Abstract
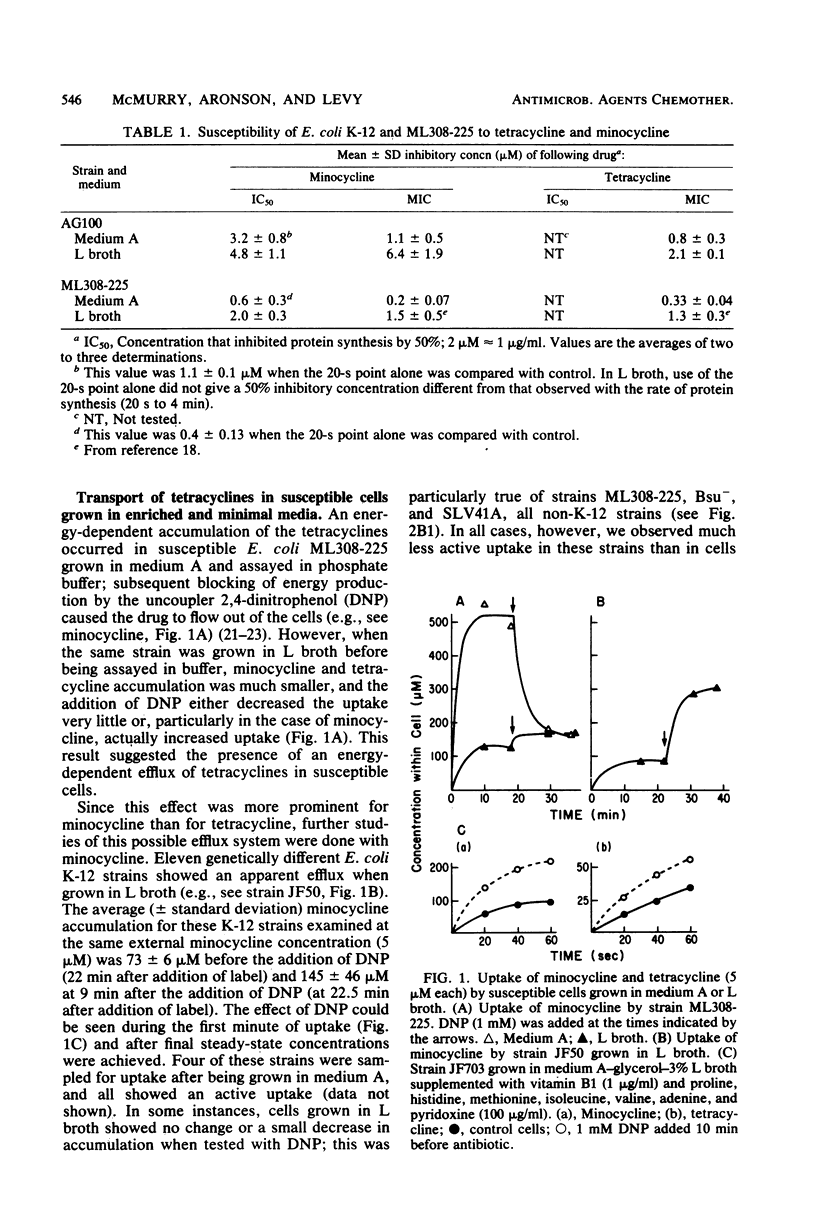
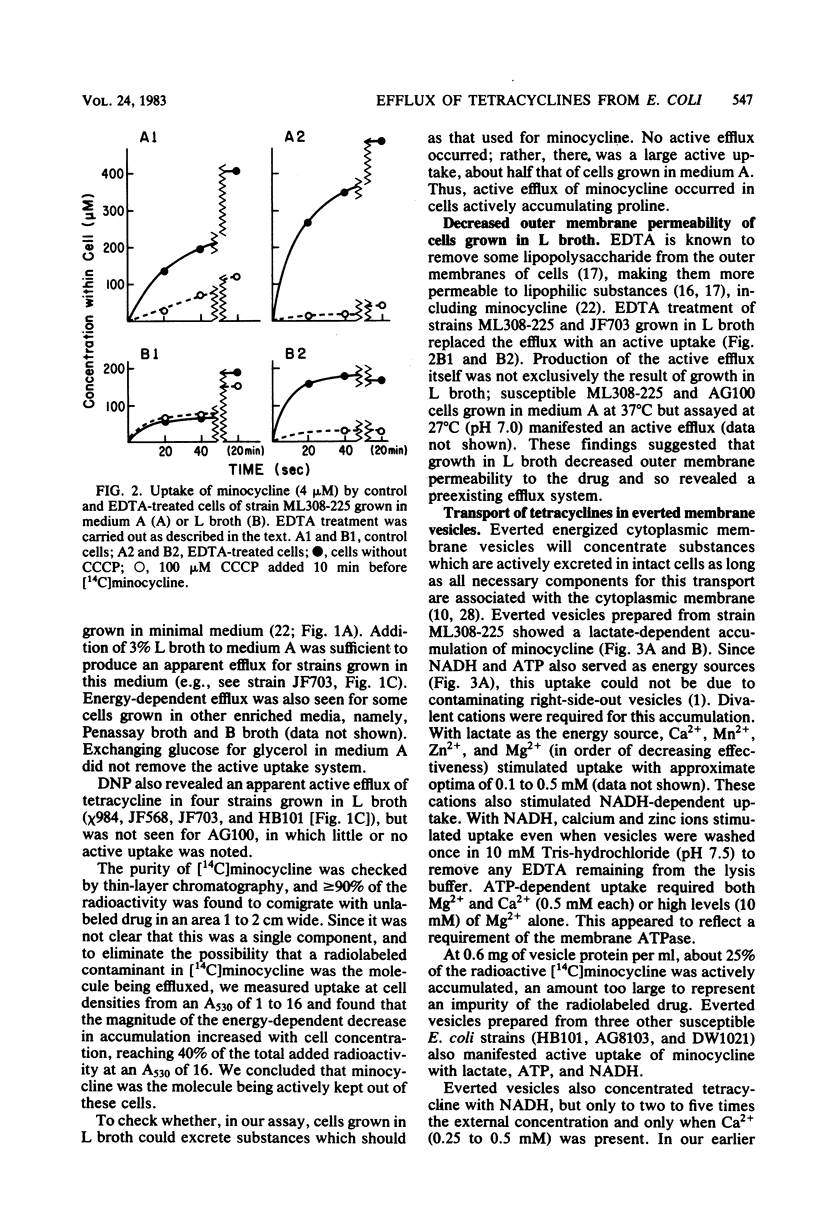
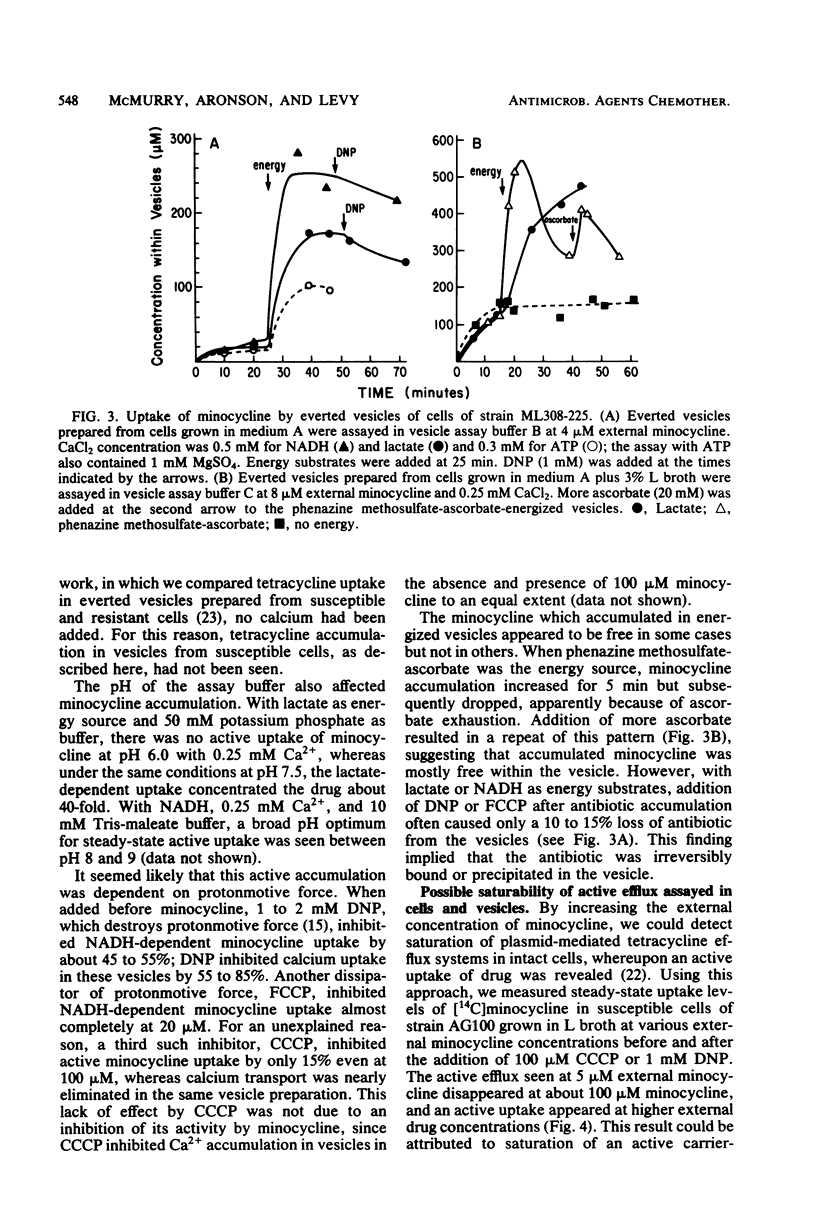
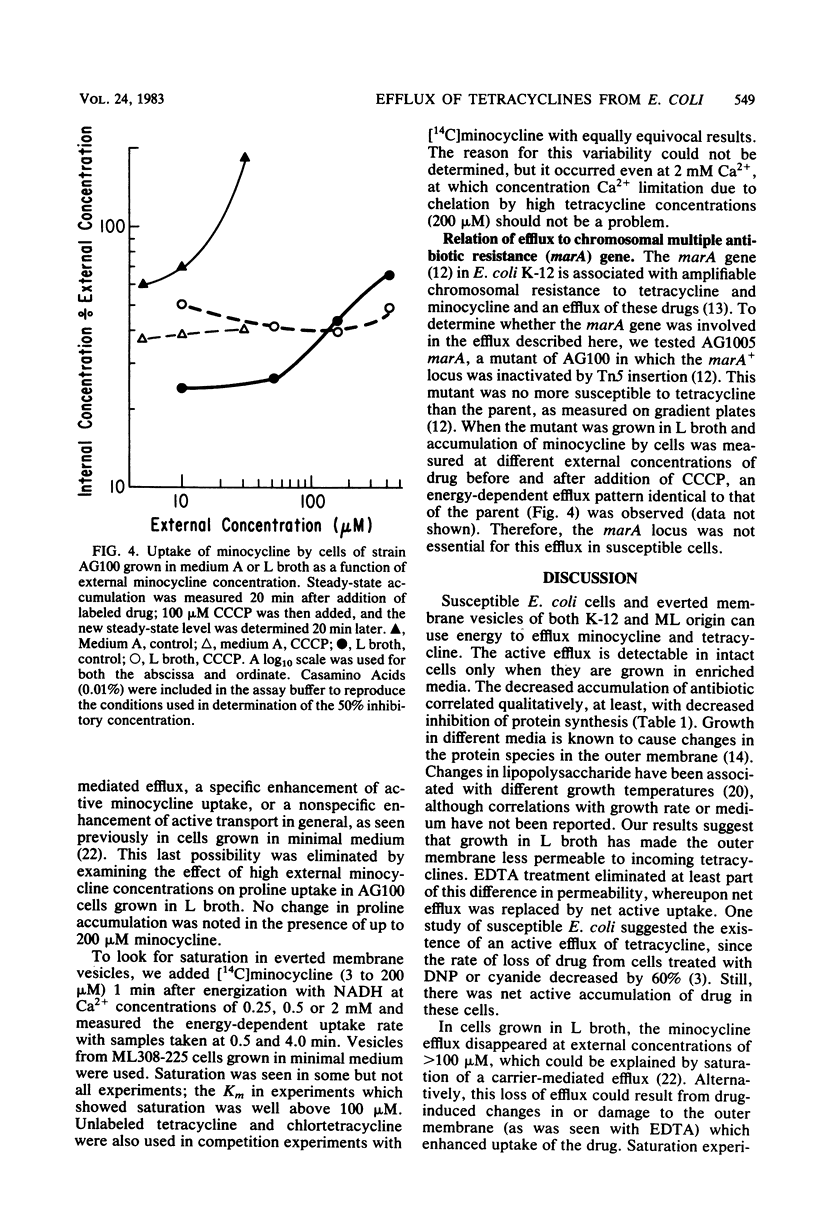
Escherichia coli shows severalfold less susceptibility to tetracyclines when grown in enriched medium than in minimal medium. Transport studies with cells harvested from these media showed different handling of the drugs. Whereas an energy-dependent uptake of tetracycline and minocycline was observed in susceptible K-12 and wild-type E. coli strains grown in minimal medium, an active efflux of minocycline and, to a lesser extent, tetracycline was seen in cells grown in L broth and other enriched media. This efflux was replaced by an active uptake system after treatment of cells grown in L broth with EDTA. When assayed at a lower temperature (27 degrees C), even cells grown in minimal medium showed an efflux of minocycline. Everted membrane vesicles prepared from susceptible cells grown in minimal medium or L broth showed an energy-dependent accumulation of minocycline and tetracycline when supplied with certain divalent cations. These results suggest that an active efflux of tetracyclines occurs in susceptible E. coli but is not detected in cells grown in minimal medium because greater permeability of the outer membrane allows a more rapid active uptake. This efflux system is distinct from that specified