Abstract
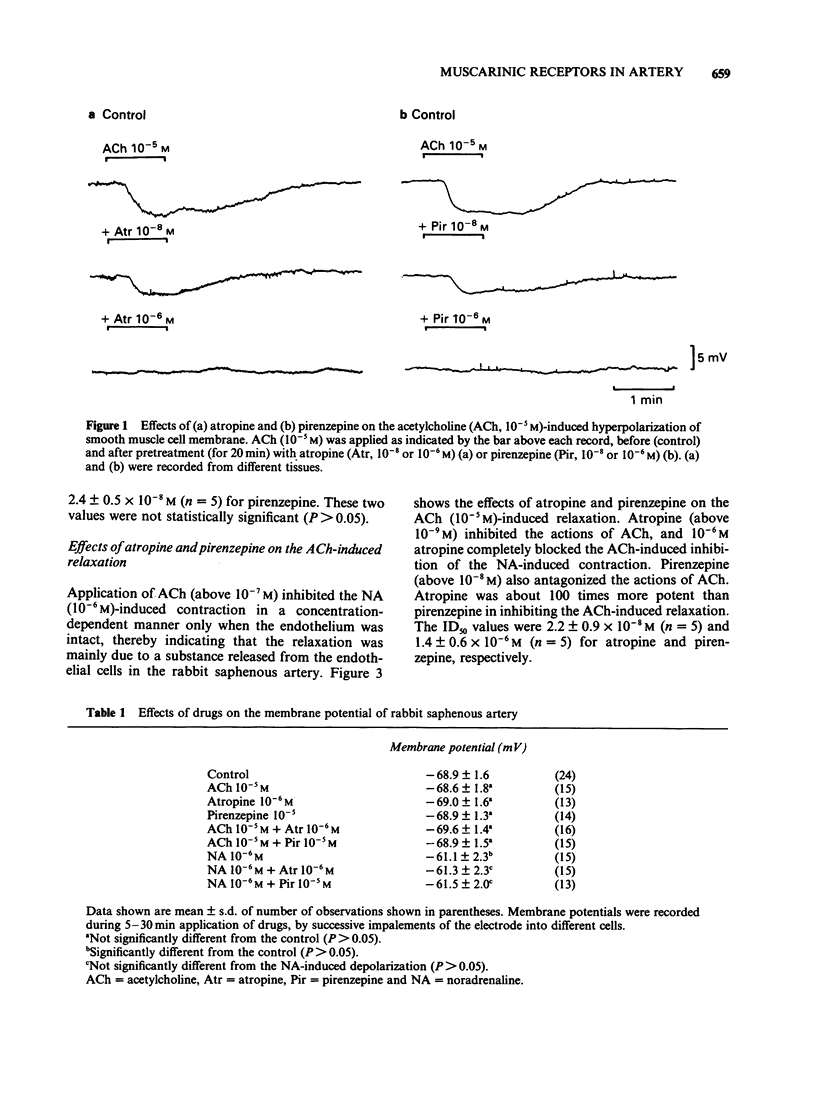
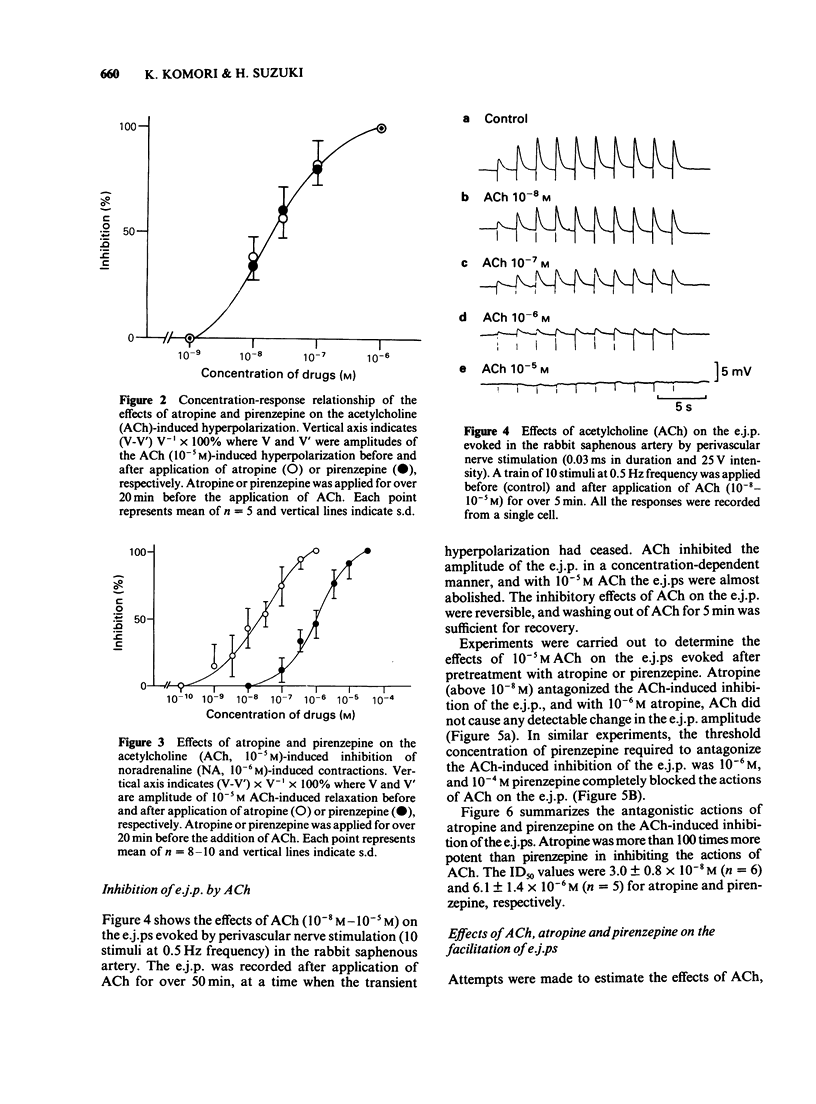
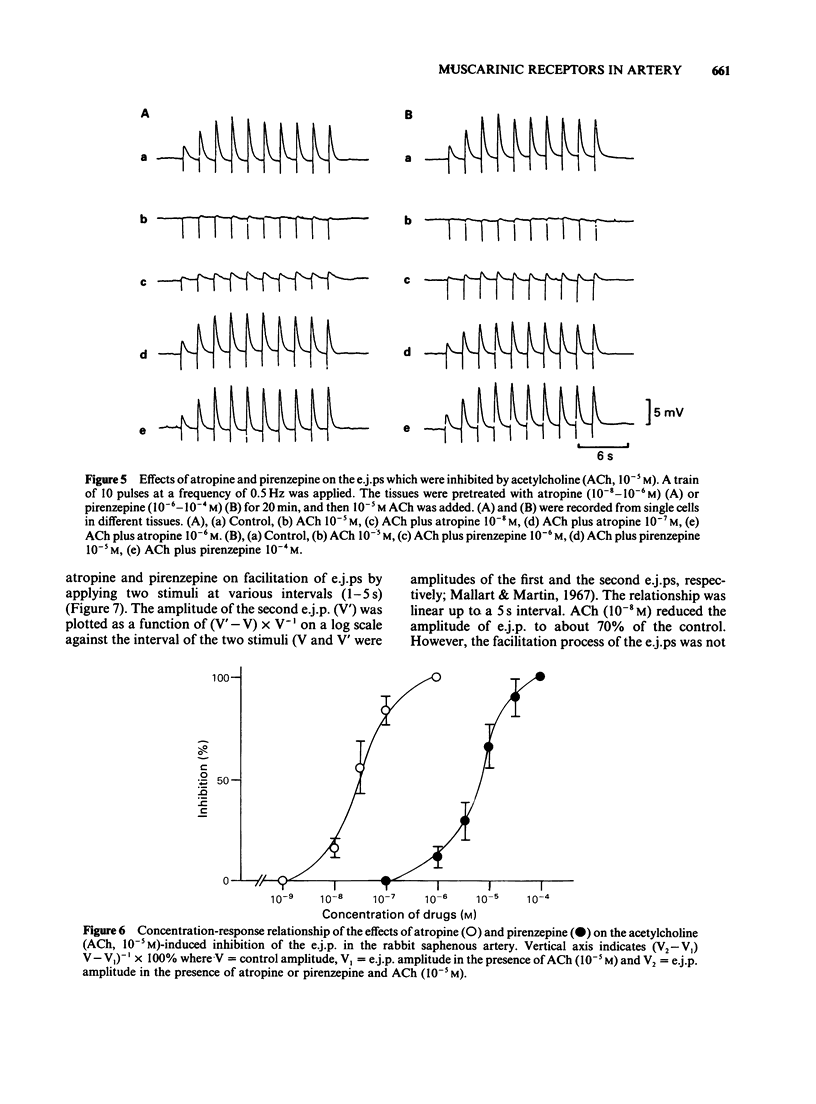
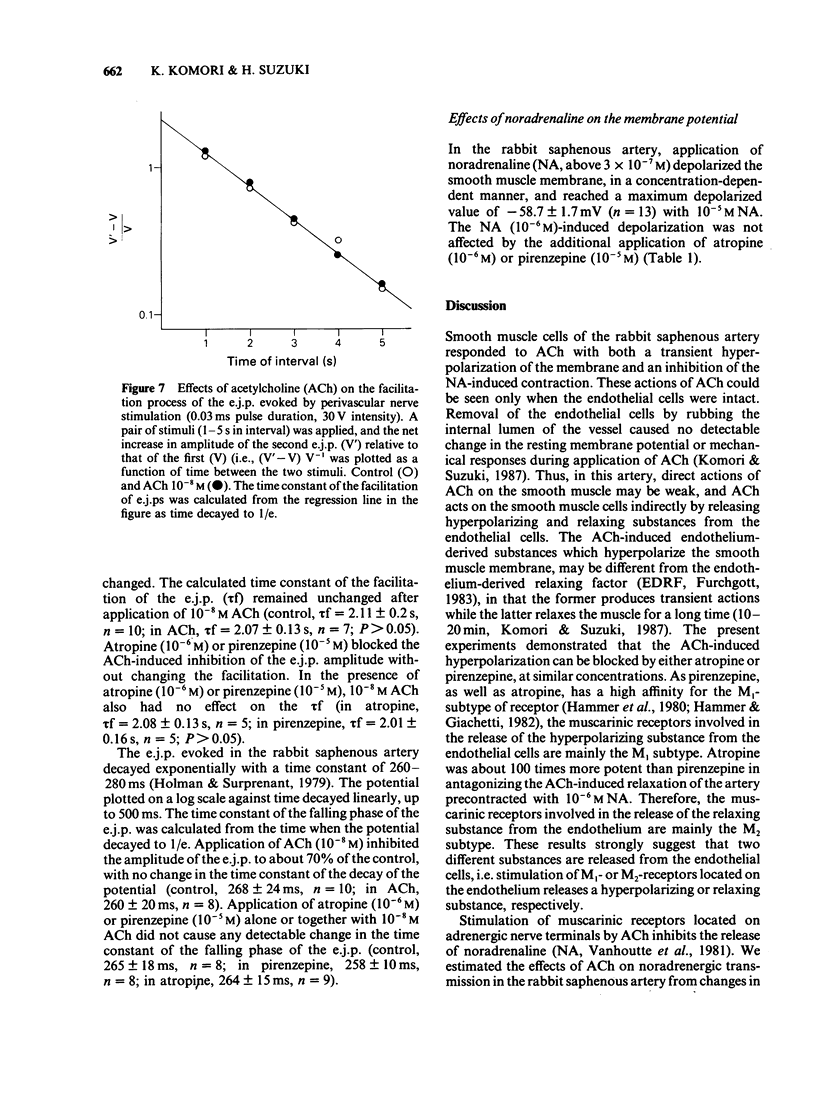
1. The properties of the muscarinic receptors in the rabbit saphenous artery were determined from electrical and mechanical responses of smooth muscle cells produced by acetylcholine (ACh). The inhibitory action of atropine and pirenzepine on the ACh-induced responses was also studied. 2. ACh produced a transient hyperpolarization of the membrane and inhibited the noradrenaline (NA)-induced contraction. These effects of ACh were apparent only when the endothelial cells were intact. 3. The ACh-induced transient hyperpolarization was antagonized by atropine or pirenzepine, with similar potencies (the ID50 values were about 2 x 10(-8) M for both antagonists). 4. The ACh-induced inhibition of the contraction to NA was antagonized by atropine more preferentially than by pirenzepine (the ID50 values were 2 x 10(-8) M for atropine and 10(-6) M for pirenzepine). 5. The excitatory junction potential (e.j.p.) evoked by perivascular nerve stimulation was inhibited by ACh (above 10(-8) M). The ACh-induced inhibition of the e.j.p. was antagonized by atropine more preferentially than by pirenzepine (the ID50 values were 3 x 10(-8) M for atropine and 6 x 10(-6) M for pirenzepine). 6. It is concluded that in the rabbit saphenous artery, two subtypes of muscarinic receptor (M1 and M2) are located on the endothelial cells. Stimulation of each subtype releases a different substance, i.e., a hyperpolarizing substance (M1-subtype) or a relaxant substance (M2-subtype). In prejunctional nerve terminals, the muscarinic receptors responsible for inhibiting the release of transmitter substances are of the M2-subtype.