Abstract
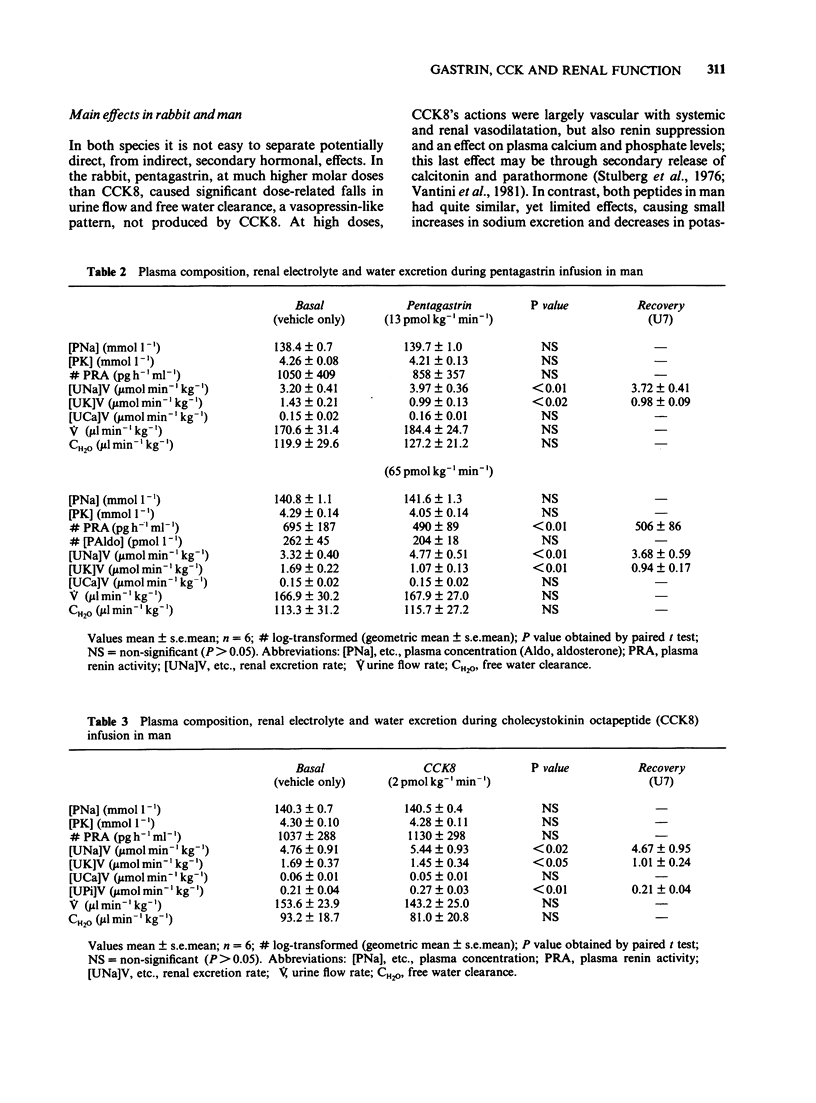
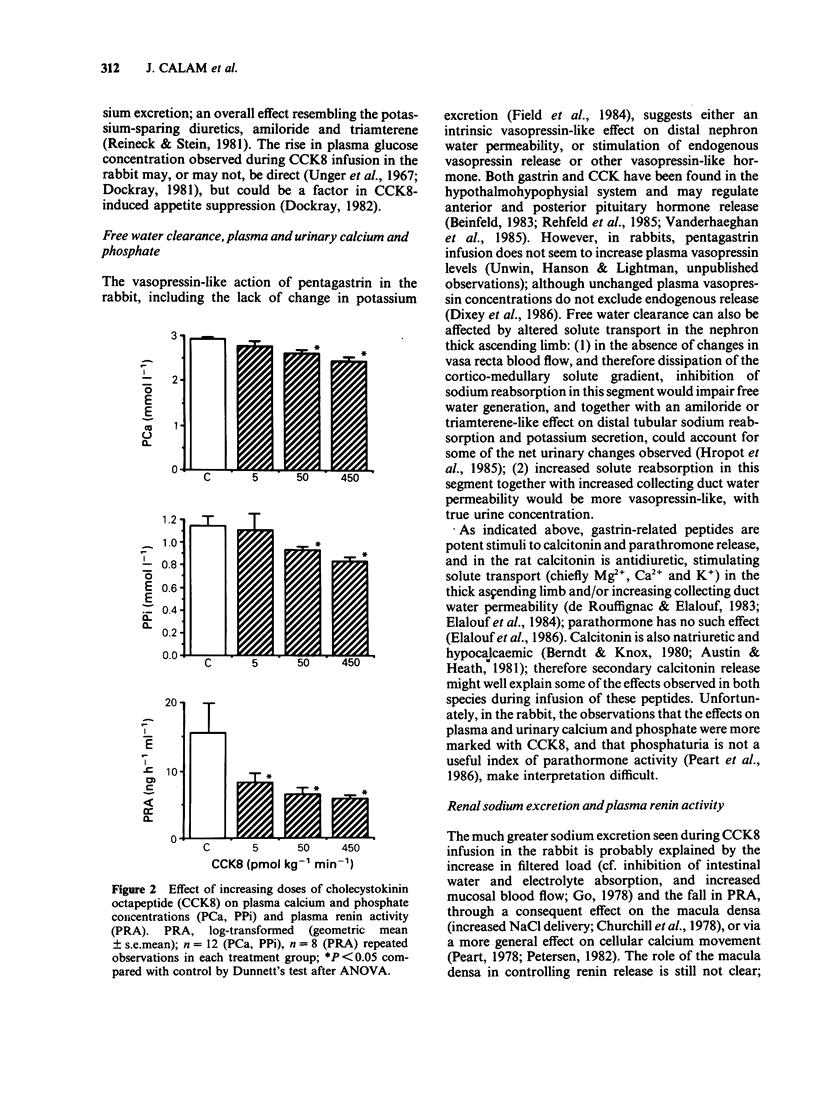
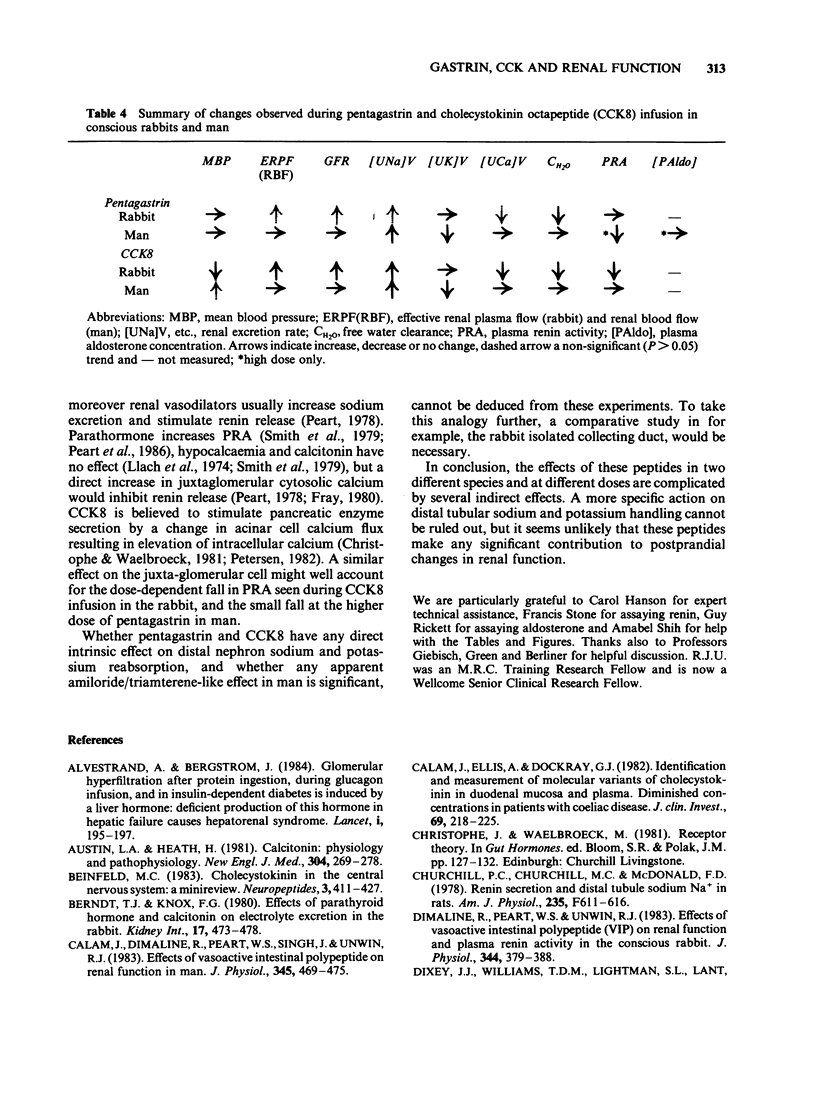
Pentagastrin and cholecystokinin octapeptide (CCK8) were infused i.v. at three different doses in two sets of 4 conscious rabbits following a repeated measurements design (130, 1,300 and 13,000 pmol kg-1 min-1 pentagastrin; 5, 50 and 450 pmol kg-1 min-1 CCK8). In man, two different doses of pentagastrin (13 and 65 pmol kg-1 min-1) were infused in two groups of 6 subjects, and CCK8 (2 pmol kg-1 min-1) in a third group. According to published human postprandial levels, plasma CCK8-like immunoreactivity concentrations were supraphysiological at all doses infused. In the rabbit, pentagastrin produced a dose-related fall in urine flow and free water clearance, but no significant change in systemic and renal haemodynamics, electrolyte excretion and measured plasma constituents; however, in human subjects, pentagastrin increased renal sodium excretion and reduced potassium excretion but did not change glomerular filtration rate. In the rabbit, CCK8 produced a dose-related fall in plasma renin activity, plasma calcium concentration and mean arterial blood pressure; dose-dependent increases in effective renal plasma flow, glomerular filtration rate and renal sodium excretion. In man, changes in sodium and potassium excretion similar to pentagastrin were observed; there were no significant changes in plasma renin activity, plasma calcium concentration, blood pressure, effective renal plasma flow or glomerular filtration rate. The pharmacological renal effects of pentagastrin in conscious water-loaded rabbits resemble vasopressin. In contrast, CCK8's most striking effect was vasodilatation and was unusual in inhibiting rather than stimulating renin release. In man the net changes in urine composition found during infusion of these peptides are similar to those produced by the potassium-sparing diuretics, amiloride and triamterene.