Abstract
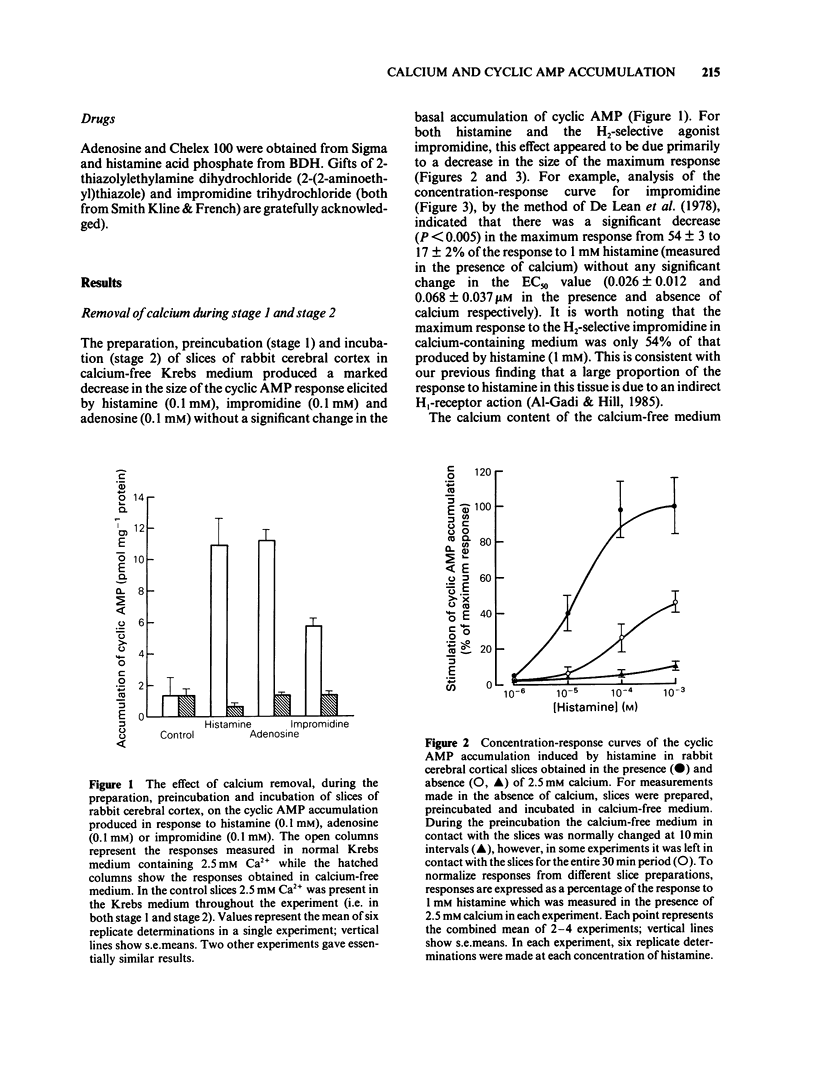
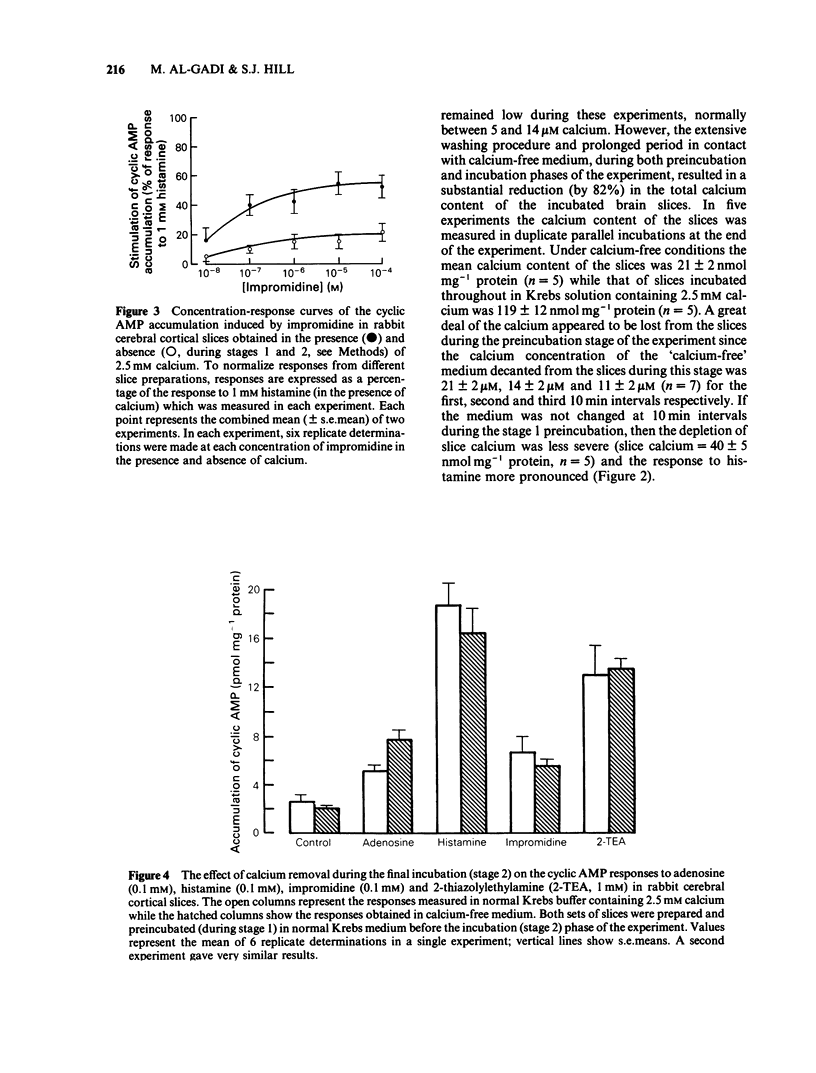
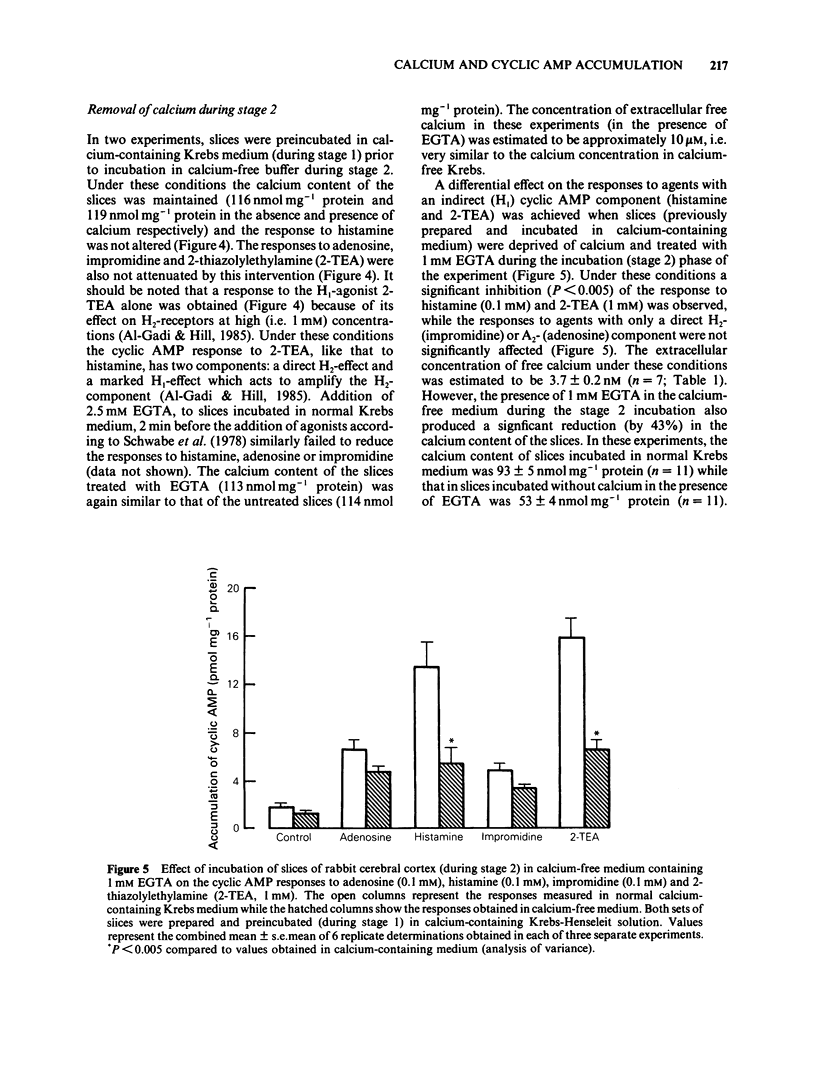
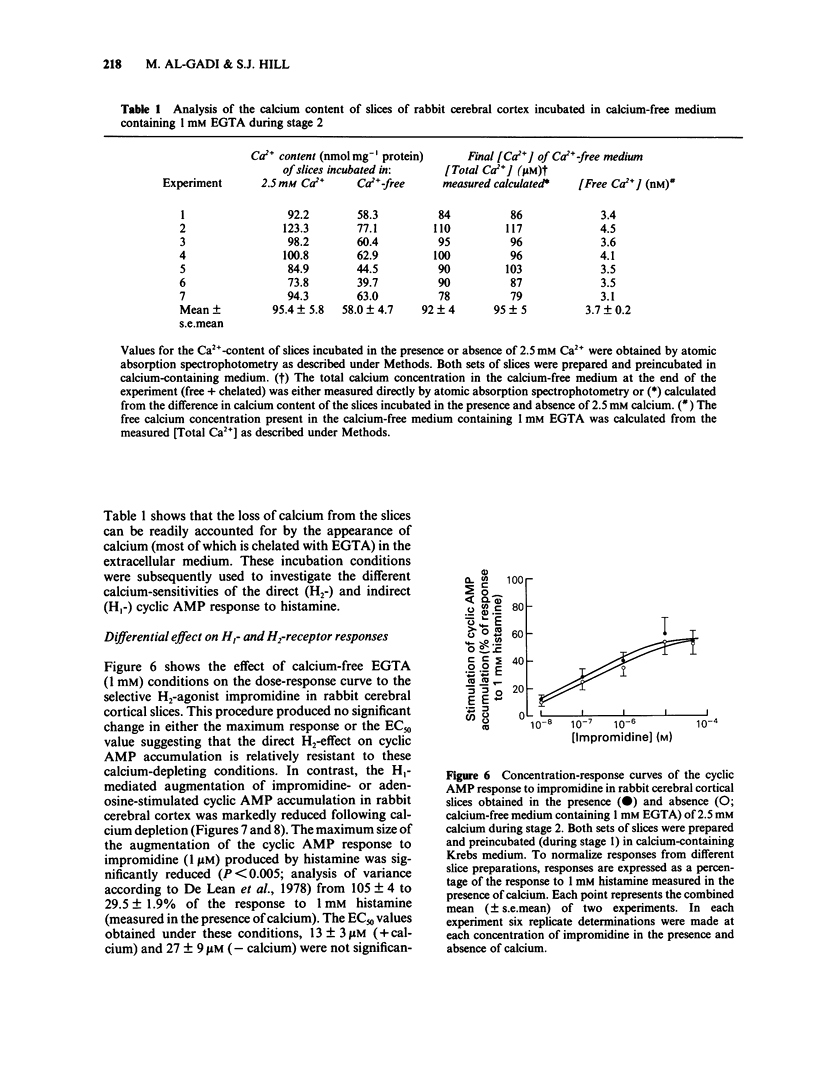
The effect of calcium on the H1- and H2-receptor components of the cyclic AMP response to histamine in rabbit cerebral cortical slices has been investigated. Removal of calcium ions from the incubation medium during the preparation, preincubation and final incubation of brain slices significantly reduced the cyclic AMP responses to adenosine, histamine and the H2-selective agonist, impromidine. Removal of calcium ions from the incubation medium during only the final incubation with agonists did not influence the responses to adenosine, histamine, impromidine and the H1-selective agonist, 2-thiazolylethylamine. Final incubation of rabbit cerebral cortical slices in calcium-free buffer containing EGTA (1 mM) however, selectively reduced the cyclic AMP responses to the H1-agonists histamine and 2-thiazolylethylamine without affecting the response to impromidine or adenosine. These latter incubation conditions significantly reduced the maximal extent of the augmentation of impromidine- or adenosine-stimulated cyclic AMP accumulation produced by H1-receptor stimulation, without affecting the EC50 values of the H1-agonists. Calcium-free/EGTA conditions did not, however, alter the dose-response parameters for the response to the H2-agonist, impromidine. These data provide further evidence that the two histamine receptor systems affect cyclic AMP accumulation in rabbit cerebral cortical slices by different mechanisms.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Gadi M., Hill S. J. Characterization of histamine receptors mediating the stimulation of cyclic AMP accumulation in rabbit cerebral cortical slices. Br J Pharmacol. 