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow R. B., Chan M. The effects of pH on the affinity of pirenzepine for muscarinic receptors in the guinea-pig ileum and rat fundus strip. Br J Pharmacol. 1982 Nov;77(3):559–563. doi: 10.1111/j.1476-5381.1982.tb09331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol. 1984 Jun;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden J. E., Large W. A. Electrophysiological analysis of neurogenic vasodilatation in the isolated lingual artery of the rabbit. Br J Pharmacol. 1986 Sep;89(1):163–171. doi: 10.1111/j.1476-5381.1986.tb11132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Forward A., Marsh S. Antagonist discrimination between ganglionic and ileal muscarinic receptors. Br J Pharmacol. 1980;71(2):362–364. doi: 10.1111/j.1476-5381.1980.tb10948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuder H., Rink D., Muscholl E. Sympathetic Nerve Stimulation on the perfused rat heart. Affinities of N-methylatropine and pirenzepine at pre- and postsynaptic muscarine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1982 Feb;318(3):210–219. doi: 10.1007/BF00500482. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Goyal R. K., Rattan S. Neurohumoral, hormonal, and drug receptors for the lower esophageal sphincter. Gastroenterology. 1978 Mar;74(3):598–619. [PubMed] [Google Scholar]
- Hammer R., Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980 Jan 3;283(5742):90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- Hammer R., Giachetti A. Muscarinic receptor subtypes: M1 and M2 biochemical and functional characterization. Life Sci. 1982 Dec 27;31(26):2991–2998. doi: 10.1016/0024-3205(82)90066-2. [DOI] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. M. Some properties of the excitatory junction potentials recorded from saphenous arteries of rabbits. J Physiol. 