Abstract
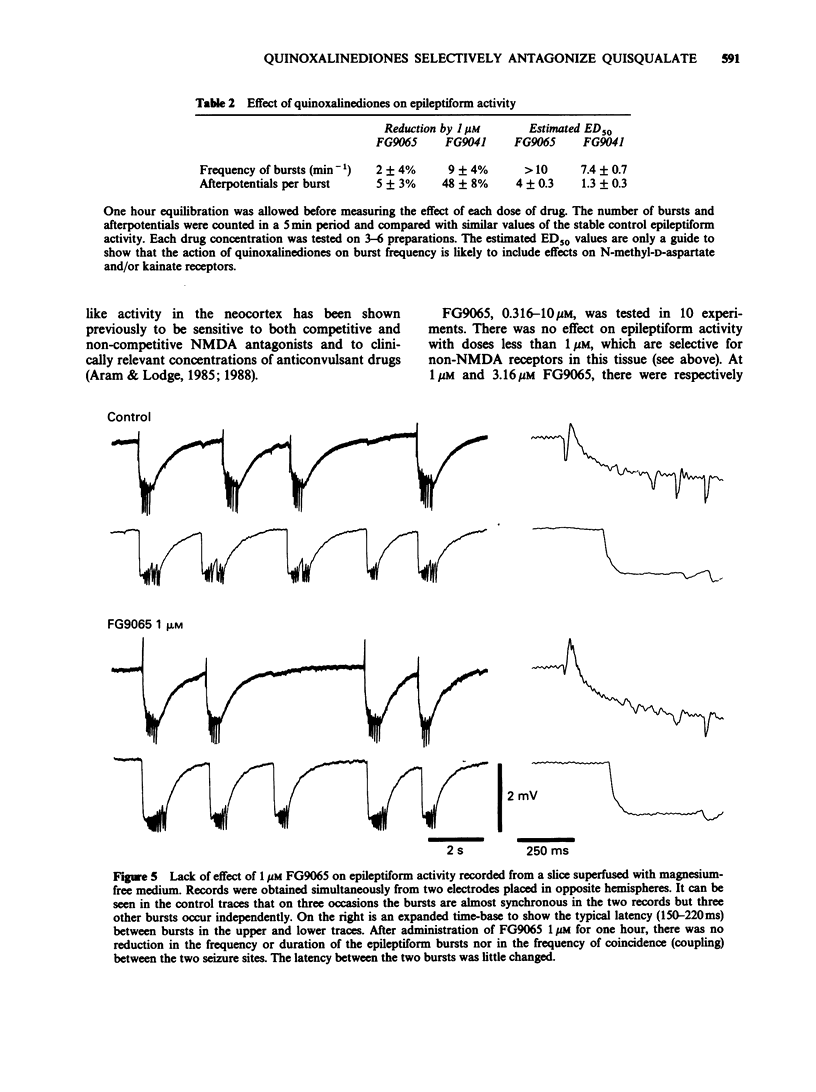
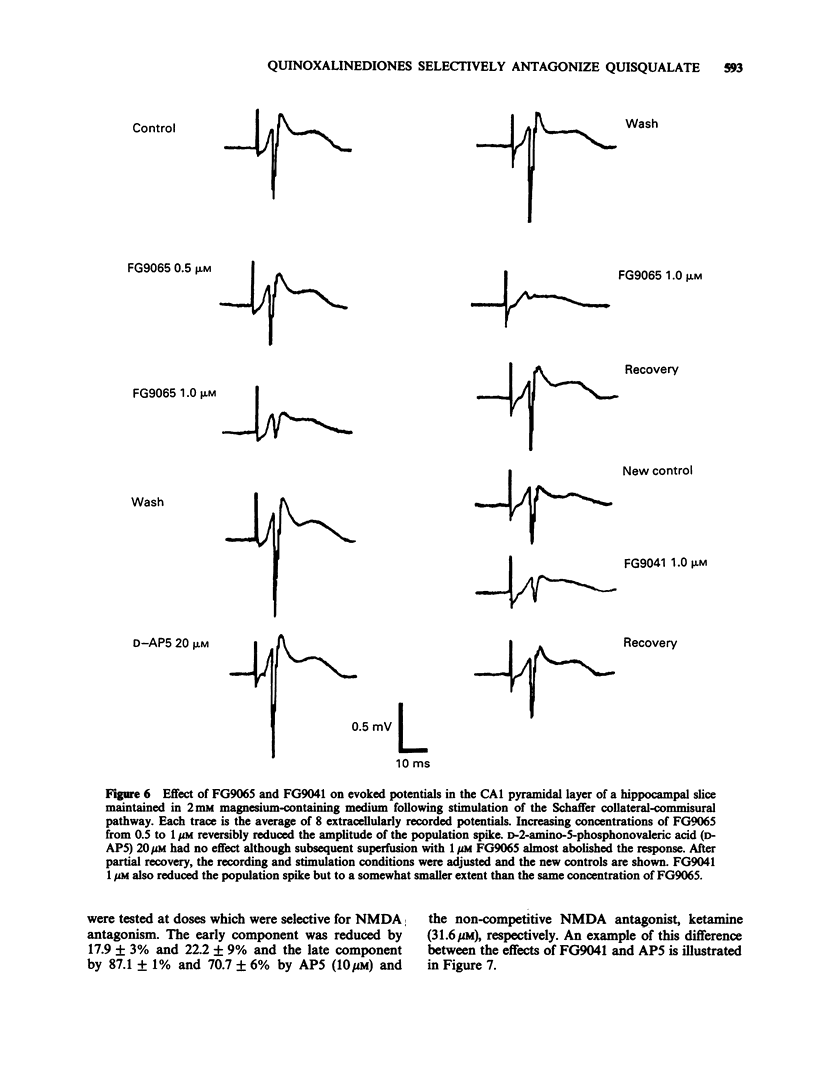
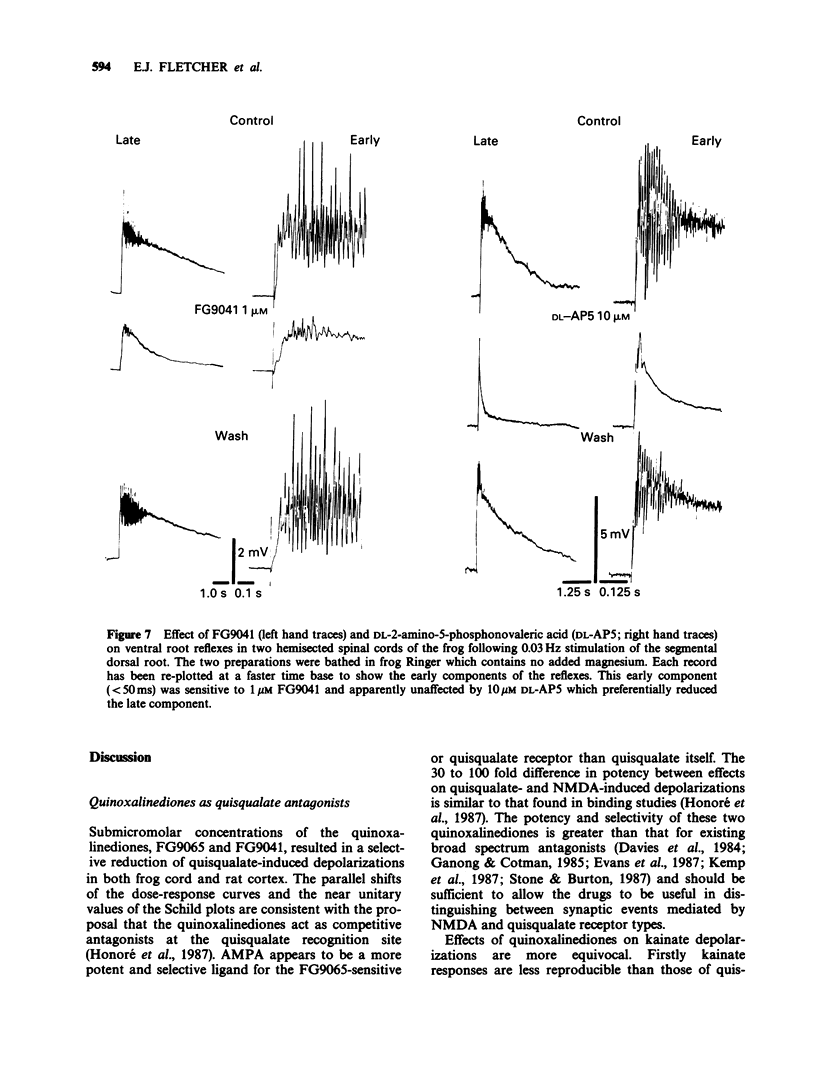
1. Two quinozalinediones, FG9041 and FG9065, which had previously been shown to displace binding to the quisqualate receptor, were tested on rat neocortex and frog spinal cord in vitro against depolarizations induced by quisqualate, kainate and N-methyl-D-aspartate (NMDA). In both preparations effects of quisqualate were reduced the most and those of NMDA the least. 2. The near unitary slopes of the Schild plots were consistent with a competitive type of interaction. pA2 values for FG9041 were estimated to be 6.6, 6.1 and 5.1 in frog cord and 5.9, 5.3 and and about 4 in the rat neocortex for quisqualate, kainate and NMDA antagonism, respectively. FG9065 gave equivalent pA2 values of 6.2, 5.6 and 4.5. 3. At concentrations, which were without effect on depolarizations induced by NMDA, FG9041 and FG9065 reduced or blocked synaptically-evoked field potentials in hippocampal and neocortical slices superfused with normal magnesium-containing medium. Since these synaptic components are also insensitive to NMDA antagonists, these results are consistent with their mediation by postsynaptic receptors of the quisqualate (or kainate) type. 4. By contrast, quinoxalinediones had only limited effects on spontaneous epileptiform activity seen in both neocortical and hippocampal preparations when superfused with magnesium-free medium. These burst discharges were, however, abolished by NMDA antagonists. 