1985 Aug;85(4):877–888. doi: 10.1111/j.1476-5381.1985.tb11087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand-Srivastava M. B., Johnson R. A. Regulation of adenosine-sensitive adenylate cyclase from rat brain striatum. J Neurochem. 1980 Oct;35(4):905–914. doi: 10.1111/j.1471-4159.1980.tb07089.x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Huang Y. C., Breckenridge B. M., Wolff D. J. Identification of a calcium-binding protein as a calcium-dependent regulator of brain adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Jan;72(1):64–68. doi: 10.1073/pnas.72.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. L., Ekins R. P., Albano J. D. Saturation assay for cyclic AMP using endogenous binding protein. Adv Cyclic Nucleotide Res. 1972;2:25–40. [PubMed] [Google Scholar]
- Brown E., Kendall D. A., Nahorski S. R. Inositol phospholipid hydrolysis in rat cerebral cortical slices: I. Receptor characterisation. J Neurochem. 1984 May;42(5):1379–1387. doi: 10.1111/j.1471-4159.1984.tb02798.x. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y., Bradham L. S., Lynch T. J., Lin Y. M., Tallant E. A. Protein activator of cyclic 3':5'-nucleotide phosphodiesterase of bovine or rat brain also activates its adenylate cyclase. Biochem Biophys Res Commun. 1975 Oct 6;66(3):1055–1062. doi: 10.1016/0006-291x(75)90747-0. [DOI] [PubMed] [Google Scholar]
- Daum P. R., Downes C. P., Young J. M. Histamine stimulation of inositol 1-phosphate accumulation in lithium-treated slices from regions of guinea pig brain. J Neurochem. 1984 Jul;43(1):25–32. doi: 10.1111/j.1471-4159.1984.tb06674.x. [DOI] [PubMed] [Google Scholar]