1979 Feb;287:337–351. doi: 10.1113/jphysiol.1979.sp012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbinger H., Halim S., Lambrecht G., Weiler W., Wessler I. Comparison of affinities of muscarinic antagonists to pre- and postjunctional receptors in the guinea-pig ileum. Eur J Pharmacol. 1984 Aug 17;103(3-4):313–320. doi: 10.1016/0014-2999(84)90492-8. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Kuriyama H. Effects of acetylcholine on the smooth muscle cell of isolated main coronary artery of the guinea-pig. J Physiol. 1979 Aug;293:119–133. doi: 10.1113/jphysiol.1979.sp012881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Ito Y., Suzuki H., Kitamura K., Itoh T. Factors modifying contraction-relaxation cycle in vascular smooth muscles. Am J Physiol. 1982 Nov;243(5):H641–H662. doi: 10.1152/ajpheart.1982.243.5.H641. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. Adrenergic transmissions in the guinea-pig mesenteric artery and their cholinergic modulations. J Physiol. 1981 Aug;317:383–396. doi: 10.1113/jphysiol.1981.sp013831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. The effects of acetylcholine on the membrane and contractile properties of smooth muscle cells of the rabbit superior mesenteric artery. Br J Pharmacol. 1978 Dec;64(4):493–501. doi: 10.1111/j.1476-5381.1978.tb17310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara H., Suzuki H. Effects of tyramine on noradrenaline outflow and electrical responses induced by field stimulation in the perfused rabbit ear artery. Br J Pharmacol. 