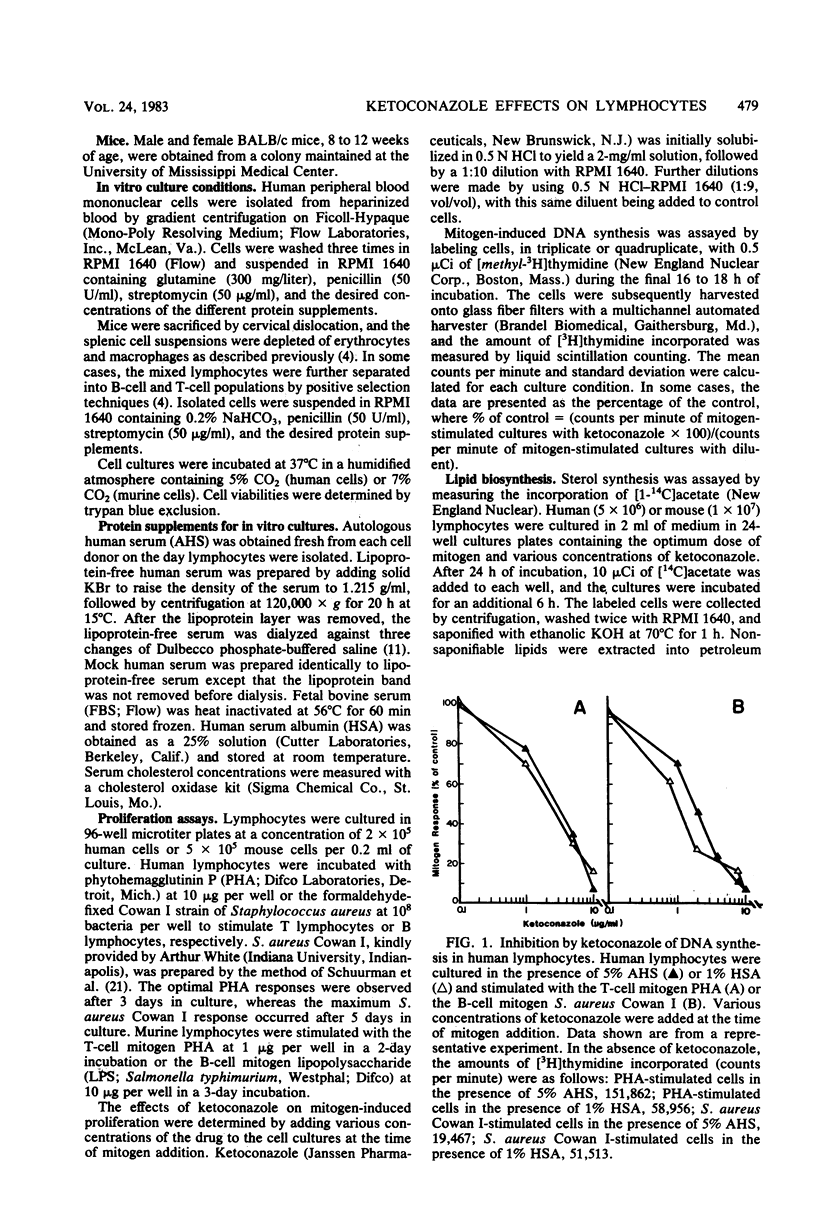
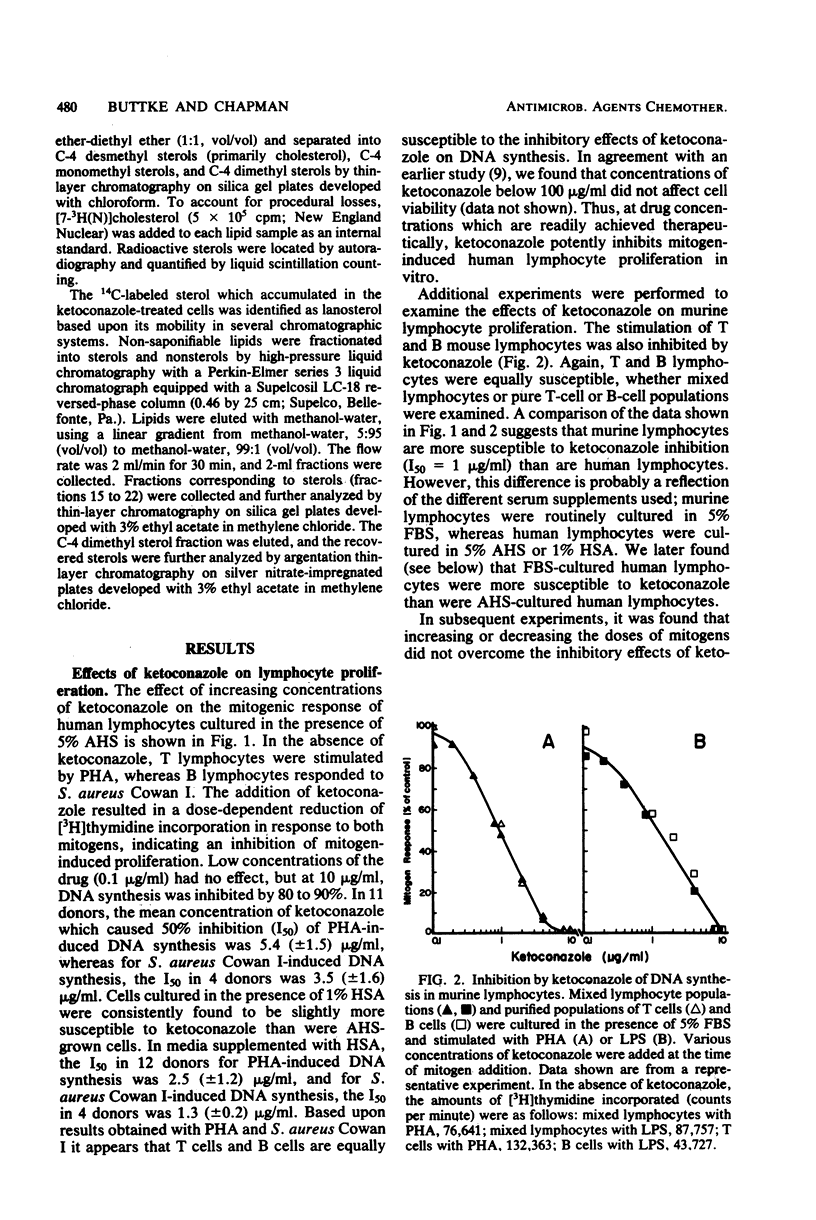
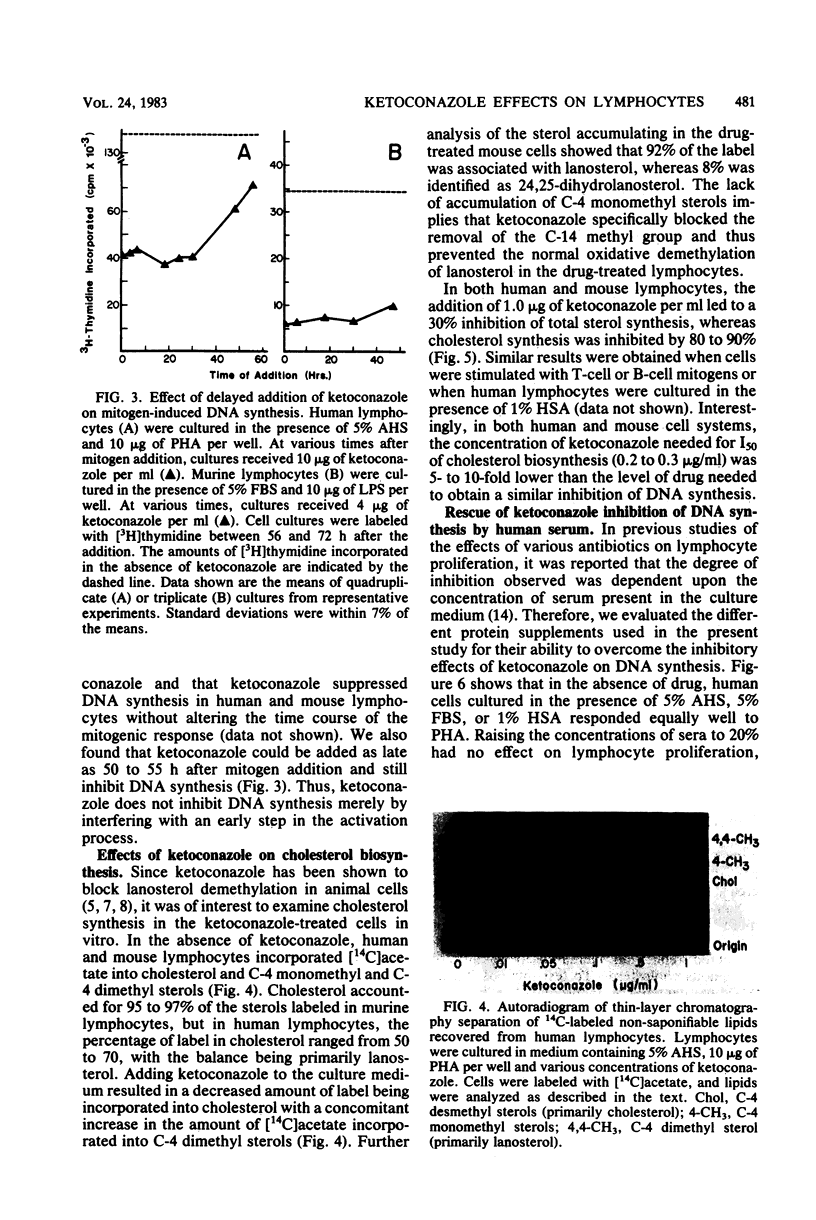
Abstract
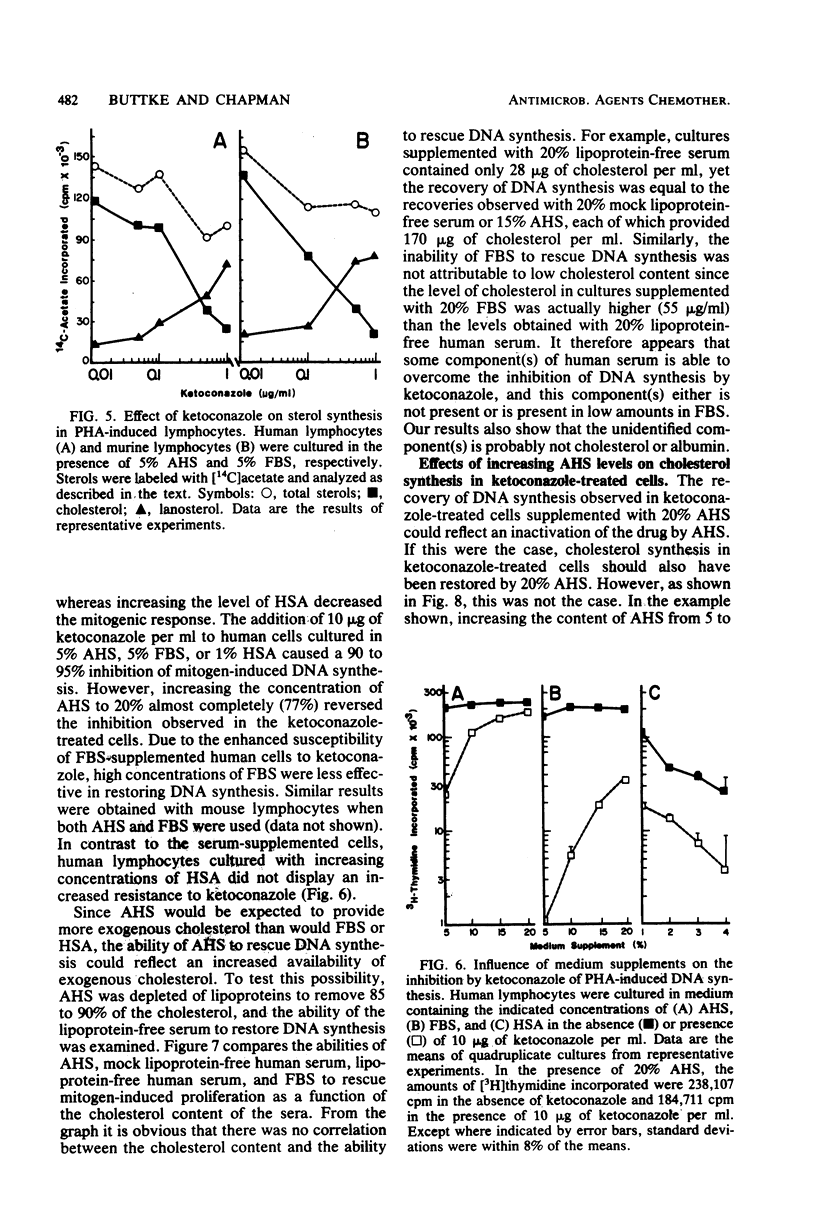
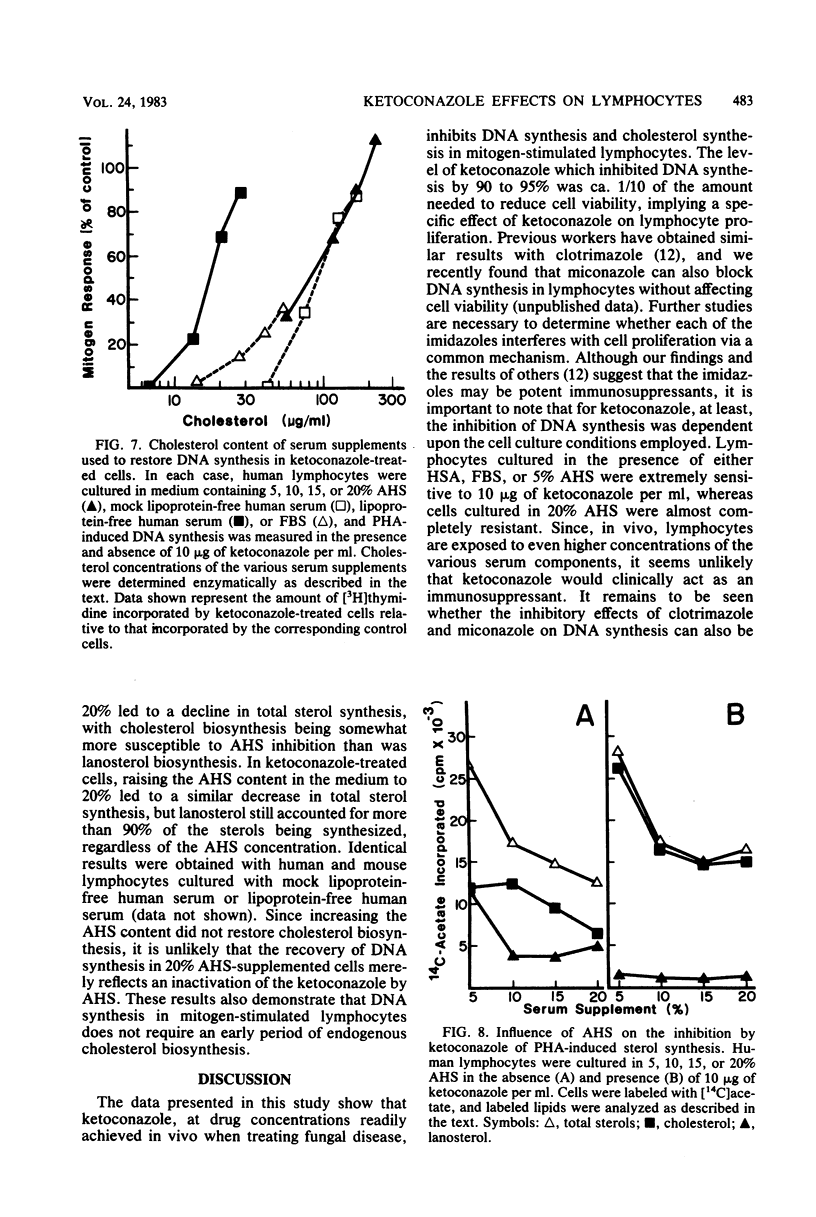
The effects of ketoconazole on mitogen-induced DNA synthesis and cholesterol biosynthesis in human and murine lymphocytes have been examined. Ketoconazole concentrations which do not affect cell viability (0.1 to 10 micrograms/ml) in culture led to a dose-dependent inhibition of DNA synthesis, as measured by [3H]thymidine incorporation, induced by either T-cell or B-cell mitogens. At drug concentrations 5- to 10-fold lower, ketoconazole inhibited the incorporation of [14C]acetate into cholesterol, with a resultant accumulation of [14C]lanosterol. The suppressive effects of ketoconazole on DNA synthesis were reversed by increasing the concentration of human serum in the culture medium from 5 to 20%. The depletion of lipoproteins in human serum by density centrifugation reduced the cholesterol content by 90% but did not affect the ability of the serum to overcome the inhibition by ketoconazole of DNA synthesis. Unlike DNA synthesis, cholesterol biosynthesis was not restored