- Daum P. R., Hill S. J., Young J. M. Histamine H1-agonist potentiation of adenosine-stimulated cyclic AMP accumulation in slices of guinea-pig cerebral cortex: comparison of response and binding parameters. Br J Pharmacol. 1982 Oct;77(2):347–357. doi: 10.1111/j.1476-5381.1982.tb09304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Donaldson J., Hill S. J. Enhancement of histamine H1-receptor agonist activity by 1,4-dithiothreitol in guinea-pig cerebellum and cerebral cortex. J Neurochem. 1986 Nov;47(5):1476–1482. doi: 10.1111/j.1471-4159.1986.tb00781.x. [DOI] [PubMed] [Google Scholar]
- Donaldson J., Hill S. J. Histamine-induced hydrolysis of polyphosphoinositides in guinea-pig ileum and brain. Eur J Pharmacol. 1986 May 27;124(3):255–265. doi: 10.1016/0014-2999(86)90226-8. [DOI] [PubMed] [Google Scholar]
- Donaldson J., Hill S. J. Histamine-induced inositol phospholipid breakdown in the longitudinal smooth muscle of guinea-pig ileum. Br J Pharmacol. 1985 Jun;85(2):499–512. doi: 10.1111/j.1476-5381.1985.tb08887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Wusteman M. M. Breakdown of polyphosphoinositides and not phosphatidylinositol accounts for muscarinic agonist-stimulated inositol phospholipid metabolism in rat parotid glands. Biochem J. 1983 Dec 15;216(3):633–640. doi: 10.1042/bj2160633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J. P., Johnson C. L., Weinstein H., Maayani S. Antagonism of histamine-activated adenylate cyclase in brain by D-lysergic acid diethylamide. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5697–5701. doi: 10.1073/pnas.74.12.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegstrand L. R., Kanof P. D., Greengard P. Histamine-sensitive adenylate cyclase in mammalian brain. Nature. 1976 Mar 11;260(5547):163–165. doi: 10.1038/260163a0. [DOI] [PubMed] [Google Scholar]
