Abstract
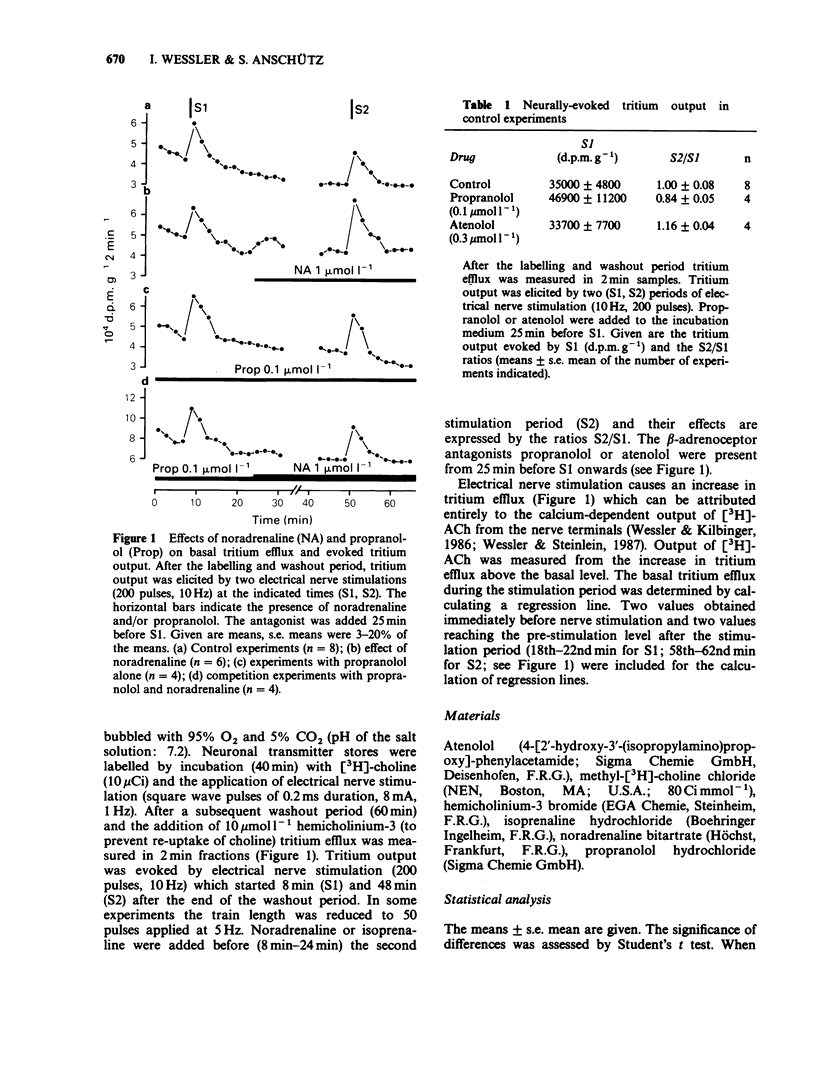
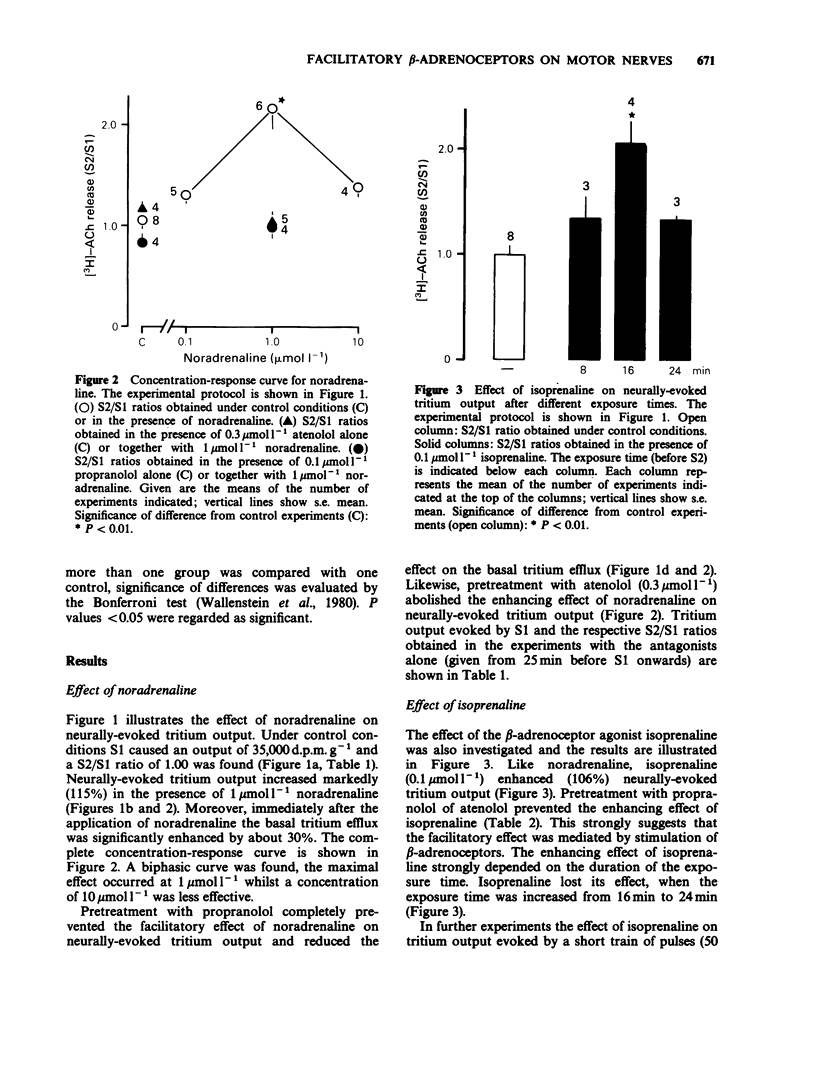
1. Neurally-evoked output of newly synthesized [3H]-acetylcholine from the rat phrenic nerve was measured in the absence of cholinesterase inhibitors. 2. Noradrenaline and isoprenaline enhanced neurally-evoked transmitter output markedly. Moreover, immediately after the application of noradrenaline the basal tritium efflux increased significantly. 3. Pretreatment with propranolol (0.1 mumol l-1) or atenolol (0.3 mumol l-1) completely prevented the stimulatory effect of noradrenaline and isoprenaline on evoked transmitter output. 4. The facilitatory effect of isoprenaline declined, when the exposure time was increased. This observation supports the assumption that beta-adrenoceptors can be desensitized or inactivated during continued exposure to agonists. 5. It was shown for the first time that stimulation of beta-adrenoceptors enhances transmitter output from the motor nerve. It is proposed that these beta-adrenoceptors are of the beta 1-subtype and are localized on the endings of motor nerves. Circulating catecholamines may facilitate neuromuscular transmission by stimulation of presynaptic beta-adrenoceptors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman W. C., Raper C. Effects of sympathomimetic amines on neuromuscular transmission. Br J Pharmacol Chemother. 1966 Aug;27(2):313–331. doi: 10.1111/j.1476-5381.1966.tb01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K. Effects of catecholamines on the neuromuscular junction in the rat diaphragm. J Physiol. 1970 Dec;211(3):551–570. doi: 10.1113/jphysiol.1970.sp009293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A. Beta-adrenergic receptors and their mode of coupling to adenylate cyclase. Physiol Rev. 1986 Jul;66(3):819–854. doi: 10.1152/physrev.1986.66.3.819. [DOI] [PubMed] [Google Scholar]
- Malta E., McPherson G. A., Raper C. Comparison of pre-junctional alpha-adrenoceptors at the neuromuscular junction with vascular post-junctional alpha-receptors in cat skeletal muscle. Br J Pharmacol. 1979 Feb;65(2):249–256. doi: 10.1111/j.1476-5381.1979.tb07825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Meadows J. C. The effect of adrenaline on the contraction of human muscle. J Physiol. 