by 20% fresh human serum or lipoprotein-depleted human serum. These results demonstrate that ketoconazole potently inhibits DNA synthesis and cholesterol synthesis in mitogen-stimulated lymphocytes at drug concentrations obtained therapeutically. Further, the uncoupling of endogenous cholesterol synthesis and DNA synthesis indicates at least two levels of action of ketoconazole in mammalian lymphocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astruc M., Laporte M., Tabacik C., Crastes de Paulet A. Effect of oxygenated sterols on 3-hydroxy-3-methylglutaryl coenzyme A reductase and DNA synthesis in phytohemagglutinin-stimulated human lymphocytes. Biochem Biophys Res Commun. 1978 Nov 29;85(2):691–700. doi: 10.1016/0006-291x(78)91217-2. [DOI] [PubMed] [Google Scholar]
- Beggs W. H., Andrews F. A., Sarosi G. A. Action of imidazole-containing antifungal drugs. Life Sci. 1981 Jan 12;28(2):111–118. doi: 10.1016/0024-3205(81)90542-7. [DOI] [PubMed] [Google Scholar]
- Borgers M. Mechanism of action of antifungal drugs, with special reference to the imidazole derivatives. Rev Infect Dis. 1980 Jul-Aug;2(4):520–534. doi: 10.1093/clinids/2.4.520. [DOI] [PubMed] [Google Scholar]
