Abstract
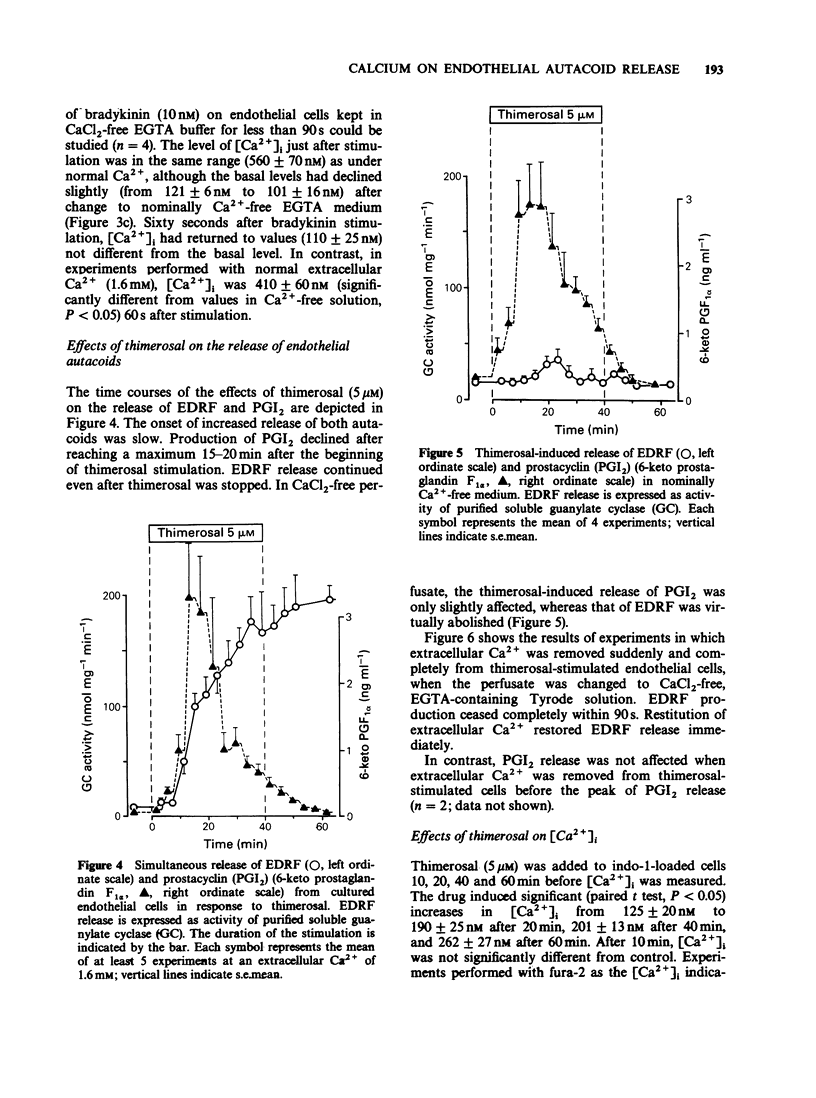
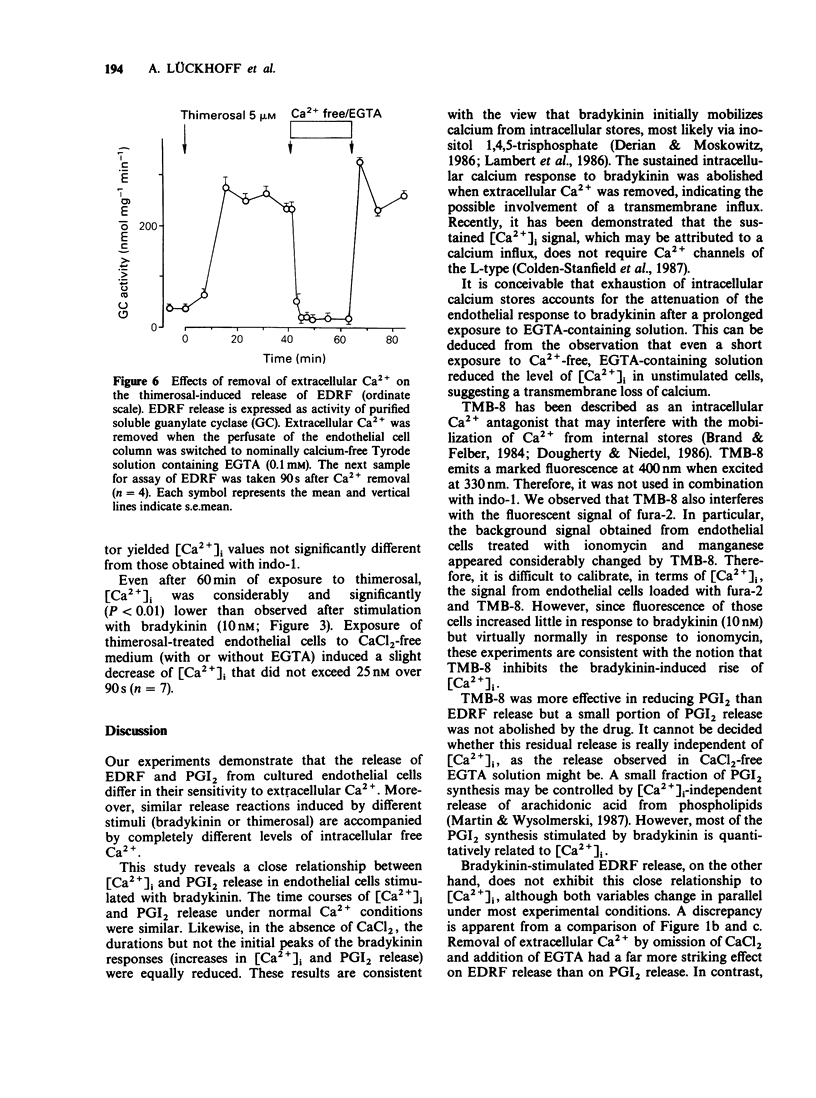
1. The effects of extracellular Ca2+ on the release of endothelium-derived relaxing factor (EDRF) and prostacyclin (PGI2), and on the intracellular free calcium concentration [( Ca2+]i), were studied in cultured bovine aortic endothelial cells. 2. Receptor-mediated stimulation of endothelial cells with bradykinin (10 nM) elicited a transient release of EDRF (assayed by its stimulant effect on purified soluble guanylate cyclase) and of PGI2 (measured by radioimmunoassay for 6-keto prostaglandin F1 alpha). 3. Bradykinin (10 nM) also increased [Ca2+]i (measured with the fluorescent probe indo-1) from 125 +/- 11 nM to 631 +/- 59 nM, with the same time course as for autacoid release. 4. In Ca2+-free medium, [Ca2+]i was still increased by bradykinin but declined faster (within 1 min) to resting levels than in the presence of extracellular Ca2+. 5. PGI2 release was almost completely abolished in Ca2+-free medium. The intracellular calcium antagonist TMB-8 evoked a similar inhibition of PGI2 release. 6. In contrast, bradykinin-induced EDRF release was not significantly affected by TMB-8 but was completely abolished in Ca2+-free medium. 7. When endothelial cells were stimulated with the receptor-independent drug thimerosal (an inhibitor of the enzyme acyl-CoA-lysolecithin-acyl-transferase; 5 microM), a long-lasting release of EDRF (greater than 90 min) and PGI2 (greater than 20 min) was observed. 8. In contrast to bradykinin stimulation, thimerosal-induced autacoid release was associated with only a slight increase of [Ca2+]i to 201 +/- 13 nM after 40 min.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