by tetracycline resistance determinants. Since the active efflux of minocycline in cells grown in L broth disappeared at external antibiotic concentrations of greater than 100 microM, it may be saturable and so mediated by a membrane carrier.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler L. W., Rosen B. P. Functional mosaicism of membrane proteins in vesicles of Escherichia coli. J Bacteriol. 1977 Feb;129(2):959–966. doi: 10.1128/jb.129.2.959-966.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asleson G. L., Frank C. W. pH dependence of carbon-13 nuclear magnetic resonance shifts of tetracycline. Microscopic dissociation constants. J Am Chem Soc. 1976 Aug 4;98(16):4745–4749. doi: 10.1021/ja00432a009. [DOI] [PubMed] [Google Scholar]
- Ball P. R., Shales S. W., Chopra I. Plasmid-mediated tetracycline resistance in Escherichia coli involves increased efflux of the antibiotic. Biochem Biophys Res Commun. 1980 Mar 13;93(1):74–81. doi: 10.1016/s0006-291x(80)80247-6. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brey R. N., Rosen B. P. Cation/proton antiport systems in Escherichia coli. Properties of the calcium/proton antiporter. J Biol Chem. 1979 Mar 25;254(6):1957–1963. [PubMed] [Google Scholar]
- Brown J. R., Ireland D. S. Structural requirements for tetracycline activity. Adv Pharmacol Chemother. 1978;15:161–202. doi: 10.1016/s1054-3589(08)60483-4. [DOI] [PubMed] [Google Scholar]
