Abstract
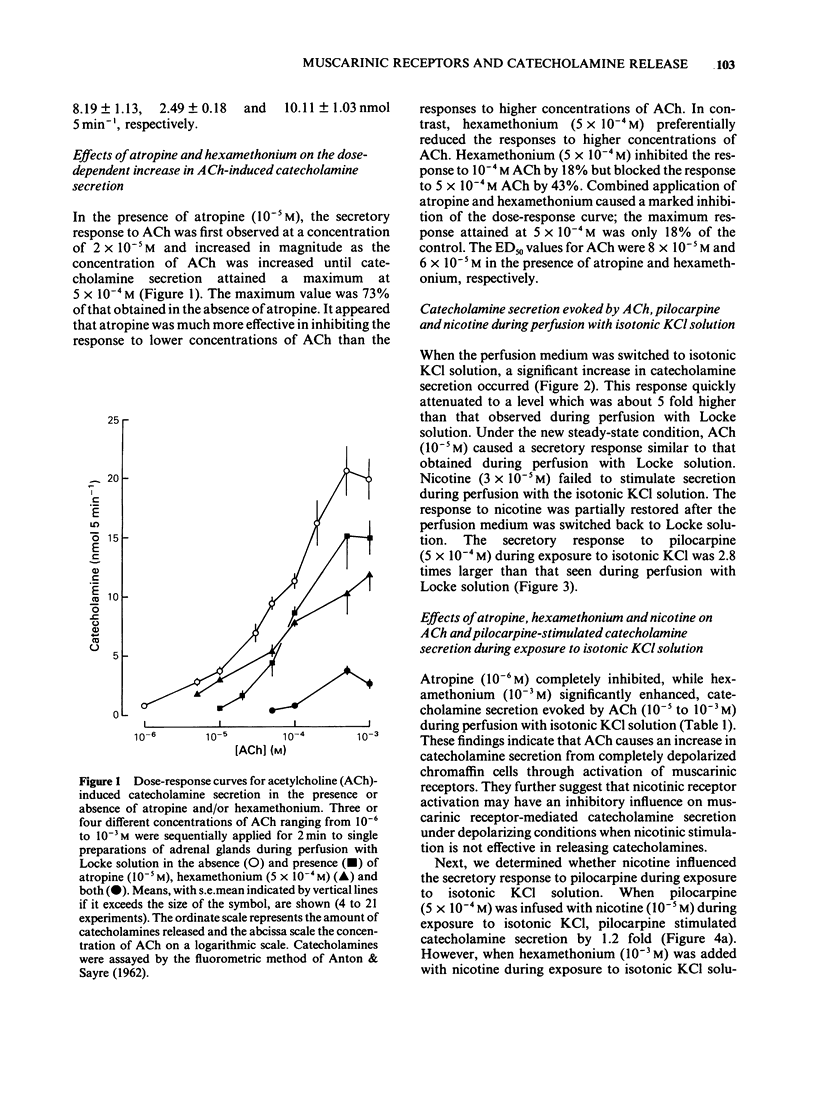
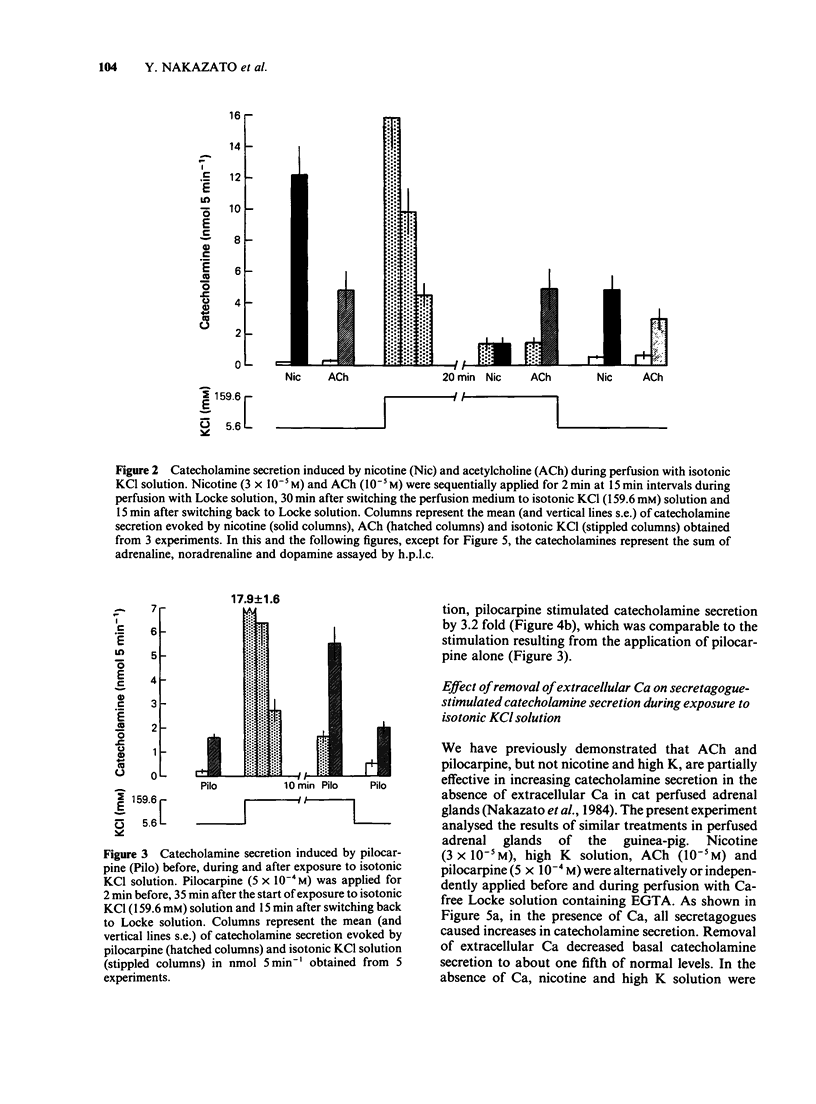
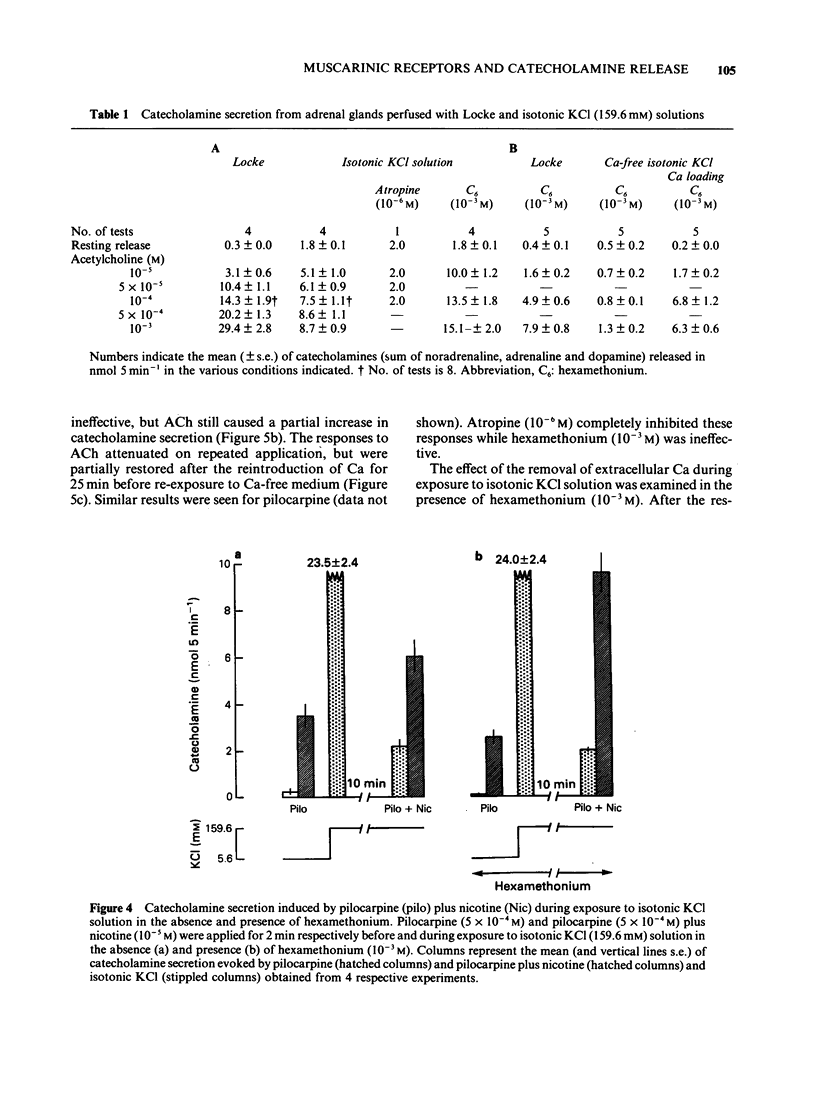
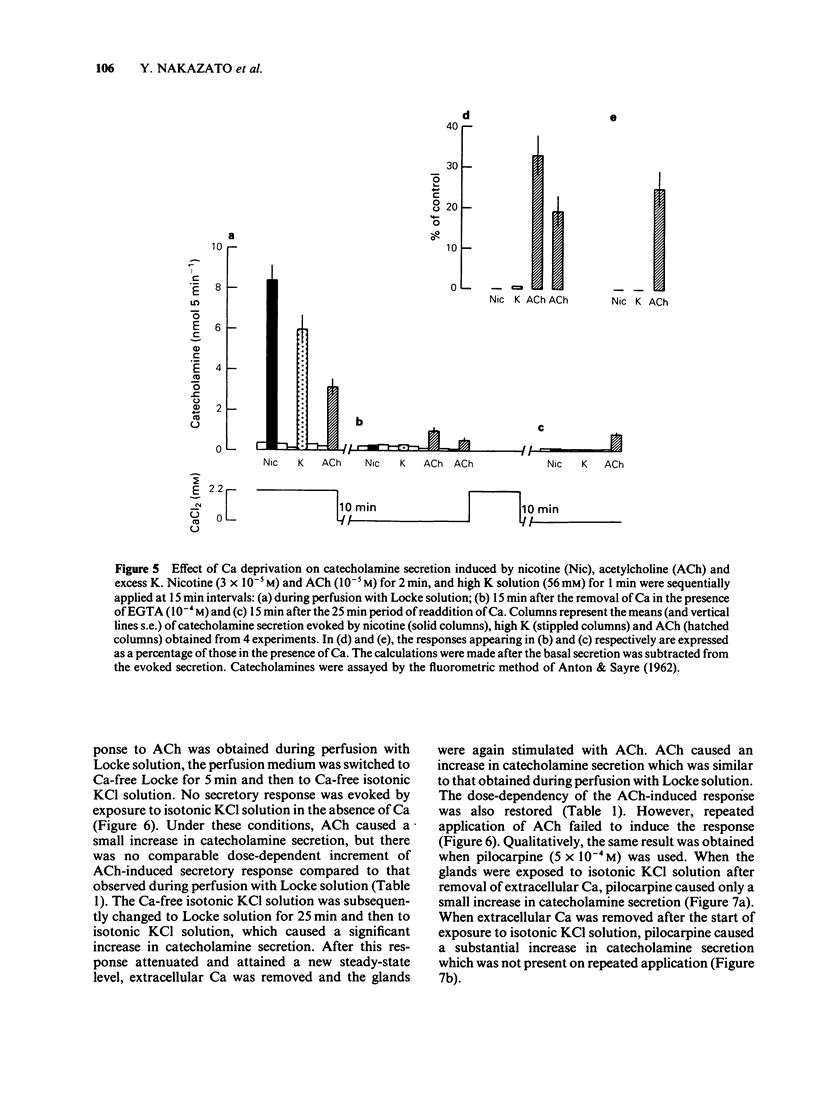
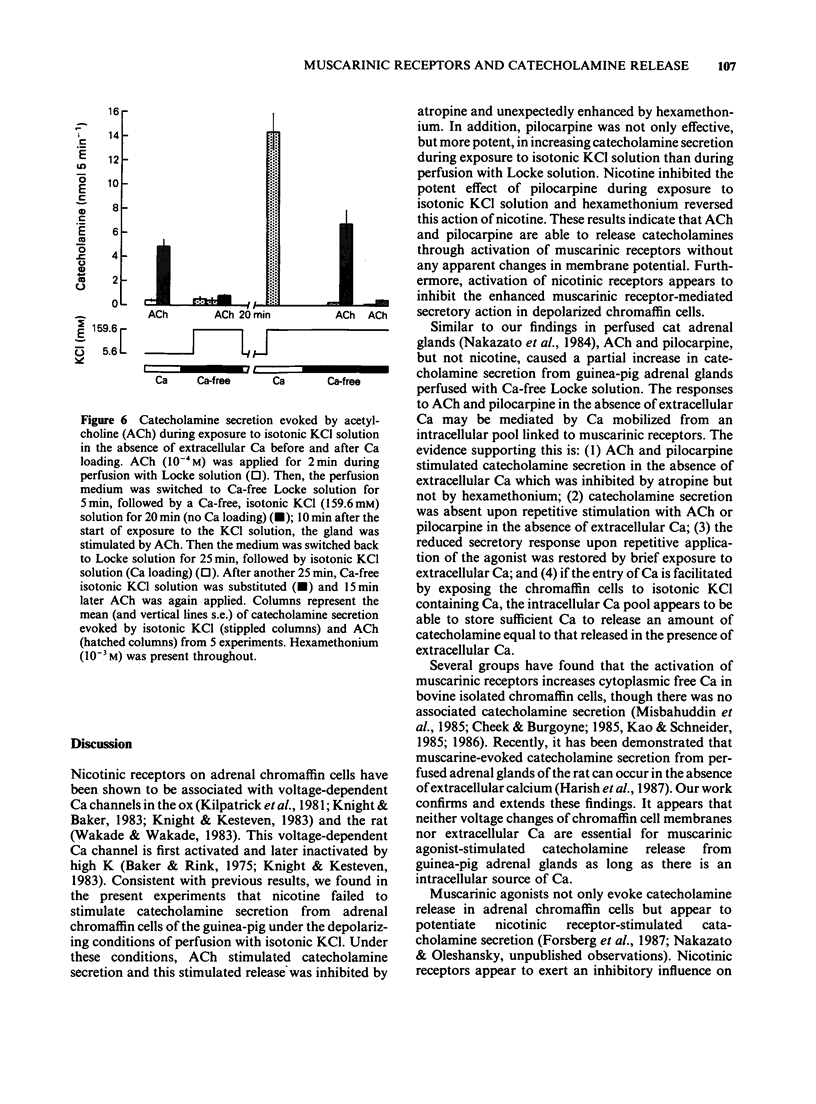
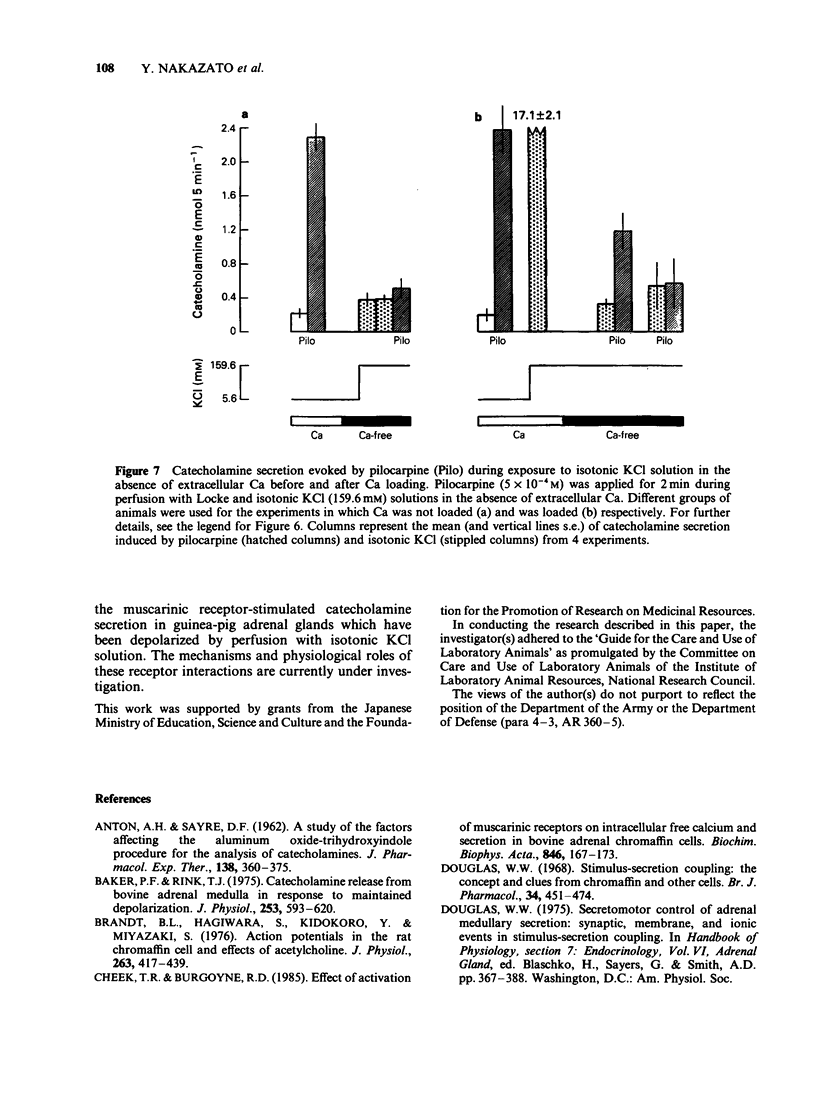
1. The differences between the mechanisms of muscarinic and nicotinic receptor-mediated catecholamine secretion with respect to their dependence on voltage changes and extracellular Ca were examined using perfused adrenal glands of the guinea-pig. 2. Acetylcholine (ACh, 10(-6) to 10(-3) M) caused a dose-dependent increase in catecholamine secretion. The ED50 value for ACh was 7 x 10(-5) M. In the presence of atropine (10(-5) M), the dose-response curve for ACh was shifted to the right. Hexamethonium (5 x 10(-4) M) preferentially reduced the responses to higher concentrations of ACh (greater than 10(-5) M). Pilocarpine (5 x 10(-4) M) and nicotine (3 x 10(-5) M) also stimulated catecholamine release. 