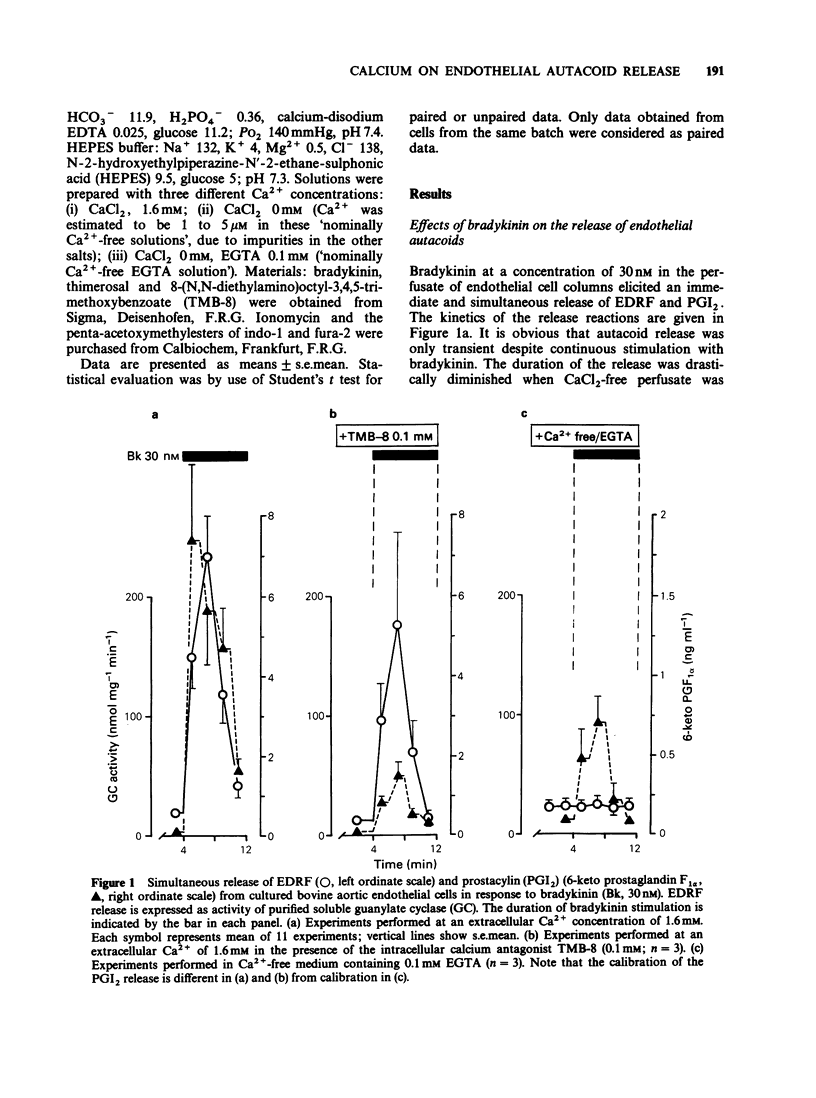
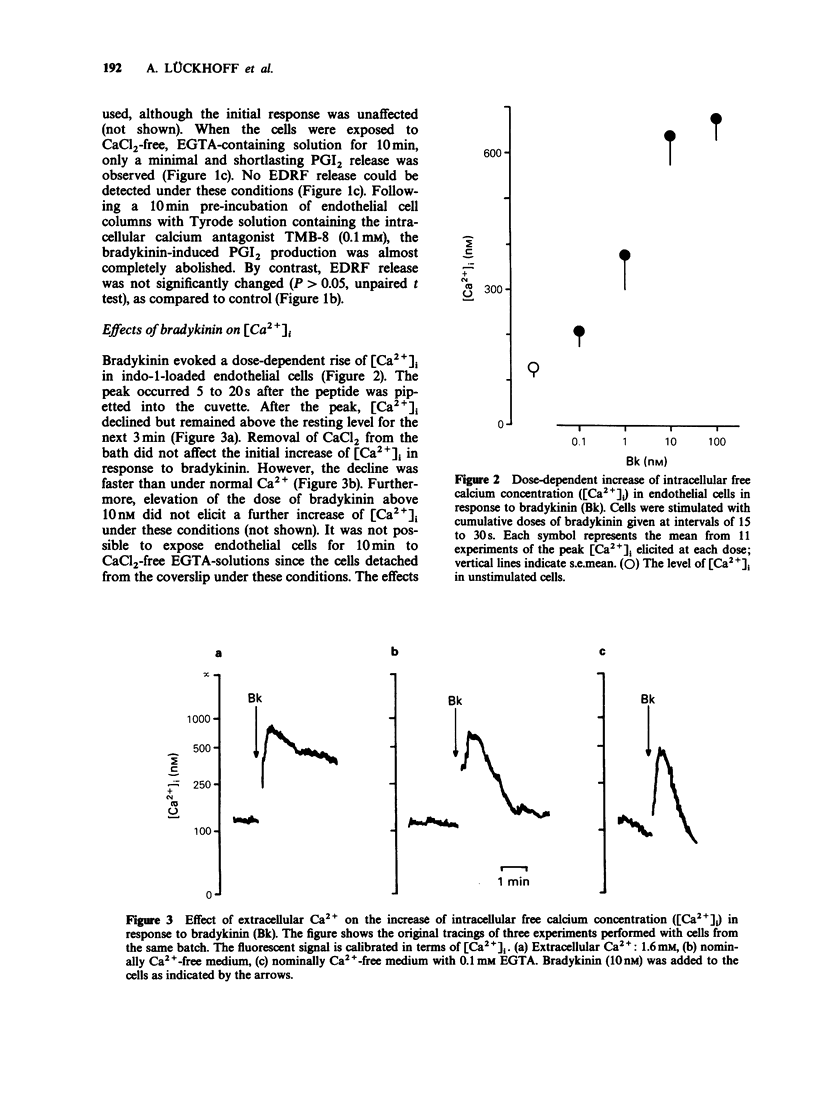


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Felber S. M. The intracellular calcium antagonist TMB-8 [8-(NN-diethylamino)octyl-3,4,5-trimethoxybenzoate] inhibits mitochondrial ATP production in rat thymocytes. Biochem J. 1984 Dec 15;224(3):1027–1030. doi: 10.1042/bj2241027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton A. F., Hoak J. C. Prostacyclin biosynthesis in cultured vascular endothelium is limited by deactivation of cyclooxygenase. J Clin Invest. 1983 Oct;72(4):1255–1261. doi: 10.1172/JCI111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Förstermann U., Matsuda H., Pohl U. The role of prostaglandins in the endothelium-mediated vasodilatory response to hypoxia. Pflugers Arch. 1984 May;401(1):77–83. doi: 10.1007/BF00581536. [DOI] [PubMed] [Google Scholar]
- Busse R., Trogisch G., Bassenge E. The role of endothelium in the control of vascular tone. Basic Res Cardiol. 1985 Sep-Oct;80(5):475–490. doi: 10.1007/BF01907912. [DOI] [PubMed] [Google Scholar]
- Colden-Stanfield M., Schilling W. P., Ritchie A. K., Eskin S. G., Navarro L. T., Kunze D. L. Bradykinin-induced increases in cytosolic calcium and ionic currents in cultured bovine aortic endothelial cells. Circ Res. 1987 Nov;61(5):632–640. doi: 10.1161/01.res.61.5.632. [DOI] [PubMed] [Google Scholar]
