A, Bruns ME, Christakos S. Expression of calbindin-D decreases with age in intestine and kidney. Endocrinology. 1989;125:2950–2956. doi: 10.1210/endo-125-6-2950. [DOI] [PubMed] [Google Scholar]
- 80.Yamamoto M, Kawanobe Y, Takahashi H, Shimazawa E, Kimura S, Ogata E. Vitamin D deficiency and renal calcium transport in the rat. J Clin Invest. 1984;74:507–513. doi: 10.1172/JCI111448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Friedman PA, Gesek FA. Vitamin D3 accelerates PTH-dependent calcium transport in distal convoluted tubule cells. Am J Physiol. 1993;265:F300–F308. doi: 10.1152/ajprenal.1993.265.2.F300. [DOI] [PubMed] [Google Scholar]
- 82.Morris HA. Strategies for interpretation of clinical laboratory data. Clin Biochem Revs. 1991;12:34–37. [Google Scholar]
- 83.Booth BE, Tsai HC, Morris RC., Jr Parathyroidectomy reduces 25-hydroxyvitamin D3-1 alpha-hydroxylase activity in the hypocalcemic vitamin D-deficient chick. J Clin Invest. 1977;60:1314–1320. doi: 10.1172/JCI108890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lobaugh B, Garner SC, Lovdal JA, et al. Parathyroidectomy abolishes the increase of renal 25-hydroxyvitamin D-1 alpha-hydroxylase in lactating rats. Am J Physiol. 1993;264:E981–E985. doi: 10.1152/ajpendo.1993.264.6.E981. [DOI] [PubMed] [Google Scholar]
- 85.Brenza HL, Kimmel-Jehan C, Jehan F, et al. Parathyroid hormone activation of the 25-hydroxyvitamin D3-1alpha-hydroxylase gene promoter. Proc Natl Acad Sci USA. 1998;95:1387–1391. doi: 10.1073/pnas.95.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shinki T, Jin CH, Nishimura A, et al. Parathyroid hormone inhibits 25-hydroxyvitamin D3-24-hydroxylase mRNA expression stimulated by 1 alpha, 25-dihydroxyvitamin D3 in rat kidney but not in intestine. J Biol Chem. 1992;267:13757–13762. [PubMed] [Google Scholar]
- 87.Zierold C, Mings J, DeLuca H. PTH regulates 24-hydroxylase mRNA by altering its stability. J Bone Miner Res. 2001;16:S556. doi: 10.1073/pnas.241516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thakker RV, Fraher LJ, Adami S, Karmali R, O'Riordan JL. Circulating concentrations of 1,25-dihydroxyvitamin D3 in patients with primary hyperparathyroidism. Bone Miner. 1986;1:137–144. [PubMed] [Google Scholar]
- 89.Weisinger JR, Favus MJ, Langman CB, Bushinsky DA. Regulation of 1,25-dihydroxyvitamin D3 by calcium in the parathyroidectomized, parathyroid hormone-replete rat. J Bone Miner Res. 1989;4:929–935. doi: 10.1002/jbmr.5650040618. [DOI] [PubMed] [Google Scholar]
- 90.Bland R, Walker EA, Hughes SV, Stewart PM, Hewison M. Constitutive expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in a transformed human proximal tubule cell line: evidence for direct regulation of vitamin D metabolism by calcium. Endocrinology. 1999;140:2027–2034. doi: 10.1210/endo.140.5.6683. [DOI] [PubMed] [Google Scholar]
- 91.Martin TJ. Calcitonin, an update. Bone. 1999;24:63S–65S. doi: 10.1016/s8756-3282(99)00068-x. [DOI] [PubMed] [Google Scholar]
- 92.Beckman MJ, Goff JP, Reinhardt TA, Beitz DC, Horst RL. In vivo regulation of rat intestinal 24-hydroxylase: potential new role of calcitonin. Endocrinology. 1994;5:1951–1955. doi: 10.1210/endo.135.5.7956916. [DOI] [PubMed] [Google Scholar]
- 93.Gao X, Dwivedi P, Morris HA, Omdahl JL, May BK. Calcitonin regulation of 25-hydroxyvitamin D-24-hydroxylase (CYP24) in kidney cells. (in preparation)
- 94.Tanaka Y, Castillo L, DeLuca HF. The 24-hydroxylation of 1,25-dihydroxyvitamin D3. J Biol Chem. 1977;2:1421–1424. [PubMed] [Google Scholar]
- 95.Turner RT, Puzas JE, Forte MD, et al. In vitro synthesis of 1 alpha,25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol by isolated calvarial cells. Proc Natl Acad Sci USA. 1980;77:5720–5724. doi: 10.1073/pnas.77.10.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reichel H, Koeffler HP, Norman AW. Synthesis in vitro of 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 by interferon-gamma-stimulated normal human bone marrow and alveolar macrophages. J Biol Chem. 1987;262:10931–10937. [PubMed] [Google Scholar]