3. During perfusion with isotonic KCl solution, ACh and pilocarpine, but not nicotine, evoked catecholamine secretion. These responses were abolished by atropine (10(-6) M). Pilocarpine-stimulated catecholamine secretion was enhanced during perfusion with isotonic KCl solution. Under these conditions, hexamethonium (10(-3) M) significantly augmented ACh-evoked catecholamine release. 4. During perfusion with either Ca-free isotonic KCl or Ca-free Locke solution, ACh and pilocarpine caused a partial increase in catecholamine secretion whereas nicotine and high K solution (56 mM) did not. The responses to ACh and pilocarpine were completely inhibited by atropine but not by hexamethonium. 5. When guinea-pig adrenal glands were perfused with isotonic KCl solution containing 2.2 mM Ca which was subsequently removed and replaced with EGTA, ACh-induced catecholamine secretion was similar in magnitude to that observed during perfusion with Locke solution.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- Baker P. F., Rink T. J. Catecholamine release from bovine adrenal medulla in response to maintained depolarization. J Physiol. 1975 Dec;253(2):593–620. doi: 10.1113/jphysiol.1975.sp011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B. L., Hagiwara S., Kidokoro Y., Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol. 1976 Dec;263(3):417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek T. R., Burgoyne R. D. Effect of activation of muscarinic receptors on intracellular free calcium and secretion in bovine adrenal chromaffin cells. Biochim Biophys Acta. 1985 Jul 30;846(1):167–173. doi: 10.1016/0167-4889(85)90122-3. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. On the mode of action of acetylcholine in evoking adrenal medullary secretion: increased uptake of calcium during the secretory response. J Physiol. 1962 Aug;162:385–392. doi: 10.1113/jphysiol.1962.sp006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. Stimulation of uptake of calcium-45 in the adrenal gland by acetylcholine. Nature. 1961 Dec 30;192:1299–1299. doi: 10.1038/1921299a0. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. J Physiol. 1967 Jul;191(1):107–121. doi: 10.1113/jphysiol.1967.sp008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg E. J., Rojas E., Pollard H. B. Muscarinic receptor enhancement of nicotine-induced catecholamine secretion may be mediated by phosphoinositide metabolism in bovine adrenal chromaffin cells. J Biol Chem. 1986 Apr 15;261(11):4915–4920. [PubMed] [Google Scholar]