- Derian C. K., Moskowitz M. A. Polyphosphoinositide hydrolysis in endothelial cells and carotid artery segments. Bradykinin-2 receptor stimulation is calcium-independent. J Biol Chem. 1986 Mar 15;261(8):3831–3837. [PubMed] [Google Scholar]
- Dougherty R. W., Niedel J. E. Cytosolic calcium regulates phorbol diester binding affinity in intact phagocytes. J Biol Chem. 1986 Mar 25;261(9):4097–4100. [PubMed] [Google Scholar]
- Egan R. W., Paxton J., Kuehl F. A., Jr Mechanism for irreversible self-deactivation of prostaglandin synthetase. J Biol Chem. 1976 Dec 10;251(23):7329–7335. [PubMed] [Google Scholar]
- Forsberg E. J., Feuerstein G., Shohami E., Pollard H. B. Adenosine triphosphate stimulates inositol phospholipid metabolism and prostacyclin formation in adrenal medullary endothelial cells by means of P2-purinergic receptors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5630–5634. doi: 10.1073/pnas.84.16.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förstermann U., Goppelt-Strübe M., Frölich J. C., Busse R. Inhibitors of acyl-coenzyme A:lysolecithin acyltransferase activate the production of endothelium-derived vascular relaxing factor. J Pharmacol Exp Ther. 1986 Jul;238(1):352–359. [PubMed] [Google Scholar]