1970 Apr;207(2):429–448. doi: 10.1113/jphysiol.1970.sp009071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi G. T., Vizi E. S., Chaudhry I. A., Nagashima H., Duncalf D., Foldes F. F., Goldiner P. L. Modulation of stimulation-evoked release of newly formed acetylcholine from mouse hemidiaphragm preparation. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jul;336(1):11–15. doi: 10.1007/BF00177744. [DOI] [PubMed] [Google Scholar]
- Starke K. Presynaptic receptors. Annu Rev Pharmacol Toxicol. 1981;21:7–30. doi: 10.1146/annurev.pa.21.040181.000255. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Wessler I., Halank M., Rasbach J., Kilbinger H. Presynaptic nicotine receptors mediating a positive feed-back on transmitter release from the rat phrenic nerve. Naunyn Schmiedebergs Arch Pharmacol. 1986 Dec;334(4):365–372. doi: 10.1007/BF00569371. [DOI] [PubMed] [Google Scholar]
- Wessler I., Karl M., Mai M., Diener A. Muscarine receptors on the rat phrenic nerve, evidence for positive and negative muscarinic feedback mechanisms. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jun;335(6):605–612. doi: 10.1007/BF00166975. [DOI] [PubMed] [Google Scholar]
- Wessler I., Kilbinger H. Release of [3H]acetylcholine from a modified rat phrenic nerve-hemidiaphragm preparation. Naunyn Schmiedebergs Arch Pharmacol. 1986 Dec;334(4):357–364. doi: 10.1007/BF00569370. [DOI] [PubMed] [Google Scholar]
- Wessler I., Sandmann J. Uptake and metabolism of [3H]choline by the rat phrenic nerve-hemidiaphragm preparation. Naunyn Schmiedebergs Arch Pharmacol. 1987 Mar;335(3):231–237. doi: 10.1007/BF00172789. [DOI] [PubMed] [Google Scholar]
- Wessler I., Scheuer B., Kilbinger H. [3H]acetylcholine release from the phrenic nerve is increased or decreased by activation or desensitization of nicotine receptors. Eur J Pharmacol. 1987 Mar 3;135(1):85–87. doi: 10.1016/0014-2999(87)90760-6. [DOI] [PubMed] [Google Scholar]
- Wessler I., Steinlein O. Differential release of [3H]acetylcholine from the rat phrenic nerve-hemidiaphragm preparation by electrical nerve stimulation and by high potassium. Neuroscience. 1987 Jul;22(1):289–299. doi: 10.1016/0306-4522(87)90219-3. [DOI] [PubMed] [Google Scholar]
- Wilson D. F. The effects of dibutyryl cyclic adenosine 3',5'-monophosphate, theophylline and aminophylline on neuromuscular transmission in the rat. J Pharmacol Exp Ther. 1974 Feb;188(2):447–452. [PubMed] [Google Scholar]