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvestrand A., Bergström J. Glomerular hyperfiltration after protein ingestion, during glucagon infusion, and in insulin-dependent diabetes is induced by a liver hormone: deficient production of this hormone in hepatic failure causes hepatorenal syndrome. Lancet. 1984 Jan 28;1(8370):195–197. doi: 10.1016/s0140-6736(84)92115-9. [DOI] [PubMed] [Google Scholar]
- Austin L. A., Heath H., 3rd Calcitonin: physiology and pathophysiology. N Engl J Med. 1981 Jan 29;304(5):269–278. doi: 10.1056/NEJM198101293040505. [DOI] [PubMed] [Google Scholar]
- Beinfeld M. C. Cholecystokinin in the central nervous system: a minireview. Neuropeptides. 1983 Oct;3(6):411–427. doi: 10.1016/0143-4179(83)90032-x. [DOI] [PubMed] [Google Scholar]
- Berndt T. J., Knox F. G. Effects of parathyroid hormone and calcitonin on electrolyte excretion in the rabbit. Kidney Int. 1980 Apr;17(4):473–478. doi: 10.1038/ki.1980.55. [DOI] [PubMed] [Google Scholar]
- Calam J., Dimaline R., Peart W. S., Singh J., Unwin R. J. Effects of vasoactive intestinal polypeptide on renal function in man. J Physiol. 1983 Dec;345:469–475. doi: 10.1113/jphysiol.1983.sp014989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calam J., Ellis A., Dockray G. J. Identification and measurement of molecular variants of cholecystokinin in duodenal mucosa and plasma. Diminished concentrations in patients with celiac disease. J Clin Invest. 1982 Jan;69(1):218–225. doi: 10.1172/JCI110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill P. C., Churchill M. C., McDonald F. D. Renin secretion and distal tubule Na+ in rats. Am J Physiol. 1978 Dec;235(6):F611–F616. doi: 10.1152/ajprenal.1978.235.6.F611. [DOI] [PubMed] [Google Scholar]