- Goppelt-Struebe M., Koerner C. F., Hausmann G., Gemsa D., Resch K. Control of prostanoid synthesis: role of reincorporation of released precursor fatty acids. Prostaglandins. 1986 Sep;32(3):373–385. doi: 10.1016/0090-6980(86)90006-7. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Newby A. C., Lewis M. J., Henderson A. H. Production of endothelium derived relaxant factor is dependent on oxidative phosphorylation and extracellular calcium. Cardiovasc Res. 1986 Jan;20(1):7–12. doi: 10.1093/cvr/20.1.7. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D. Exogenous ATP raises cytoplasmic free calcium in fura-2 loaded piglet aortic endothelial cells. FEBS Lett. 1986 Oct 20;207(1):95–99. doi: 10.1016/0014-5793(86)80019-9. [DOI] [PubMed] [Google Scholar]
- Lambert T. L., Kent R. S., Whorton A. R. Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. J Biol Chem. 1986 Nov 15;261(32):15288–15293. [PubMed] [Google Scholar]
- Long C. J., Stone T. W. The release of endothelium-derived relaxant factor is calcium dependent. Blood Vessels. 1985;22(4):205–208. doi: 10.1159/000158602. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Increased free calcium in endothelial cells under stimulation with adenine nucleotides. J Cell Physiol. 1986 Mar;126(3):414–420. doi: 10.1002/jcp.1041260312. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R., Winter I., Bassenge E. Characterization of vascular relaxant factor released from cultured endothelial cells. Hypertension. 1987 Mar;9(3):295–303. doi: 10.1161/01.hyp.9.3.295. [DOI] [PubMed] [Google Scholar]
