Abstract
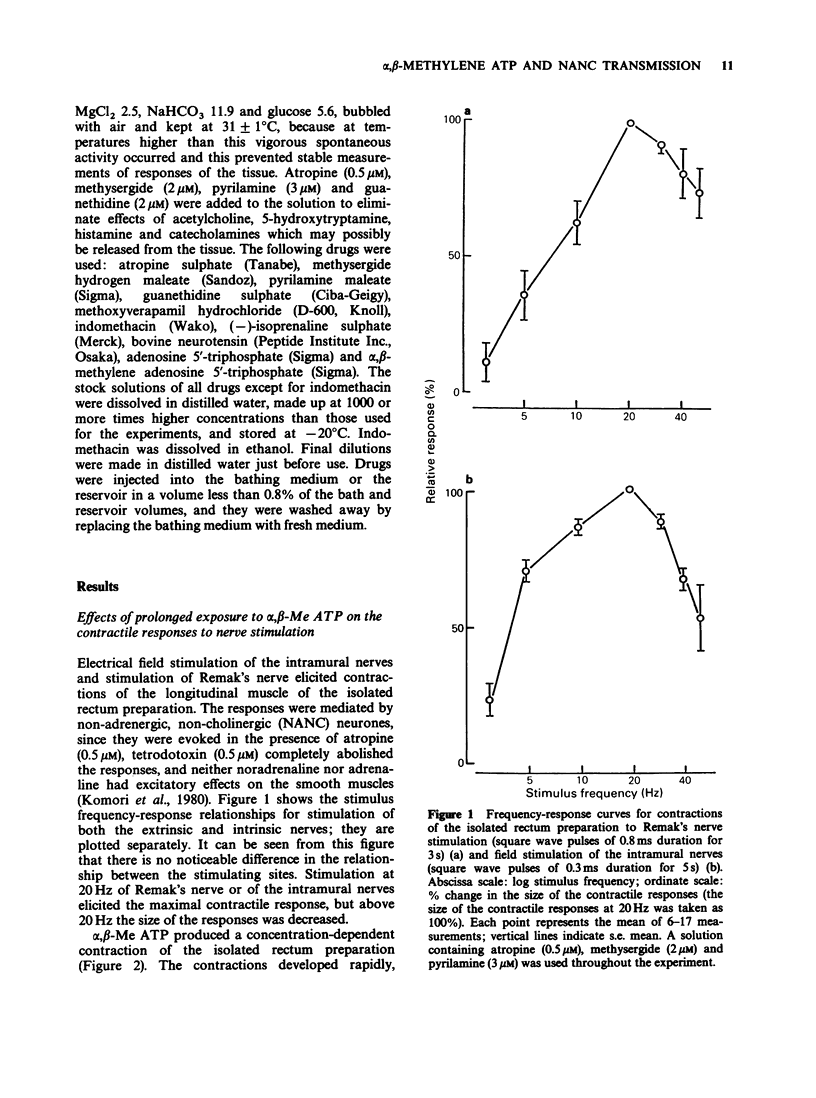
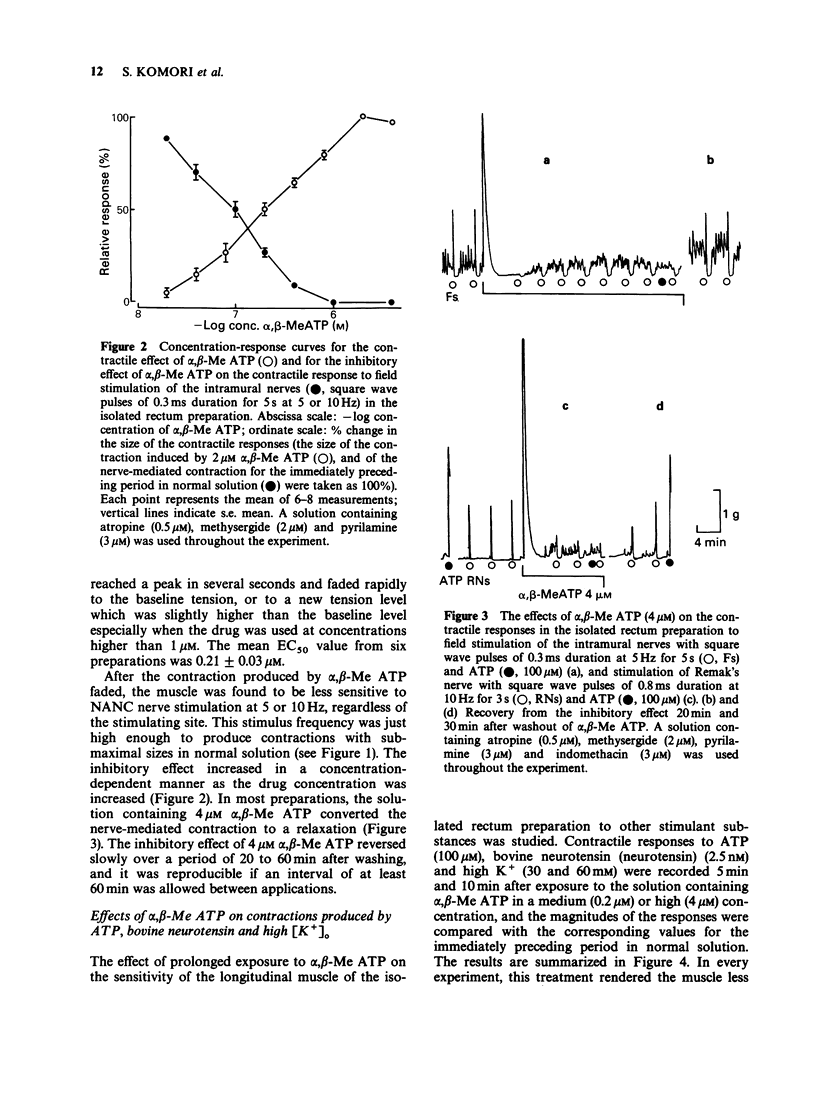
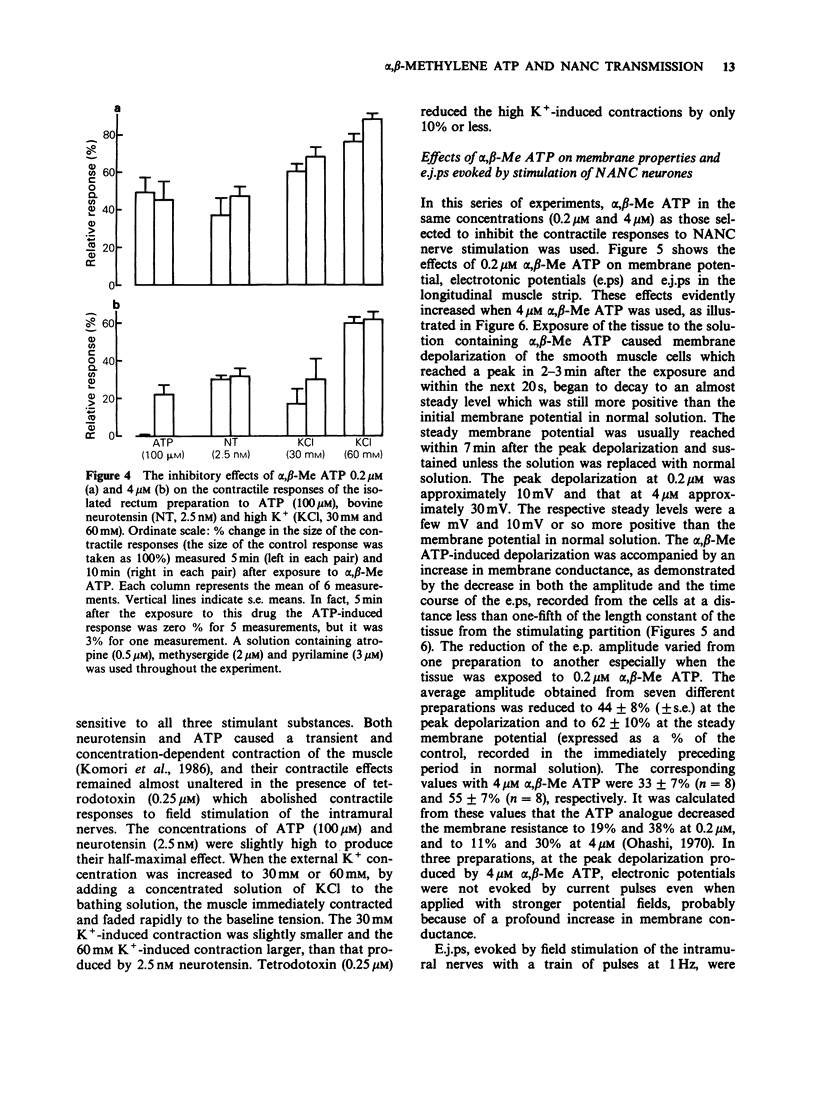
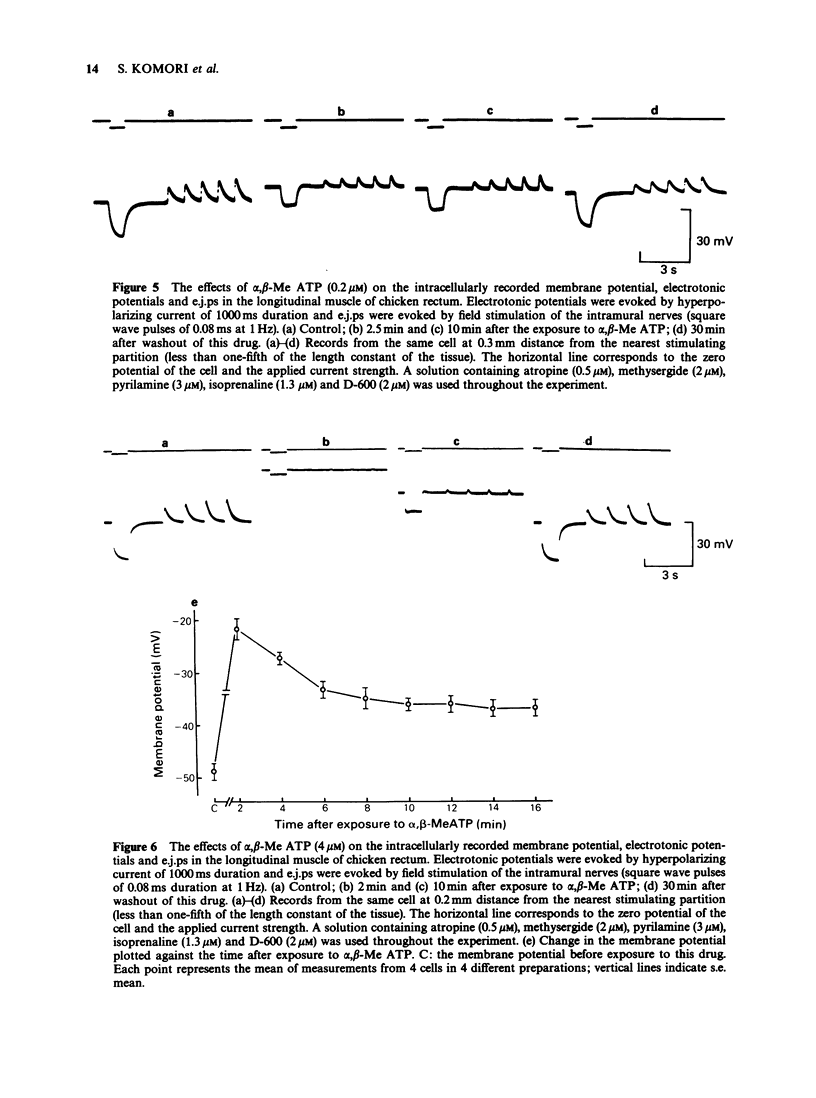
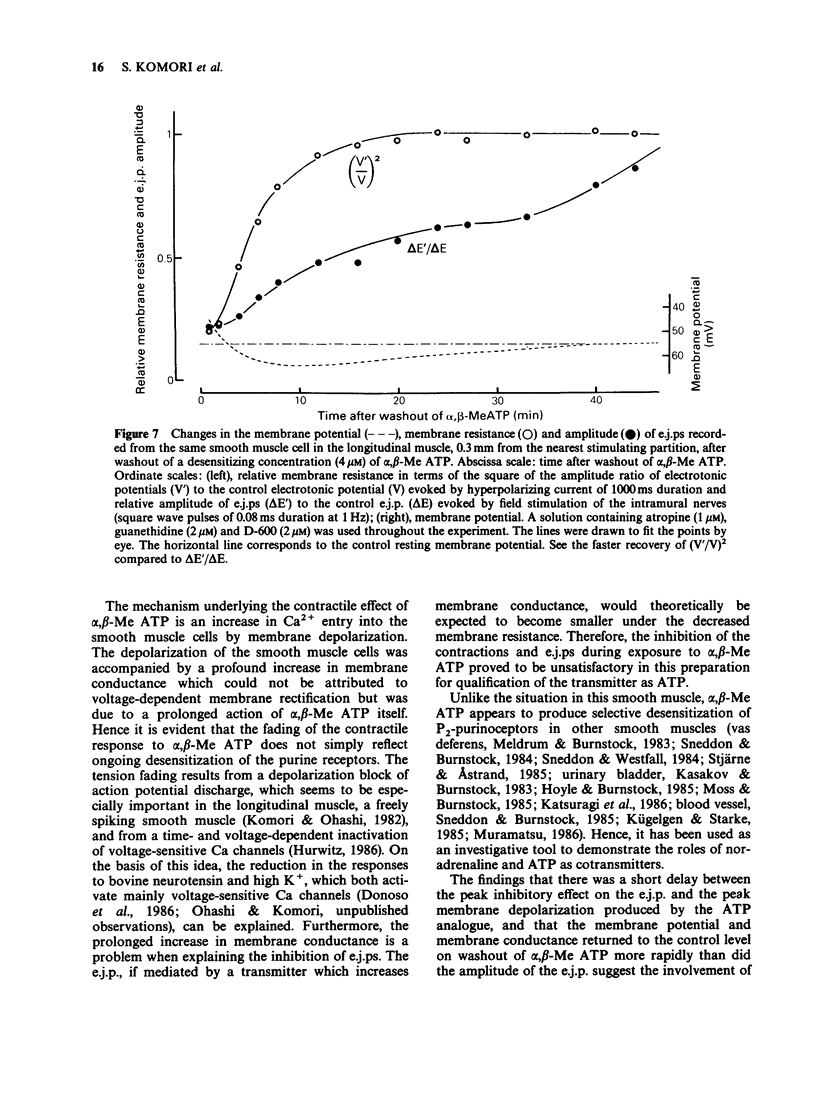
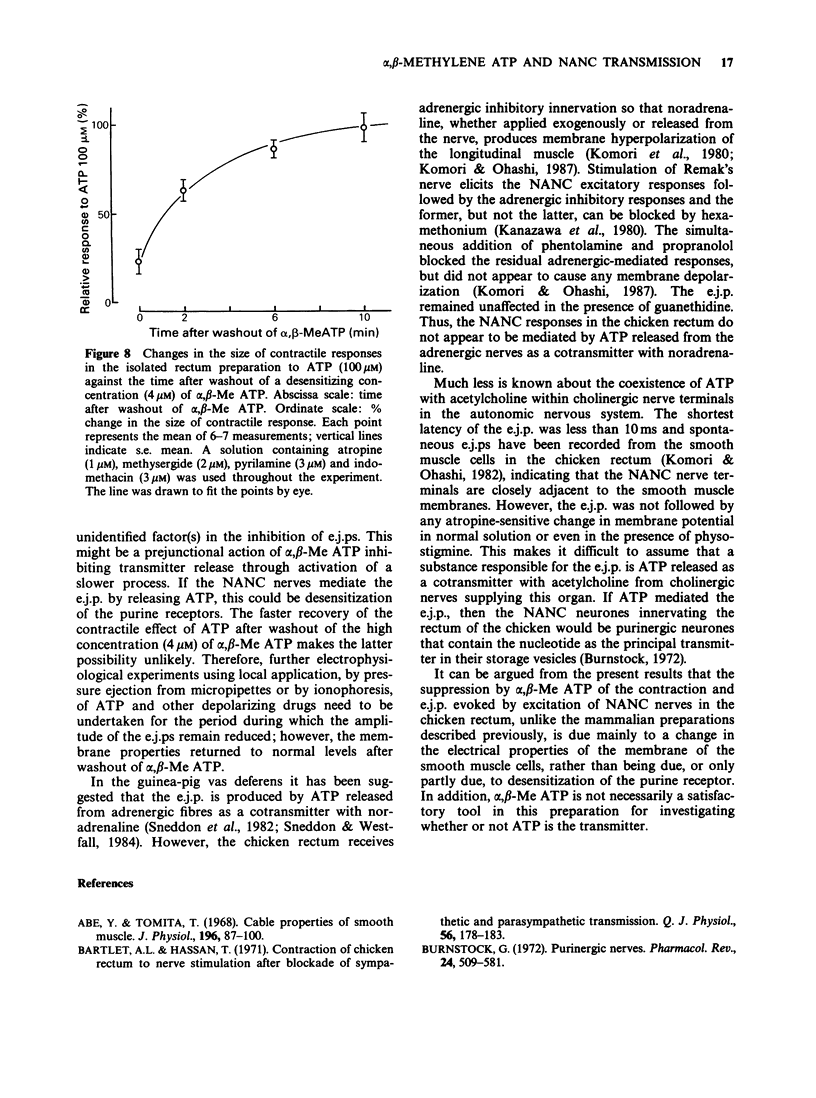
1. Effects of prolonged exposure to alpha,beta-methylene ATP (alpha,beta-Me ATP) on contractions and excitatory junction potentials (e.j.ps) evoked by non-adrenergic, non-cholinergic (NANC) excitatory nerve stimulation have been investigated in the chicken isolated rectum and longitudinal muscle strip from chicken rectum pretreated with atropine (0.5 microM), methysergide (2 microM) and pyrilamine (3 microM). 2. Alpha,beta-Me ATP (20 nM-4 microM) caused a rapid rise in tension of the longitudinal muscle of the isolated rectum preparation which returned to the baseline levels after a few minutes. The magnitude of the contractile response to NANC nerve stimulation was reduced after exposure to the drug. The inhibitory effect was related to the drug concentration; at 4 microM the nerve-mediated contraction was abolished and frequently converted to a relaxation. 3. Adenosine 5'-triphosphate (ATP, 100 microM), bovine neurotensin (2.5 nM) and K+-rich solutions (30 nM and 60 nM) all produced a transient contraction of the isolated rectum preparation. The exposure to alpha,beta-Me ATP (0.2 and 4 microM) also rendered the preparation less sensitive to these stimulant substances. 