- Caswell A. H., Hutchison J. D. Visualization of membrane bound cations by a fluorescent technique. Biochem Biophys Res Commun. 1971 Jan 8;42(1):43–49. doi: 10.1016/0006-291x(71)90359-7. [DOI] [PubMed] [Google Scholar]
- George A. M., Levy S. B. Amplifiable resistance to tetracycline, chloramphenicol, and other antibiotics in Escherichia coli: involvement of a non-plasmid-determined efflux of tetracycline. J Bacteriol. 1983 Aug;155(2):531–540. doi: 10.1128/jb.155.2.531-540.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A. M., Levy S. B. Gene in the major cotransduction gap of the Escherichia coli K-12 linkage map required for the expression of chromosomal resistance to tetracycline and other antibiotics. J Bacteriol. 1983 Aug;155(2):541–548. doi: 10.1128/jb.155.2.541-548.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the major outer membrane proteins of Escherichia coli. Annu Rev Genet. 1981;15:91–142. doi: 10.1146/annurev.ge.15.120181.000515. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Plasmid-determined tetracycline resistance involves new transport systems for tetracycline. Nature. 1978 Nov 2;276(5683):90–92. doi: 10.1038/276090a0. [DOI] [PubMed] [Google Scholar]
- McConnell M., Wright A. Variation in the structure and bacteriophage-inactivating capacity of Salmonella anatum lipopolysaccharide as a function of growth temperature. J Bacteriol. 1979 Feb;137(2):746–751. doi: 10.1128/jb.137.2.746-751.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L. M., Cullinane J. C., Levy S. B. Transport of the lipophilic analog minocycline differs from that of tetracycline in susceptible and resistant Escherichia coli strains. Antimicrob Agents Chemother. 1982 Nov;22(5):791–799. doi: 10.1128/aac.22.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L., Levy S. B. Two transport systems for tetracycline in sensitive Escherichia coli: critical role for an initial rapid uptake system insensitive to energy inhibitors. Antimicrob Agents Chemother. 1978 Aug;14(2):201–209. doi: 10.1128/aac.14.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L., Petrucci R. E., Jr, Levy S. B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Rosen B. P. Energetics of plasmid-mediated arsenate resistance in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6119–6122. doi: 10.1073/pnas.79.20.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. C., Frank C. W. Circular dichroism spectra of tetracycline complexes with Mg+2 and Ca+2. J Pharm Sci. 1976 Dec;65(12):1728–1732. doi: 10.1002/jps.2600651209. [DOI] [PubMed] [Google Scholar]