- Harish O. E., Kao L. S., Raffaniello R., Wakade A. R., Schneider A. S. Calcium dependence of muscarinic receptor-mediated catecholamine secretion from the perfused rat adrenal medulla. J Neurochem. 1987 Jun;48(6):1730–1735. doi: 10.1111/j.1471-4159.1987.tb05730.x. [DOI] [PubMed] [Google Scholar]
- Ito S., Nakazato Y., Ohga A. The effect of veratridine on the release of catecholamines from the perfused adrenal gland. Br J Pharmacol. 1979 Feb;65(2):319–330. doi: 10.1111/j.1476-5381.1979.tb07833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao L. S., Schneider A. S. Calcium mobilization and catecholamine secretion in adrenal chromaffin cells. A Quin-2 fluorescence study. J Biol Chem. 1986 Apr 15;261(11):4881–4888. [PubMed] [Google Scholar]
- Kao L. S., Schneider A. S. Muscarinic receptors on bovine chromaffin cells mediate a rise in cytosolic calcium that is independent of extracellular calcium. J Biol Chem. 1985 Feb 25;260(4):2019–2022. [PubMed] [Google Scholar]
- Kidokoro Y., Miyazaki S., Ozawa S. Acetylcholine-induced membrane depolarization and potential fluctuations in the rat adrenal chromaffin cell. J Physiol. 1982 Mar;324:203–220. doi: 10.1113/jphysiol.1982.sp014107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y., Ritchie A. K. Chromaffin cell action potentials and their possible role in adrenaline secretion from rat adrenal medulla. J Physiol. 1980 Oct;307:199–216. doi: 10.1113/jphysiol.1980.sp013431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Slepetis R., Kirshner N. Ion channels and membrane potential in stimulus-secretion coupling in adrenal medulla cells. J Neurochem. 1981 Mar;36(3):1245–1255. doi: 10.1111/j.1471-4159.1981.tb01724.x. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Stimulus-secretion coupling in isolated bovine adrenal medullary cells. Q J Exp Physiol. 1983 Jan;68(1):123–143. doi: 10.1113/expphysiol.1983.sp002691. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Kesteven N. T. Evoked transient intracellular free Ca2+ changes and secretion in isolated bovine adrenal medullary cells. Proc R Soc Lond B Biol Sci. 1983 May 23;218(1211):177–199. doi: 10.1098/rspb.1983.0033. [DOI] [PubMed] [Google Scholar]
- Misbahuddin M., Isosaki M., Houchi H., Oka M. Muscarinic receptor-mediated increase in cytoplasmic free Ca2+ in isolated bovine adrenal medullary cells. Effects of TMB-8 and phorbol ester TPA. FEBS Lett. 1985 Oct 7;190(1):25–28. doi: 10.1016/0014-5793(85)80419-1. [DOI] [PubMed] [Google Scholar]
- Salzman S. K., Sellers M. S. Determination of norepinephrine in brain perfusates using high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1982 Oct 8;232(1):29–37. doi: 10.1016/s0378-4347(00)86004-1. [DOI] [PubMed] [Google Scholar]
- Ungar A., Phillips J. H. Regulation of the adrenal medulla. Physiol Rev. 1983 Jul;63(3):787–843. doi: 10.1152/physrev.1983.63.3.787. [DOI] [PubMed] [Google Scholar]
- Wakade A. R., Wakade T. D. Contribution of nicotinic and muscarinic receptors in the secretion of catecholamines evoked by endogenous and exogenous acetylcholine. Neuroscience. 1983 Nov;10(3):973–978. doi: 10.1016/0306-4522(83)90235-x. [DOI] [PubMed] [Google Scholar]


