Abstract
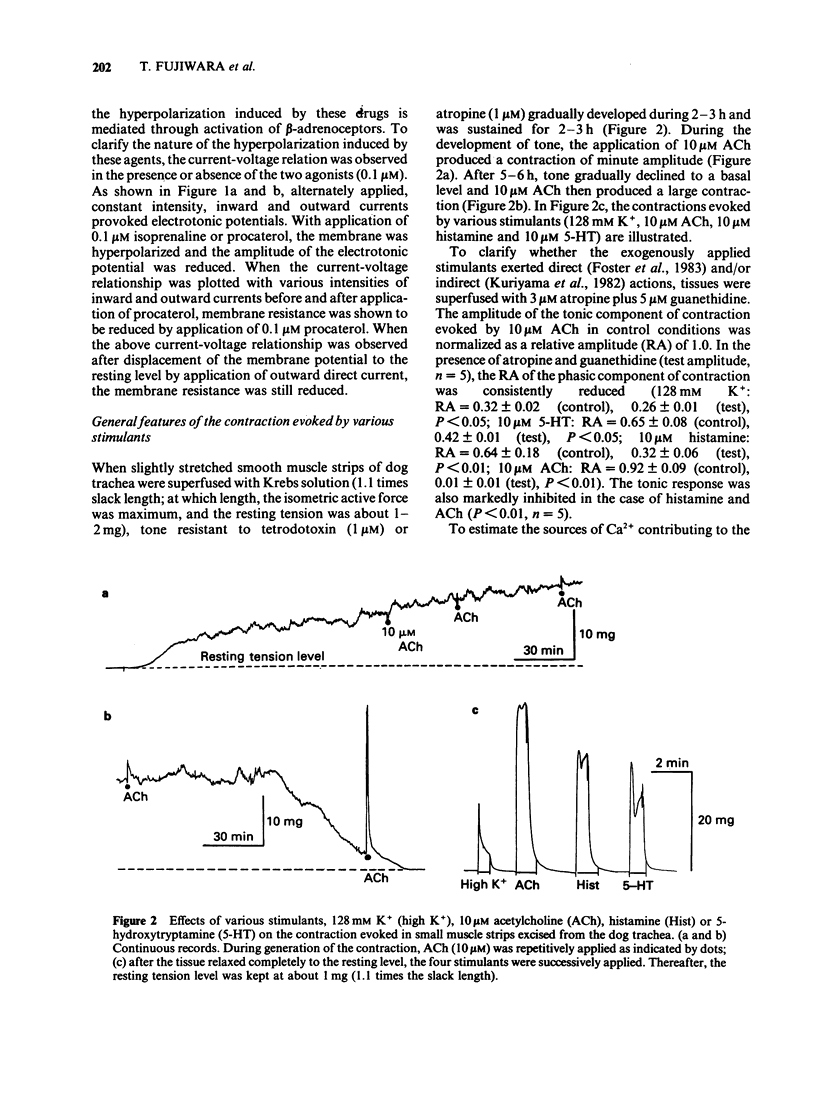
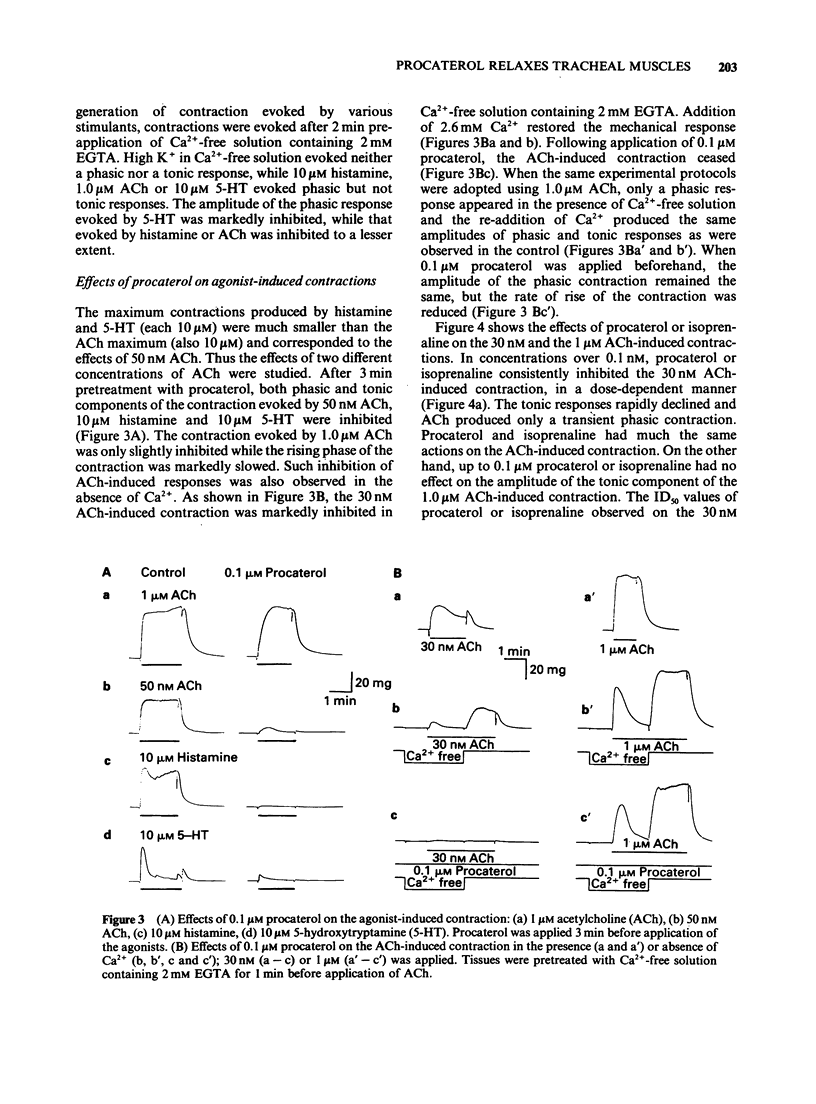
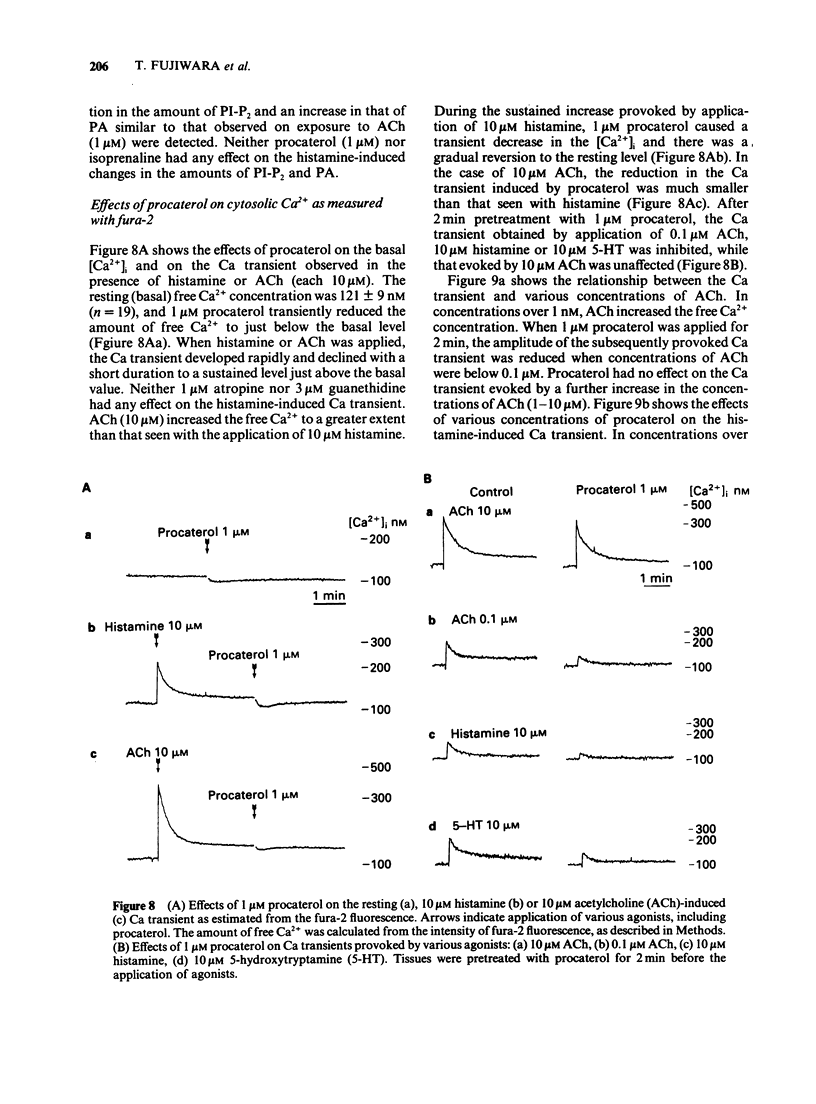
1. The effects of procaterol, a beta 2-adrenoceptor agonist, on smooth muscle cells of the dog trachea were investigated by use of microelectrode and isometric tension recording methods, and by measurement of Ca transients as estimated from the fura-2 fluorescence, adenosine 3':5'-cyclic monophosphate (cyclic AMP) and breakdown of phosphatidylinositols. 2. Procaterol hyperpolarized the membrane and increased the ionic conductance (above 10 nM) in a dose-dependent manner. These actions were inhibited by propranolol. 3. Procaterol inhibited the mechanical responses evoked by acetylcholine (ACh), histamine or 5-hydroxytryptamine (5-HT), in the presence or absence of Ca2+ in the bath solution, but not that evoked by high concentrations of ACh (1 microM). The ID50 value of procaterol for the peak amplitude of the ACh-induced contraction (30 nM) was 0.3 nM. The equivalent values for the histamine-induced phasic and tonic responses (10 microM) were 0.15 and 0.01 nM), respectively. 4. Procaterol (over 1 nM) increased the amount of cyclic AMP in a dose-dependent manner which was blocked by prior application of propranolol. 5. Procaterol did not alter the changes in the amounts of phosphatidylinositol 4,5-bisphosphate (PI-P2) and phosphatidic acid (PA) induced by ACh, histamine or 5-HT. Thus, the synthesis of inositol 1,4,5-trisphosphate is not affected by stimulation of the beta 2-adrenoceptor. 6. ACh increased the free Ca2+ concentration to a greater extent than that produced by histamine or 5-HT. These changes were reduced by procaterol, except for those induced by high concentrations of ACh (over 1 microM). 