Abstract
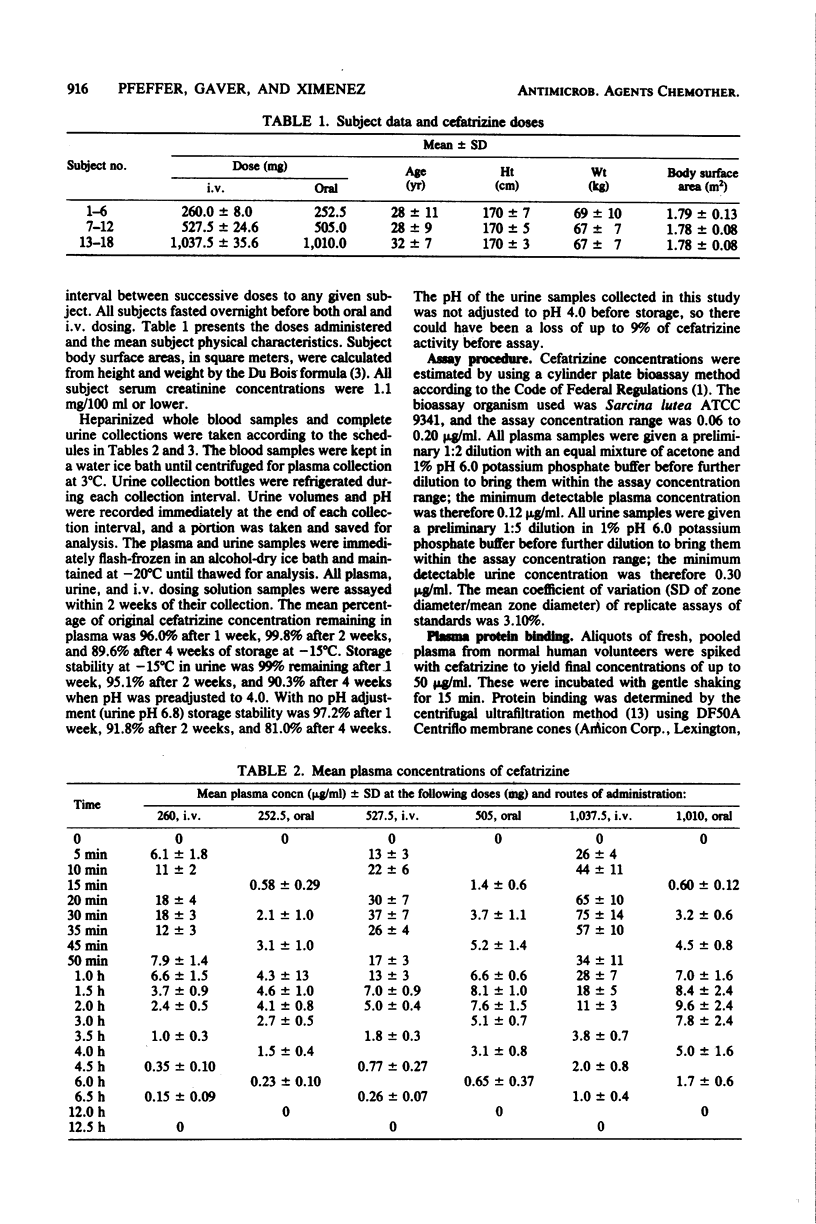
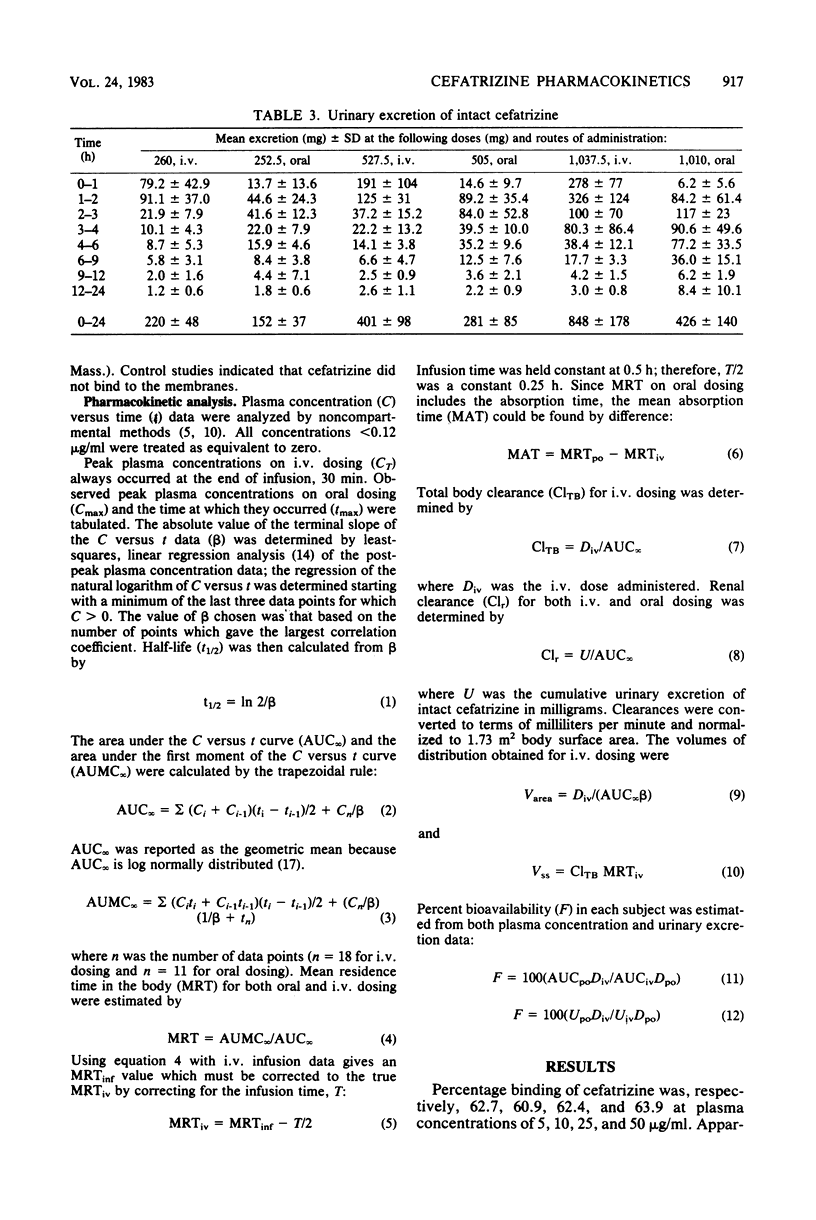
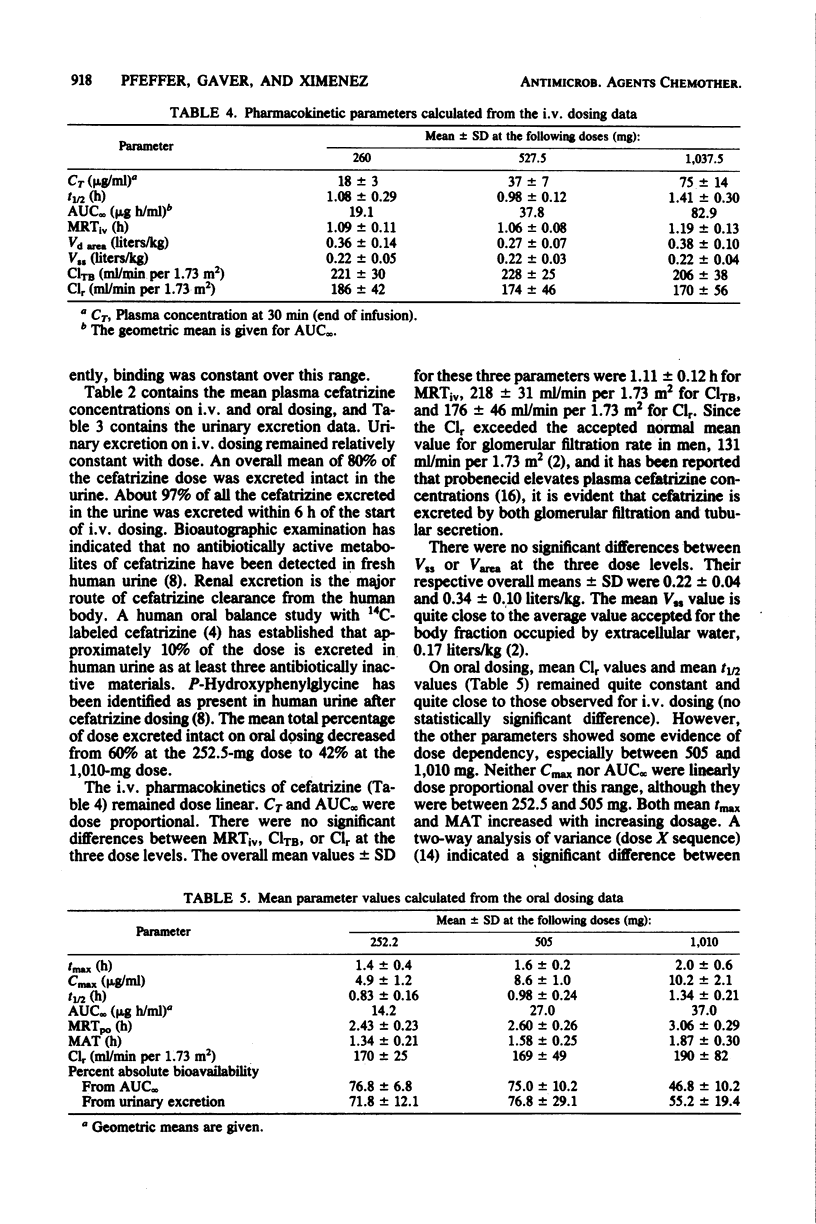
Cefatrizine was administered intravenously and orally at dose levels of 250, 500, and 1,000 mg to normal male volunteers in a crossover study. Intravenous pharmacokinetics were dose linear over this range; mean peak plasma concentrations at the end of 30-min infusions were, respectively, 18, 37, and 75 micrograms/ml, total body clearance was 218 ml/min per 1.73 m2, renal clearance was 176 ml/min per 1.73 m2, and mean retention time in the body was 1.11 h. Cumulative urinary excretion of intact cefatrizine was 80% of the dose, and half-lives ranged from 1 to 1.4 h. Steady-state volume of distribution was 0.22 liters/kg. On oral administration, the absolute bioavailabilities of cefatrizine were 75% at 250 and 500 mg and 50% at 1,000 mg. The mean peak plasma concentrations and peak times were, respectively, 4.9, 8.6, and 10.2 micrograms/ml at 1.4, 1.6, and 2.0 h, mean residence times were 2.4, 2.6, and 3.1 h, and mean absorption times were 1.3, 1.6, and 1.9 h. Oral renal clearance and half-life values corresponded