4. Alpha,beta-Me ATP (0.2 and 4 microM) caused a membrane depolarization in cells of the longitudinal muscle strip. The depolarization reached a peak within 2-3 min after application and then decayed to a steady level that was still more positive than the baseline level. The electrotonic potentials were reduced in amplitude to 44 +/- 8% (n = 7) of the normal amplitude if measured at the peak depolarization produced with 0.2 microM alpha,beta-Me ATP, and to 62 +/- 10% (n = 7) if measured at the steady-state depolarization. With 4 microM, the corresponding percentages were 33 +/- 7% (n = 8) and 55 +/- 7% (n = 8), indicating a decrease in membrane resistance. 5. The e.j.ps in response to field stimulation of the intramural nerves and Remak's nerve stimulation were decreased in amplitude and duration during exposure to alpha,beta-Me ATP (0.2 and 4 microM). 6. The smooth muscle cells regained normal membrane resistance and sensitivity to ATP on washout of alpha,beta-Me ATP (4 microM) more rapidly than the responses to NANC nerve stimulation. 7. It can be argued from the results that the suppression by alpha,beta-Me ATP of the contraction and e.j.p. evoked by NANC nerve stimulation in the chicken rectum, unlike the mammalian preparation described previously, is due mainly to a change in the electrical properties of the membrane of the smooth muscle cells, rather than being due, or only partly due, to desensitization of the purine receptor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlet A. L., Hassan T. Contraction of chicken rectum to nerve stimulation after blockade of sympathetic and parasympathetic transmission. Q J Exp Physiol Cogn Med Sci. 1971 Jul;56(3):178–183. doi: 10.1113/expphysiol.1971.sp002117. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. The effect of alpha, beta-methylene ATP on the depolarization evoked by noradrenaline (gamma-adrenoceptor response) and ATP in the immature rat basilar artery. Br J Pharmacol. 1986 May;88(1):6–8. doi: 10.1111/j.1476-5381.1986.tb09464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso M. V., Huidobro-Toro J. P., Kullak A. Involvement of calcium channels in the contractile activity of neurotensin but not acetylcholine: studies with calcium channel blockers and Bay K 8644 on the rat fundus. Br J Pharmacol. 1986 Aug;88(4):837–846. doi: 10.1111/j.1476-5381.1986.tb16257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle C. H., Burnstock G. Atropine-resistant excitatory junction potentials in rabbit bladder are blocked by alpha,beta-methylene ATP. Eur J Pharmacol. 