- Buttke T. M., Mallett G. S., Cuchens M. A. Positive selection of mouse B and T lymphocytes and analysis of isolated populations by flow cytometry. J Immunol Methods. 1983 Mar 11;58(1-2):193–207. doi: 10.1016/0022-1759(83)90275-2. [DOI] [PubMed] [Google Scholar]
- Chang T. Y., Telakowski C., Heuvel W. V., Alberts A. W., Vagelos P. R. Isolation and partial characterization of a cholesterol-requiring mutant of Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):832–836. doi: 10.1073/pnas.74.3.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. W., Heiniger H. J., Kandutsch A. A. Relationship between sterol synthesis and DNA synthesis in phytohemagglutinin-stimulated mouse lymphocytes. Proc Natl Acad Sci U S A. 1975 May;72(5):1950–1954. doi: 10.1073/pnas.72.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert J. A., Lipsky P. E. Sterol metabolism and lymphocyte function: inhibition of endogenous sterol biosynthesis does not prevent mitogen-induced human T lymphocyte activation. J Immunol. 1980 May;124(5):2240–2246. [PubMed] [Google Scholar]
- Cuthbert J. A., Lipsky P. E. Sterol metabolism and lymphocyte responsiveness: inhibition of endogenous sterol synthesis prevents mitogen-induced human T cell proliferation. J Immunol. 1981 Jun;126(6):2093–2099. [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defay R., Astruc M. E., Roussillon S., Descomps B., Crastes de Paulet A. DNA synthesis and 3-hydroxy-3-methylglutaryl CoA reductase activity in PHA stimulated human lymphocytes: a comparative study of the inhibitory effects of some oxysterols with special reference to side chain hydroxylated derivatives. Biochem Biophys Res Commun. 1982 May 31;106(2):362–372. doi: 10.1016/0006-291x(82)91118-4. [DOI] [PubMed] [Google Scholar]
- Gow P. J., Corrigall V., Panayi G. S. The effect of clotrimazole on human lymphocyte responsiveness to plant mitogens. Agents Actions. 1979 Dec;9(5-6):543–548. doi: 10.1007/BF01968125. [DOI] [PubMed] [Google Scholar]
- Grosso D. S., Boyden T. W., Pamenter R. W., Johnson D. G., Stevens D. A., Galgiani J. N. Ketoconazole inhibition of testicular secretion of testosterone and displacement of steroid hormones from serum transport proteins. Antimicrob Agents Chemother. 1983 Feb;23(2):207–212. doi: 10.1128/aac.23.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser W. E., Jr, Remington J. S. Effect of antibiotics on the immune response. Am J Med. 1982 May;72(5):711–716. doi: 10.1016/0002-9343(82)90534-4. [DOI] [PubMed] [Google Scholar]