well to the intravenous values. Cumulative urinary excretion of intact cefatrizine (as percentage of dose) was 60 at 250 mg, 56 at 500 mg, and 42 at 1,000 mg. It is hypothesized that the lack of oral dose linearity between the 500- and 1,000-mg doses is due to a component of cefatrizine absorption by a saturable transport process. Relative absorption at the high dose would be sufficiently slow that an absorption "window" would be passed before maximum bioavailability could be attained. It is not expected that the observed bioavailability decrease at doses exceeding 500 mg will have any therapeutic significance, since clinical studies are establishing efficacy for a recommended unit dosage regimen of 500 mg.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gaver R. C., Deeb G. Disposition of 14C-cefatrizine in man. Drug Metab Dispos. 1980 May-Jun;8(3):157–162. [PubMed] [Google Scholar]
- Kimura T., Yamamoto T., Mizuno M., Suga Y., Kitade S., Sezaki H. Characterization of aminocephalosporin transport across rat small intestine. J Pharmacobiodyn. 1983 Apr;6(4):246–253. doi: 10.1248/bpb1978.6.246. [DOI] [PubMed] [Google Scholar]
- Leitner F., Buck R. E., Misiek M., Pursiano T. A., Price K. E. BL-S 640, a cephalosporin with a broad spectrum of antibacterial activity: properties in vitro. Antimicrob Agents Chemother. 1975 Mar;7(3):298–305. doi: 10.1128/aac.7.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M., Ohtawa M., Akiyama I., Yamamato M., Tomioka J. [Metabolism of cefatrizine (S-640P) in rat, rabbit, dog, monkey and human (author's transl)]. Jpn J Antibiot. 1976 Jan;29(1):90–106. [PubMed] [Google Scholar]
- Neu H. C., Fu K. P. Cefatrizine activity compared with that of other cephalosporins. Antimicrob Agents Chemother. 1979 Feb;15(2):209–212. doi: 10.1128/aac.15.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadomy S., Wagner G., Carver M. In vitro activities of five oral cephalosporins against aerobic pathogenic bacteria. Antimicrob Agents Chemother. 1977 Nov;12(5):609–613. doi: 10.1128/aac.12.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhvi S. M., Heald A. F., Gadebusch H. H., Resnick M. E., Difazio L. T., Leitz M. A. Human serum protein binding of cephalosporin antibiotics in vitro. J Lab Clin Med. 1977 Feb;89(2):414–420. [PubMed] [Google Scholar]