- Rosen B. P., Tsuchiya T. Preparation of everted membrane vesicles from Escherichia coli for the measurement of calcium transport. Methods Enzymol. 1979;56:233–241. doi: 10.1016/0076-6879(79)56026-1. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Fishkes H. Sodium-proton antiport in isolated membrane vesicles of Escherichia coli. Biochemistry. 1978 Feb 21;17(4):706–711. doi: 10.1021/bi00597a023. [DOI] [PubMed] [Google Scholar]
- Schumacher G. E., Linn E. E. Kinetic and thermodynamic aspects of in vitro interphase transfer of tetracyclines II: influence of divalent metal salts. J Pharm Sci. 1978 Dec;67(12):1717–1720. doi: 10.1002/jps.2600671222. [DOI] [PubMed] [Google Scholar]
- Silver S., Keach D. Energy-dependent arsenate efflux: the mechanism of plasmid-mediated resistance. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6114–6118. doi: 10.1073/pnas.79.20.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stezowski J. J. Chemical-structural properties of tetracycline derivatives. 1. Molecular structure and conformation of the free base derivatives. J Am Chem Soc. 1976 Sep 15;98(19):6012–6018. doi: 10.1021/ja00435a039. [DOI] [PubMed] [Google Scholar]
- Tritton T. R. Ribosome-tetracycline interactions. Biochemistry. 1977 Sep 6;16(18):4133–4138. doi: 10.1021/bi00637a029. [DOI] [PubMed] [Google Scholar]
- Tynecka Z., Gos Z., Zajac J. Energy-dependent efflux of cadmium coded by a plasmid resistance determinant in Staphylococcus aureus. J Bacteriol. 1981 Aug;147(2):313–319. doi: 10.1128/jb.147.2.313-319.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