1985 Oct;86(2):405–416. doi: 10.1111/j.1476-5381.1985.tb08910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Slack B. E., Surprenant A. Muscarinic M1 and M2 receptors mediate depolarization and presynaptic inhibition in guinea-pig enteric nervous system. J Physiol. 1985 Nov;368:435–452. doi: 10.1113/jphysiol.1985.sp015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H. Adrenergic transmission in the dog mesenteric vein and its modulation by alpha-adrenoceptor antagonists. Br J Pharmacol. 1984 Mar;81(3):479–489. doi: 10.1111/j.1476-5381.1984.tb10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szelenyi I. Does pirenzepine distinguish between 'subtypes' of muscarinic receptors? Br J Pharmacol. 1982 Dec;77(4):567–569. doi: 10.1111/j.1476-5381.1982.tb09332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata Y. Regional differences in electrical and mechanical properties of guinea-pig mesenteric vessels. Jpn J Physiol. 1980;30(5):709–728. doi: 10.2170/jjphysiol.30.709. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M. Inhibition by acetylcholine of adrenergic neurotransmission in vascular smooth muscle. Circ Res. 1974 Mar;34(3):317–326. doi: 10.1161/01.res.34.3.317. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Lorenz R. R., Tyce G. M. Inhibition of norepinephrine- 3 H release from sympathetic nerve endings in veins by acetylcholine. J Pharmacol Exp Ther. 1973 May;185(2):386–394. [PubMed] [Google Scholar]
- Vanhoutte P. M., Verbeuren T. J., Webb R. C. Local modulation of adrenergic neuroeffector interaction in the blood vessel well. Physiol Rev. 1981 Jan;61(1):151–247. doi: 10.1152/physrev.1981.61.1.151. [DOI] [PubMed] [Google Scholar]