- Lückhoff A. Measuring cytosolic free calcium concentration in endothelial cells with indo-1: the pitfall of using the ratio of two fluorescence intensities recorded at different wavelengths. Cell Calcium. 1986 Aug;7(4):233–248. doi: 10.1016/0143-4160(86)90003-5. [DOI] [PubMed] [Google Scholar]
- Martin T. W., Wysolmerski R. B. Ca2+-dependent and Ca2+-independent pathways for release of arachidonic acid from phosphatidylinositol in endothelial cells. J Biol Chem. 1987 Sep 25;262(27):13086–13092. [PubMed] [Google Scholar]
- Morgan-Boyd R., Stewart J. M., Vavrek R. J., Hassid A. Effects of bradykinin and angiotensin II on intracellular Ca2+ dynamics in endothelial cells. Am J Physiol. 1987 Oct;253(4 Pt 1):C588–C598. doi: 10.1152/ajpcell.1987.253.4.C588. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Böhme E., Busse R. Stimulation of soluble guanylate cyclase by endothelium-derived relaxing factor from cultured endothelial cells. Eur J Pharmacol. 1987 Mar 17;135(2):247–250. doi: 10.1016/0014-2999(87)90620-0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Peach M. J., Singer H. A., Izzo N. J., Jr, Loeb A. L. Role of calcium in endothelium-dependent relaxation of arterial smooth muscle. Am J Cardiol. 1987 Jan 23;59(2):35A–43A. doi: 10.1016/0002-9149(87)90174-3. [DOI] [PubMed] [Google Scholar]
- Pirotton S., Raspe E., Demolle D., Erneux C., Boeynaems J. M. Involvement of inositol 1,4,5-trisphosphate and calcium in the action of adenine nucleotides on aortic endothelial cells. J Biol Chem. 1987 Dec 25;262(36):17461–17466. [PubMed] [Google Scholar]