1985 Aug 15;114(2):239–240. doi: 10.1016/0014-2999(85)90635-1. [DOI] [PubMed] [Google Scholar]
- Hurwitz L. Pharmacology of calcium channels and smooth muscle. Annu Rev Pharmacol Toxicol. 1986;26:225–258. doi: 10.1146/annurev.pa.26.040186.001301. [DOI] [PubMed] [Google Scholar]
- Ishikawa S. Actions of ATP and alpha, beta-methylene ATP on neuromuscular transmission and smooth muscle membrane of the rabbit and guinea-pig mesenteric arteries. Br J Pharmacol. 1985 Dec;86(4):777–787. doi: 10.1111/j.1476-5381.1985.tb11099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T., Ohashi H., Takewaki T. Evidence that cell bodies of non-cholinergic, excitatory neurones which supply the smooth muscle of the chicken rectum are located in the ganglia of Remak's nerve. Br J Pharmacol. 1980;71(2):519–524. doi: 10.1111/j.1476-5381.1980.tb10966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasakov L., Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982 Dec 24;86(2):291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Katsuragi T., Kuratomi L., Furukawa T. Clonidine-evoked selective P1-purinoceptor antagonism of contraction of guinea-pig urinary bladder. Eur J Pharmacol. 1986 Feb 11;121(1):119–122. doi: 10.1016/0014-2999(86)90400-0. [DOI] [PubMed] [Google Scholar]