- Hill S. J., Daum P., Young J. M. Affinities of histamine H1-antagonists in guinea pig brain: similarity of values determined from [3H]mepyramine binding and from inhibition of a functional response. J Neurochem. 1981 Nov;37(5):1357–1360. doi: 10.1111/j.1471-4159.1981.tb04692.x. [DOI] [PubMed] [Google Scholar]
- Hollingsworth E. B., Daly J. W. Accumulation of inositol phosphates and cyclic AMP in guinea-pig cerebral cortical preparations. Effects of norepinephrine, histamine, carbamylcholine and 2-chloroadenosine. Biochim Biophys Acta. 1985 Nov 20;847(2):207–216. doi: 10.1016/0167-4889(85)90022-9. [DOI] [PubMed] [Google Scholar]
- Hollingsworth E. B., Sears E. B., Daly J. W. An activator of protein kinase C (phorbol-12-myristate-13-acetate) augments 2-chloroadenosine-elicited accumulation of cyclic AMP in guinea pig cerebral cortical particulate preparations. FEBS Lett. 1985 May 20;184(2):339–342. doi: 10.1016/0014-5793(85)80634-7. [DOI] [PubMed] [Google Scholar]
- Hough L. B., Weinstein H., Green J. P. One agonist and two receptors mediating the same effect: histamine receptors linked to adenylate cyclase in the brain. Adv Biochem Psychopharmacol. 1980;21:183–192. [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Downes C. P. Inositol trisphosphates in carbachol-stimulated rat parotid glands. Biochem J. 1984 Oct 1;223(1):237–243. doi: 10.1042/bj2230237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi S., Rall T. W. Studies on adenosine 3',5'-phosphate in rabbit cerebral cortex. Mol Pharmacol. 1968 Jul;4(4):379–388. [PubMed] [Google Scholar]