- 97.Adams JS, Beeker TG, Hongo T, Clemens TL. Constitutive expression of a vitamin D 1-hydroxylase in a myelomonocytic cell line: a model for studying 1,25-dihydroxyvitamin D production in vitro. J Bone Miner Res. 1990;5:1265–1269. doi: 10.1002/jbmr.5650051212. [DOI] [PubMed] [Google Scholar]
- 98.Panda DK, Al Kawas S, Seldin MF, Hendy GN, Goltzman D. 25-hydroxyvitamin D 1alpha-hydroxylase: structure of the mouse gene, chromosomal assignment, and developmental expression. J Bone Miner Res. 2001;16:46–56. doi: 10.1359/jbmr.2001.16.1.46. [DOI] [PubMed] [Google Scholar]
- 99.St Arnaud R, Dardene O, Prud'homme J, Hocking SA, Glorieux FH. Coventional and tissue-specific inactivation of the 25-hydroxyvitamin D-1alpha-hydroxylase (CYP27B1) J Cell Biochem. 2003;88:245–251. doi: 10.1002/jcb.10348. [DOI] [PubMed] [Google Scholar]
- 100.Sagiv P, Lidor C, Hallel T, Edelstein S. Decrease in bone level of 1,25-dihydroxyvitamin D in women over 45 years old. Calcif Tissue Int. 1992;51:24–26. doi: 10.1007/BF00296212. [DOI] [PubMed] [Google Scholar]
- 101.Lidor C, Sagiv P, Amdur B, et al. Decrease in bone levels of 1,25-dihydroxyvitamin D in women with subcapital fracture of the femur. Calcif Tissue Int. 1993;52:146–148. doi: 10.1007/BF00308324. [DOI] [PubMed] [Google Scholar]
- 102.Ohyama Y, Noshiro M, Eggertsen G, et al. Structural characterization of the gene encoding rat 25-hydroxyvitamin D3 24-hydroxylase. Biochemistry. 1993;32:76–82. doi: 10.1021/bi00052a011. [DOI] [PubMed] [Google Scholar]
- 103.St-Arnaud R, Arabian A, Travers R, et al. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology. 2000;141:2658–2666. doi: 10.1210/endo.141.7.7579. [DOI] [PubMed] [Google Scholar]
- 104.Nishimura A, Shinki T, Jin CH, et al. Regulation of messenger ribonucleic acid expression of 1 alpha,25-dihydroxyvitamin D3-24-hydroxylase in rat osteoblasts. Endocrinology. 1994;134:1794–1799. doi: 10.1210/endo.134.4.8137744. [DOI] [PubMed] [Google Scholar]
- 105.Hollis BW. Comparison of commercially available (125)I-based RIA methods for the determination of circulating 25-hydroxyvitamin D. Clin Chem. 2000;46:1657–1661. [PubMed] [Google Scholar]
- 106.Roth HJ, Zahn I, Alkier R, Schmidt H. Validation of the first automated chemiluminescence protein-binding assay for the detection of 25-hydroxycalciferol. Clin Lab. 2001;47:357–365. [PubMed] [Google Scholar]
- 107.Morris HA, Need AG, Nordin BEC. Editorial: the hip fracture threat. Med L Aust. 1999;1170:459–460. doi: 10.5694/j.1326-5377.1999.tb127840.x. [DOI] [PubMed] [Google Scholar]
- 108.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Revs. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 109.Weatherall M. A meta-analysis of 25 hydroxyvitamin D in older people with fracture of the proximal femur. NZ Med J. 2000;113:137–140. [PubMed] [Google Scholar]
- 110.Mason RS, Posen S. The relevance of 25-hydroxycalciferol measurements in the treatment of hypoparathyroidism. Clin Endocrinol. 1979;10:265–269. doi: 10.1111/j.1365-2265.1979.tb02080.x. [DOI] [PubMed] [Google Scholar]
- 111.Lips P, Chapuy MC, Dawson-Hughes B, Pols HA, Holick MF. An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos Int. 1999;9:394–397. doi: 10.1007/s001980050162. [DOI] [PubMed] [Google Scholar]
- 112.Malabanan A, Veronokis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 113.Jesudason D, Need AG, Horowitz M, O'Loughlin PD, Morris HA, Nordin BE. Relationship between serum 25-hydroxyvitamin D and bone resorption markers in vitamin D insufficiency. Bone. 2002;31:626–630. doi: 10.1016/s8756-3282(02)00866-9. [DOI] [PubMed] [Google Scholar]
- 114.Cochran M. Bone disease in renal failure. In Nordin BEC, Need AG, Morris HA (eds) Metabolic Bone and Stome Disease 3rd edition. Churchill Livingstone, Edinburgh. 1993; 163–180.