- Komori S., Fukutome T., Ohashi H. Isolation of a peptide material showing strong rectal muscle-contracting activity from chicken rectum and its identification as chicken neurotensin. Jpn J Pharmacol. 1986 Apr;40(4):577–589. doi: 10.1254/jjp.40.577. [DOI] [PubMed] [Google Scholar]
- Komori S., Ohashi H. Nerve pathways involved in adrenergic regulation of electrical and mechanical activities in the chicken rectum. Br J Pharmacol. 1987 Jan;90(1):121–129. doi: 10.1111/j.1476-5381.1987.tb16831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori S., Ohashi H. Some characteristics of transmission from non-adrenergic, non-cholinergic excitatory nerves to the smooth muscle of the chicken. J Auton Nerv Syst. 1982 Sep;6(2):199–210. doi: 10.1016/0165-1838(82)90051-0. [DOI] [PubMed] [Google Scholar]
- Komori S., Ohashi H., Takewaki T. The effects of alpha- and beta-adrenoceptor activation on tension and membrane properties of the longitudinal smooth muscle of the chicken rectum. Br J Pharmacol. 1980;71(2):479–488. doi: 10.1111/j.1476-5381.1980.tb10961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum L. A., Burnstock G. Evidence that ATP acts as a co-transmitter with noradrenaline in sympathetic nerves supplying the guinea-pig vas deferens. Eur J Pharmacol. 1983 Aug 19;92(1-2):161–163. doi: 10.1016/0014-2999(83)90126-7. [DOI] [PubMed] [Google Scholar]