- Kanof P. D., Greengard P. Pharmacological properties of histamine-sensitive adenylate cyclase from mammalian brain. J Pharmacol Exp Ther. 1979 Apr;209(1):87–96. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Magistretti P. J., Schorderet M. Norepinephrine and histamine potentiate the increases in cyclic adenosine 3':5'-monophosphate elicited by vasoactive intestinal polypeptide in mouse cerebral cortical slices: mediation by alpha 1-adrenergic and H1-histaminergic receptors. J Neurosci. 1985 Feb;5(2):362–368. doi: 10.1523/JNEUROSCI.05-02-00362.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nahorski S. R., Kendall D. A., Batty I. Receptors and phosphoinositide metabolism in the central nervous system. Biochem Pharmacol. 1986 Aug 1;35(15):2447–2453. doi: 10.1016/0006-2952(86)90038-9. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Palacios J. M., Garbarg M., Barbin G., Schwartz J. C. Pharmacological characterization of histamine receptors mediating the stimulation of cyclic AMP accumulation in slices from guinea-pig hippocampus. Mol Pharmacol. 1978 Nov;14(6):971–982. [PubMed] [Google Scholar]
- Piascik M. T., Piascik M. F., Hitzemann R. J., Potter J. D. Ca2+-dependent regulation of rat caudate nucleus adenylate cyclase and effects on the response to dopamine. Mol Pharmacol. 1981 Sep;20(2):319–325. [PubMed] [Google Scholar]
- Piascik M. T., Wisler P. L., Johnson C. L., Potter J. D. Ca2+-dependent regulation of guinea pig brain adenylate cyclase. J Biol Chem. 1980 May 10;255(9):4176–4181. [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Ohga Y., Daly J. W. The role of calcium in the regulation of cyclic nucleotide levels in brain slices of rat and guinea pig. Naunyn Schmiedebergs Arch Pharmacol. 1978 Apr;302(2):141–151. doi: 10.1007/BF00517981. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Bond M., Somlyo A. P., Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5231–5235. doi: 10.1073/pnas.82.15.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]