- Dimaline R., Peart W. S., Unwin R. J. Effects of vasoactive intestinal polypeptide (VIP) on renal function and plasma renin activity in the conscious rabbit. J Physiol. 1983 Nov;344:379–388. doi: 10.1113/jphysiol.1983.sp014946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixey J. J., Williams T. D., Lightman S. L., Lant A. F., Brewerton D. A. The effect of indomethacin on the renal response to arginine vasopressin in man. Clin Sci (Lond) 1986 May;70(5):409–416. doi: 10.1042/cs0700409. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. The physiology of cholecystokinin in brain and gut. Br Med Bull. 1982 Sep;38(3):253–258. doi: 10.1093/oxfordjournals.bmb.a071769. [DOI] [PubMed] [Google Scholar]
- Elalouf J. M., Roinel N., de Rouffignac C. ADH-like effects of calcitonin on electrolyte transport by Henle's loop of rat kidney. Am J Physiol. 1984 Feb;246(2 Pt 2):F213–F220. doi: 10.1152/ajprenal.1984.246.2.F213. [DOI] [PubMed] [Google Scholar]
- Elalouf J. M., Roinel N., de Rouffignac C. Effects of glucagon and PTH on the loop of Henle of rat juxtamedullary nephrons. Kidney Int. 1986 Apr;29(4):807–813. doi: 10.1038/ki.1986.70. [DOI] [PubMed] [Google Scholar]
- Eysselein V. E., Maxwell V., Reedy T., Wünsch E., Walsh J. H. Similar acid stimulatory potencies of synthetic human big and little gastrins in man. J Clin Invest. 1984 May;73(5):1284–1290. doi: 10.1172/JCI111330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrington K., El Nahas A. M., Hilson A. J., Gornacz G., Bloom S. R., Moorhead J. F. Gut hormones and glomerular hyperfiltration. Lancet. 1984 Mar 17;1(8377):636–636. doi: 10.1016/s0140-6736(84)91036-5. [DOI] [PubMed] [Google Scholar]
- Field M. J., Stanton B. A., Giebisch G. H. Influence of ADH on renal potassium handling: a micropuncture and microperfusion study. Kidney Int. 1984 Mar;25(3):502–511. doi: 10.1038/ki.1984.46. [DOI] [PubMed] [Google Scholar]