- Meldrum L. A., Burnstock G. Investigations into the identity of the non-adrenergic, non-cholinergic excitatory transmitter in the smooth muscle of chicken rectum. Comp Biochem Physiol C. 1985;81(2):307–309. doi: 10.1016/0742-8413(85)90011-8. [DOI] [PubMed] [Google Scholar]
- Moss H. E., Burnstock G. A comparative study of electrical field stimulation of the guinea-pig, ferret and marmoset urinary bladder. Eur J Pharmacol. 1985 Aug 27;114(3):311–316. doi: 10.1016/0014-2999(85)90375-9. [DOI] [PubMed] [Google Scholar]
- Muramatsu I. Evidence for sympathetic, purinergic transmission in the mesenteric artery of the dog. Br J Pharmacol. 1986 Mar;87(3):478–480. doi: 10.1111/j.1476-5381.1986.tb10187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oashi H. An estimate of the proportion of the resting membrane conductance of the smooth muscle of guinea-pog taenia coli attributable to chloride. J Physiol. 1970 Sep;210(2):405–419. doi: 10.1113/jphysiol.1970.sp009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi H., Naito K., Takewaki T., Okada T. Non-cholinergic, excitatory junction potentials in smooth muscle of chicken rectum. Jpn J Pharmacol. 1977 Jun;27(3):379–387. doi: 10.1254/jjp.27.379. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984 Oct 30;106(1):149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984 Apr 13;100(1):85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P., Fedan J. S. Cotransmitters in the motor nerves of the guinea pig vas deferens: electrophysiological evidence. Science. 1982 Nov 12;218(4573):693–695. doi: 10.1126/science.6291151. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984 Feb;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjärne L., Astrand P. Relative pre- and postjunctional roles of noradrenaline and adenosine 5'-triphosphate as neurotransmitters of the sympathetic nerves of guinea-pig and mouse vas deferens. Neuroscience. 1985 Mar;14(3):929–946. doi: 10.1016/0306-4522(85)90155-1. [DOI] [PubMed] [Google Scholar]
- Takewaki T., Ohashi O. Non-cholinergic excitatory transmission to intestinal smooth muscle cells. Nature. 1977 Aug 25;268(5622):749–750. doi: 10.1038/268749a0. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline and adenosine triphosphate as co-transmitters of neurogenic vasoconstriction in rabbit mesenteric artery. J Physiol. 1985 Oct;367:435–455. doi: 10.1113/jphysiol.1985.sp015834. [DOI] [PMC free article] [PubMed] [Google Scholar]