7. It is concluded that procaterol relaxes tissues precontracted by various agonists due to a reduction in the free Ca2+. This inhibitory action may be due to an increase in the amount of cyclic AMP but does not result from an inhibition of the hydrolysis of phosphatidyl inositols. The hyperpolarization induced by procaterol may partly contribute to the observed relaxation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. L., Beech D. J., Foster R. W., Morgan G. P., Small R. C. Electrophysiological and other aspects of the relaxant action of isoprenaline in guinea-pig isolated trachealis. Br J Pharmacol. 1985 Dec;86(4):843–854. doi: 10.1111/j.1476-5381.1985.tb11106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkowski K. R., Quinn P. Facilitation by beta-adrenoreceptors of stimulation-induced vasoconstriction in pithed spontaneously hypertensive rats. J Auton Pharmacol. 1984 Jun;4(2):127–131. doi: 10.1111/j.1474-8673.1984.tb00089.x. [DOI] [PubMed] [Google Scholar]
- Conti M. A., Adelstein R. S. Phosphorylation by cyclic adenosine 3':5'-monophosphate-dependent protein kinase regulates myosin light chain kinase. Fed Proc. 1980 Apr;39(5):1569–1573. [PubMed] [Google Scholar]
- Delhaye M., Taton G., Camus J. C., Chatelain P., Robberecht P., Waelbroeck M., Christophe J. Effects of full and partial beta-adrenergic agonists and antagonists on human lung adenylate cyclase. Biochem Pharmacol. 1983 Jun 15;32(12):1831–1835. doi: 10.1016/0006-2952(83)90046-1. [DOI] [PubMed] [Google Scholar]
- Foster R. W., Small R. C., Weston A. H. The spasmogenic action of potassium chloride in guinea-pig trachealis. Br J Pharmacol. 1983 Nov;80(3):553–559. doi: 10.1111/j.1476-5381.1983.tb10728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Ito Y. A role for inositol 1,4,5-trisphosphate in the initiation of agonist-induced contractions of dog tracheal smooth muscle. Br J Pharmacol. 1985 Sep;86(1):191–199. doi: 10.1111/j.1476-5381.1985.tb09449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata F., Noguchi Y., Ishikawa Y., Koda N., Ishida H. Supersensitivity of amylase secretion from rat parotid tissue--its selective nature for the beta 2-adrenergic response. Jpn J Pharmacol. 1985 Jan;37(1):129–132. doi: 10.1254/jjp.37.129. [DOI] [PubMed] [Google Scholar]