5. In the frog spinal cord the early component of the dorsal root to ventral root reflexes was selectively reduced by FG9041 whereas NMDA antagonists reduced the longer latency components. 6. Our results suggest that the quinoxalinediones are likely to be useful pharmacological probes for elucidating the role of non-NMDA receptors in the vertebrate central nervous system.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aram J. A., Lodge D. Validation of a neocortical slice preparation for the study of epileptiform activity. J Neurosci Methods. 1988 Apr;23(3):211–224. doi: 10.1016/0165-0270(88)90005-2. [DOI] [PubMed] [Google Scholar]
- Ault B., Evans R. H., Francis A. A., Oakes D. J., Watkins J. C. Selective depression of excitatory amino acid induced depolarizations by magnesium ions in isolated spinal cord preparations. J Physiol. 1980 Oct;307:413–428. doi: 10.1113/jphysiol.1980.sp013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan E. J., Collingridge G. L. Magnesium ions block an N-methyl-D-aspartate receptor-mediated component of synaptic transmission in rat hippocampus. Neurosci Lett. 1985 Jan 7;53(1):21–26. doi: 10.1016/0304-3940(85)90091-6. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A. Amino acid transmitters in the mammalian central nervous system. Ergeb Physiol. 1974;69(0):97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- Davies J., Jones A. W., Sheardown M. J., Smith D. A., Watkins J. C. