Abstract
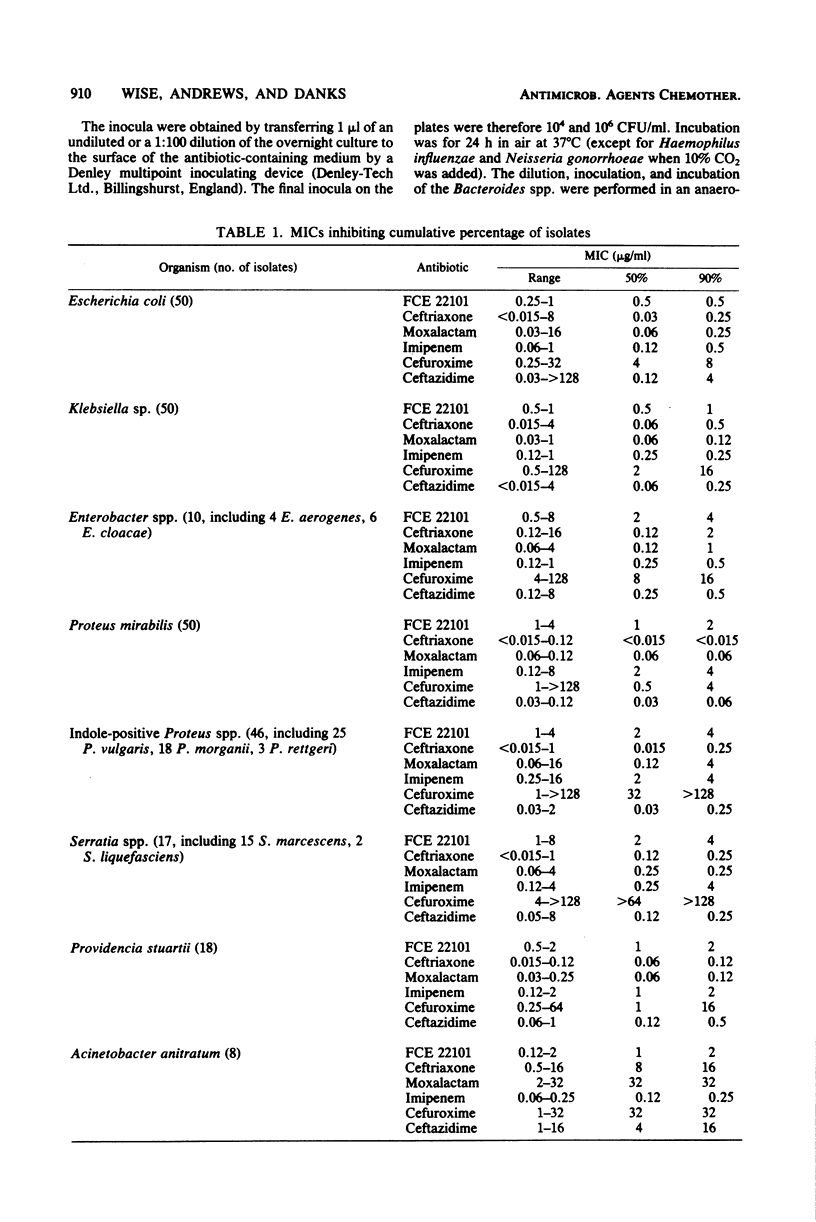
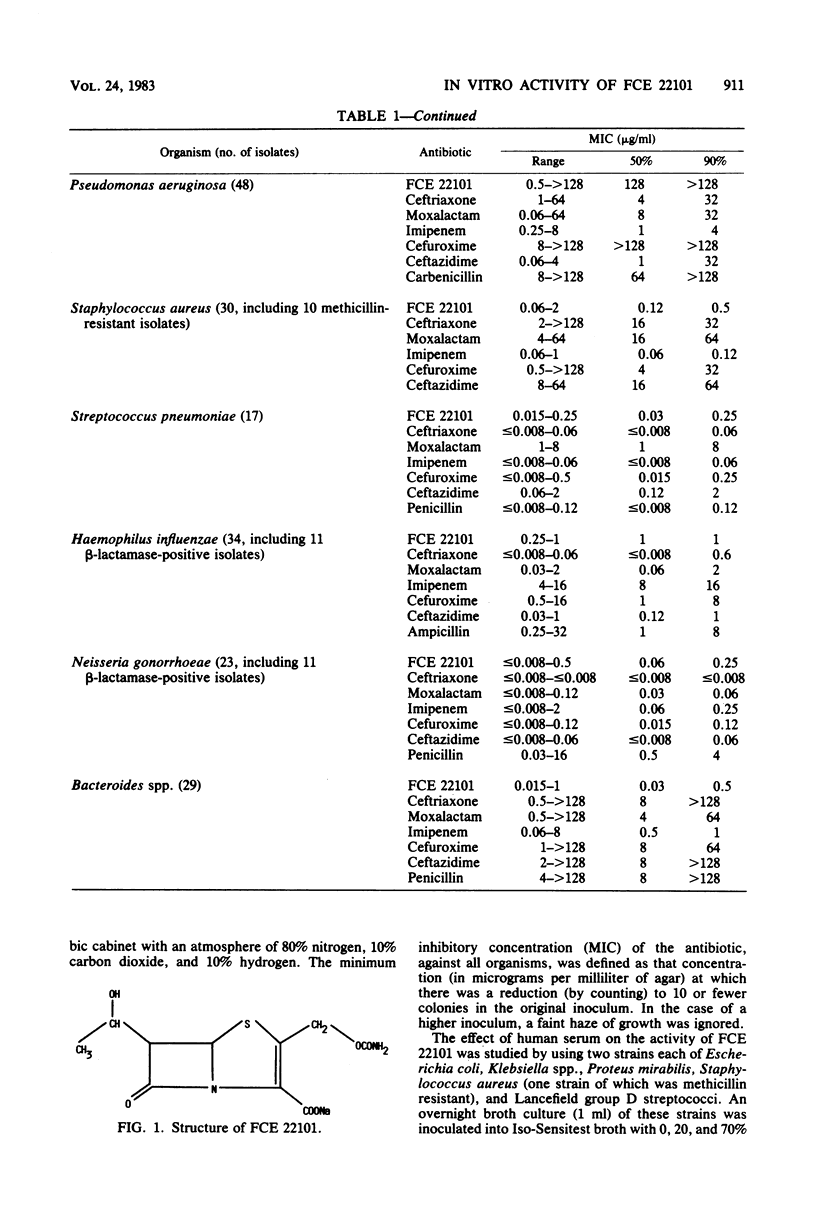
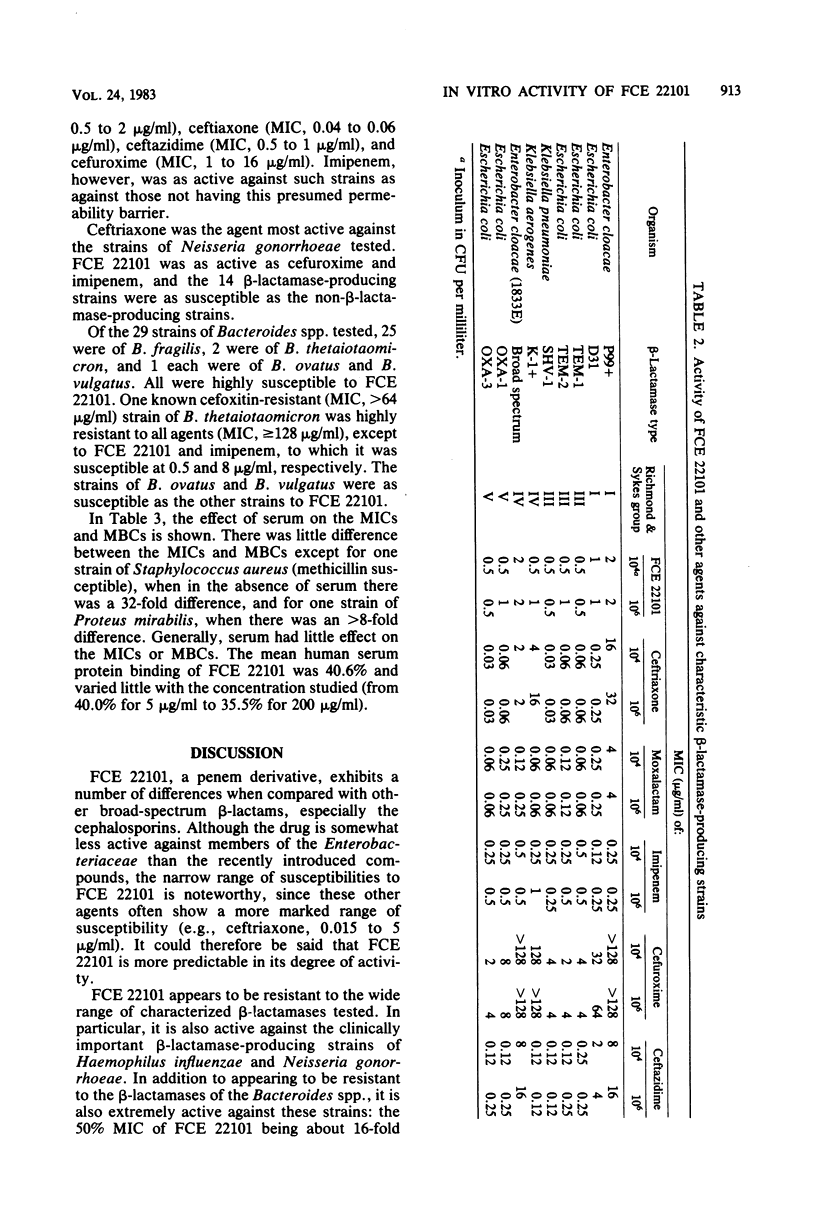
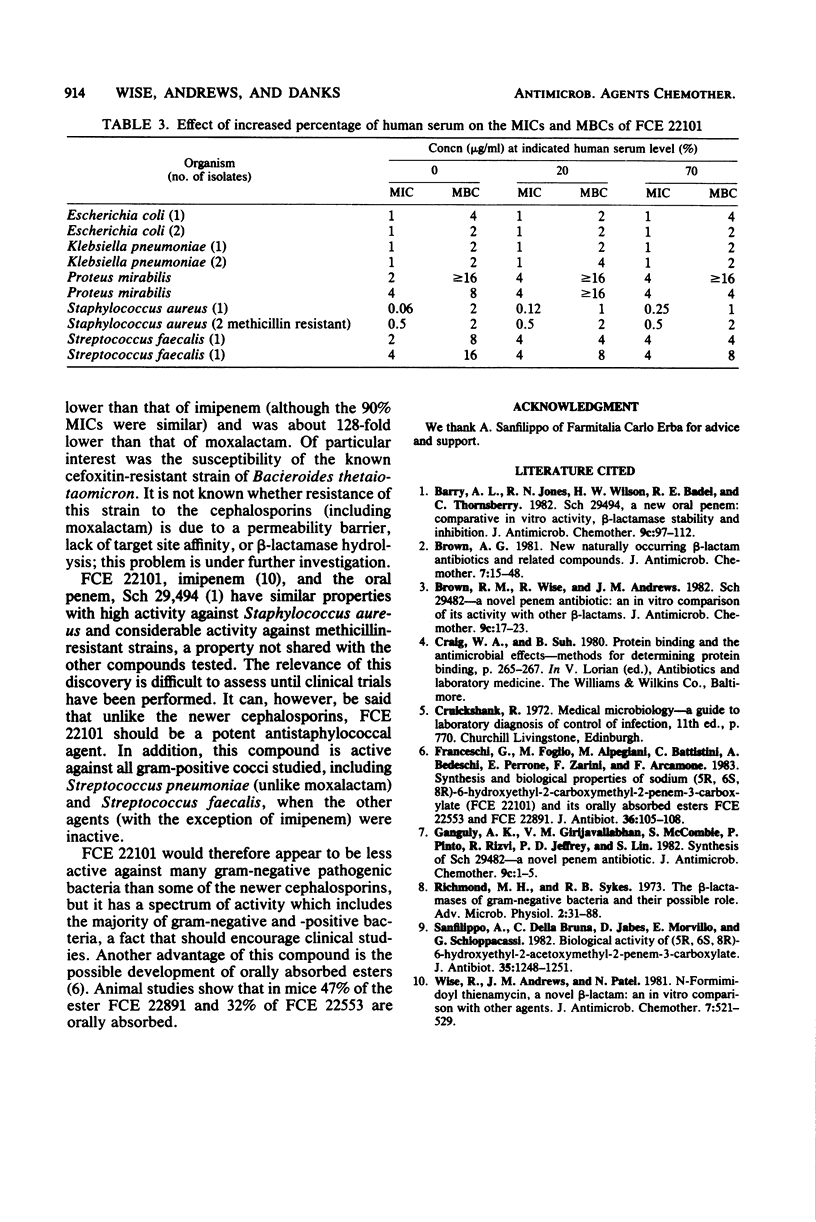
The in vitro activity of FCE 22101, a new semisynthetic penem derivative, was compared with that of ceftriaxone, moxalactam, imipenem (formerly imipemide, N-formimidoyl thienamycin, or MK 0787), cefuroxime, ceftazidime, and other beta-lactams, when appropriate, against 472 recent isolates and known beta-lactam-resistant strains. The minimum inhibitory concentrations of FCE 22101 against 90% of the members of the family Enterobacteriaceae, Haemophilus influenzae, Staphylococcus aureus, Lancefield group D streptococci, and Bacteroides spp. were between 0.5 and 4 micrograms/ml. Methicillin-resistant strains of Staphylococcus aureus were susceptible. Ninety percent of the Neisseria gonorrhoeae and Streptococcus pneumoniae strains were susceptible to 0.25 microgram of FCE 22101 per ml. Pseudomonas aeruginosa strains were resistant to FCE 22101 (minimum inhibitory concentration, greater than 128 micrograms/ml). The susceptibility of known, characterized beta-lactamase-producing strains of the Enterobacteriaceae suggested that FCE 22101 is resistant to many beta-lactamases. Generally, FCE 22101 was slightly less active than imipenem, moxalactam, ceftriaxone, and ceftazidime against members of the Enterobacteriaceae and considerably more active than the cephalosporins (including moxalactam) against Staphylococcus aureus. The human serum protein binding of FCE 22101 was about 40%, and human serum had little effect on the activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Jones R. N., Wilson H. W., Badal R. E., Thornsberry C. Sch 29482, a new oral penem: comparative in-vitro activity, beta-lactamase stability and inhibition. J Antimicrob Chemother. 1982 Feb;9 (Suppl 100):97–112. doi: 10.1093/jac/9.suppl_c.97. [DOI] [PubMed] [Google Scholar]
- Brown A. G. New naturally occurring beta-lactam antibiotics and related compounds. J Antimicrob Chemother. 1981 Jan;7(1):15–48. doi: 10.1093/jac/7.1.15. [DOI] [PubMed] [Google Scholar]
- Brown R. M., Wise R., Andrews J. M. Sch 29482--a novel penem antibiotic: an in-vitro comparison of its activity with other beta-lactams. J Antimicrob Chemother. 1982 Feb;9 (Suppl 100):17–23. doi: 10.1093/jac/9.suppl_c.17. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Sanfilippo A., Della Bruna C., Jabes D., Morvillo E., Schioppacassi G., Franceschi G., Arcamone F., Battistini C., Foglio M., Zarini F. Biological activity of (5R,6S,8R)-6-alpha-hydroxyethyl-2-acetoxymethyl-2-penem-3-carboxylate. J Antibiot (Tokyo) 1982 Sep;35(9):1248–1251. doi: 10.7164/antibiotics.35.1248. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Patel N. N-formimidoyl-thienamycin a novel beta-lactam: an in-vitro comparison with other beta-lactam antibiotics. J Antimicrob Chemother. 1981 May;7(5):521–529. doi: 10.1093/jac/7.5.521. [DOI] [PubMed] [Google Scholar]