- Himori N., Taira N. Assessment of the selectivity of OPC-2009, a new beta2-adrenoceptor stimulatn, by the use of the blood-perfused trachea in situ and of the isolated blood-perfused papillary muscle of the dog. Br J Pharmacol. 1977 Sep;61(1):9–17. doi: 10.1111/j.1476-5381.1977.tb09734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Kuriyama H. Does activation of cyclic AMP dependent phosphorylation induced by beta-adrenergic agent control the tone of vascular muscle? J Physiol. 1980 Oct;307:143–161. doi: 10.1113/jphysiol.1980.sp013428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie Y., Igawa T., Hosokawa T., Saitoh Y. Alterations in blood levels of carbohydrate and lipid metabolites and of cyclic AMP mediated by beta1- and beta2-adrenoceptors in beagle dogs: effects of procaterol, a new selective beta2-adrenoceptor agonist. Eur J Pharmacol. 1979 Feb 1;53(4):351–358. doi: 10.1016/0014-2999(79)90459-x. [DOI] [PubMed] [Google Scholar]
- Ito Y., Itoh T. Effects of isoprenaline on the contraction-relaxation cycle in the cat trachea. Br J Pharmacol. 1984 Nov;83(3):677–686. doi: 10.1111/j.1476-5381.1984.tb16221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H., Sasaguri T. Nitroglycerine- and isoprenaline-induced vasodilatation: assessment from the actions of cyclic nucleotides. Br J Pharmacol. 1985 Feb;84(2):393–406. doi: 10.1111/j.1476-5381.1985.tb12923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Excitation--contraction coupling in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1981 Dec;321:513–535. doi: 10.1113/jphysiol.1981.sp014000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrick W. G., Hoar P. E. Inhibition of smooth muscle tension by cyclic AMP-dependent protein kinase. Nature. 1981 Jul 16;292(5820):253–255. doi: 10.1038/292253a0. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Ito Y., Suzuki H., Kitamura K., Itoh T. Factors modifying contraction-relaxation cycle in vascular smooth muscles. Am J Physiol. 