- Fray J. C. Stimulus-secretion coupling of renin. Role of hemodynamic and other factors. Circ Res. 1980 Oct;47(4):485–492. doi: 10.1161/01.res.47.4.485. [DOI] [PubMed] [Google Scholar]
- Fronek K., Stahlgren L. H. Systemic and regional hemodynamic changes during food intake and digestion in nonanesthetized dogs. Circ Res. 1968 Dec;23(6):687–692. doi: 10.1161/01.res.23.6.687. [DOI] [PubMed] [Google Scholar]
- Gregory R. A. The Bayliss-Starling lecture 1973. The gastrointestinal hormones: a review of recent advances. J Physiol. 1974 Aug;241(1):1–32. doi: 10.1113/jphysiol.1974.sp010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hropot M., Fowler N., Karlmark B., Giebisch G. Tubular action of diuretics: distal effects on electrolyte transport and acidification. Kidney Int. 1985 Sep;28(3):477–489. doi: 10.1038/ki.1985.154. [DOI] [PubMed] [Google Scholar]
- Llach F., Weidmann P., Reinhart R., Maxwell M. H., Coburn J. W., Massry S. G. Effect of acute and long-standing hypocalcemia on blood pressure and plasma renin activity in man. J Clin Endocrinol Metab. 1974 May;38(5):841–847. doi: 10.1210/jcem-38-5-841. [DOI] [PubMed] [Google Scholar]
- PULLMAN T. N., ALVING A. S., DERN R. J., LANDOWNE M. The influence of dietary protein intake on specific renal functions in normal man. J Lab Clin Med. 1954 Aug;44(2):320–332. [PubMed] [Google Scholar]
- Peart W. S. Renin release. Gen Pharmacol. 1978;9(2):65–72. doi: 10.1016/0306-3623(78)90001-0. [DOI] [PubMed] [Google Scholar]
- Peart W. S., Roddis S. A., Unwin R. J. Renal electrolyte excretion and renin release during calcium and parathormone infusions in conscious rabbits. J Physiol. 1986 Apr;373:329–341. doi: 10.1113/jphysiol.1986.sp016050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H. Mechanisms of action of hormonal and neuronal peptides on exocrine gland cells. Br Med Bull. 1982 Sep;38(3):297–302. doi: 10.1093/oxfordjournals.bmb.a071776. [DOI] [PubMed] [Google Scholar]