- Hidaka K., Akiyama S. I., Kuwano M. Growth of amphotericin B-resistant hamster cell line requires exogenous cholesterol. Exp Cell Res. 1980 Jul;128(1):215–221. doi: 10.1016/0014-4827(80)90405-x. [DOI] [PubMed] [Google Scholar]
- Marriott M. S. Inhibition of sterol biosynthesis in Candida albicans by imidazole-containing antifungals. J Gen Microbiol. 1980 Mar;117(1):253–255. doi: 10.1099/00221287-117-1-253. [DOI] [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Beck J. P., Ourisson G. Regulation of sterol biosynthesis and lysis of cultured hepatoma cells: inhibition of lanosterol demethylation by hydroxysterols. Biochem Biophys Res Commun. 1979 Oct 12;90(3):897–903. doi: 10.1016/0006-291x(79)91912-0. [DOI] [PubMed] [Google Scholar]
- Schuurman R. K., Gelfand E. W., Dosch H. M. Polyclonal activation of human lymphocytes in vitro. I. Characterization of the lymphocyte response to a T cell-independent B cell mitogen. J Immunol. 1980 Aug;125(2):820–826. [PubMed] [Google Scholar]
- Uno J., Shigematsu M. L., Arai T. Primary site of action of ketoconazole on Candida albicans. Antimicrob Agents Chemother. 1982 Jun;21(6):912–918. doi: 10.1128/aac.21.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bossche H., Willemsens G., Cools W., Cornelissen F. Inhibition of ergosterol synthesis in Candida albicans by ketoconazole [proceedings]. Arch Int Physiol Biochim. 1979 Oct;87(4):849–851. [PubMed] [Google Scholar]
- Van den Bossche H., Willemsens G., Cools W., Cornelissen F., Lauwers W. F., van Cutsem J. M. In vitro and in vivo effects of the antimycotic drug ketoconazole on sterol synthesis. Antimicrob Agents Chemother. 1980 Jun;17(6):922–928. doi: 10.1128/aac.17.6.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brabander M., Aerts F., van Cutsem J., van den Bossche H., Borgers M. The activity of ketoconazole in mixed cultures of leukocytes and Candida albicans. Sabouraudia. 1980 Sep;18(3):197–210. doi: 10.1080/00362178085380351. [DOI] [PubMed] [Google Scholar]
- van den Bossche H., Willemsens G., Cools W., Lauwers W. F., Le Jeune L. Biochemical effects of miconazole on fungi. II. Inhibition of ergosterol biosynthesis in Candida albicans. Chem Biol Interact. 1978 Apr;21(1):59–78. doi: 10.1016/0009-2797(78)90068-6. [DOI] [PubMed] [Google Scholar]