1982 Nov;243(5):H641–H662. doi: 10.1152/ajpheart.1982.243.5.H641. [DOI] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984 Jun;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold C. M., Murphy R. A. Myoplasmic calcium, myosin phosphorylation, and regulation of the crossbridge cycle in swine arterial smooth muscle. Circ Res. 1986 Jun;58(6):803–815. doi: 10.1161/01.res.58.6.803. [DOI] [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Kuriyama H. Effects of cAMP- and cGMP-dependent protein kinases, and calmodulin on Ca2+ uptake by highly purified sarcolemmal vesicles of vascular smooth muscle. Biochim Biophys Acta. 1984 Jun 13;773(1):83–90. doi: 10.1016/0005-2736(84)90552-2. [DOI] [PubMed] [Google Scholar]
- Sumimoto K., Kuriyama H. Mobilization of free Ca2+ measured during contraction-relaxation cycles in smooth muscle cells of the porcine coronary artery using quin2. Pflugers Arch. 1986 Feb;406(2):173–180. doi: 10.1007/BF00586679. [DOI] [PubMed] [Google Scholar]
- Takayanagi I., Kondo N., Yamashita H., Hongo T., Takagi K. New selective beta2-adrenoreceptor stimulants 5(1-hydroxy-2-isopropylaminobutyl)- and 5-(1-hydroxy-2-ethylaminobutyl)-8-hydroxycarbostyril hydrochlorides (OPC-2009 and OPC-2030) and cyclic AMP concentration. J Pharm Pharmacol. 1977 Mar;29(3):187–189. doi: 10.1111/j.2042-7158.1977.tb11284.x. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]
- Yabuuchi Y., Yamashita S., Tei S. S. Pharmacological studies of OPC-2009, a newly synthesized selective beta adrenoceptor stimulant, in the broncho-motor and cardiovascular system of the anesthetized dog. J Pharmacol Exp Ther. 1977 Aug;202(2):326–336. [PubMed] [Google Scholar]
- Yamashita S., Takai M., Yabuuchi Y. Actions of procaterol (OPC-2009), a new beta2-adrenoceptor stimulant, on pulmonary resistance, contractions of the soleus muscle, and cardiovascular system of the anaesthetized cat. J Pharm Pharmacol. 1978 May;30(5):273–279. doi: 10.1111/j.2042-7158.1978.tb13228.x. [DOI] [PubMed] [Google Scholar]