- Premen A. J., Hall J. E., Smith M. J., Jr Postprandial regulation of renal hemodynamics: role of pancreatic glucagon. Am J Physiol. 1985 May;248(5 Pt 2):F656–F662. doi: 10.1152/ajprenal.1985.248.5.F656. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Goltermann N., Larsson L. I., Emson P. M., Lee C. M. Gastrin and cholecystokinin in central and peripheral neurons. Fed Proc. 1979 Aug;38(9):2325–2329. [PubMed] [Google Scholar]
- Rehfeld J. F., Hansen H. F., Marley P. D., Stengaard-Pedersen K. Molecular forms of cholecystokinin in the brain and the relationship to neuronal gastrins. Ann N Y Acad Sci. 1985;448:11–23. doi: 10.1111/j.1749-6632.1985.tb29902.x. [DOI] [PubMed] [Google Scholar]
- Reinhardt H. W., Kaczmarczyk G., Fahrenhorst K., Blendinger I., Gatzka M., Kuhl U., Riedel J. Postprandial changes of renal blood flow. Studies on conscious dogs on a high and low sodium intake. Pflugers Arch. 1975;354(4):287–297. doi: 10.1007/BF00587848. [DOI] [PubMed] [Google Scholar]
- Smith J. M., Mouw D. R., Vander A. J. Effect of parathyroid hormone on plasma renin activity and sodium excretion. Am J Physiol. 1979 Mar;236(3):F311–F319. doi: 10.1152/ajprenal.1979.236.3.F311. [DOI] [PubMed] [Google Scholar]
- Stulberg B., Norberg H. P., Kaplan E. L. Cholecystokinin, a new hypocalcemic agent. Surg Forum. 1976;27(62):430–431. [PubMed] [Google Scholar]
- Vantini I., Cominacini L., Piubello W., Ghidini O., Fattovich G., Ederle A., Benini L., Cocchetto R., Cavallini G., LoCascio V. Effect of exogenous gastrointestinal peptides containing the C-terminal tetrapeptide of gastrin on calcium, calcitonin and parathormone serum levels in man. Hepatogastroenterology. 1981 Feb;28(1):43–48. [PubMed] [Google Scholar]
- de Rouffignac C., Elalouf J. M. Effects of calcitonin on the renal concentrating mechanism. Am J Physiol. 1983 Oct;245(4):F506–F511. doi: 10.1152/ajprenal.1983.245.4.F506. [DOI] [PubMed] [Google Scholar]


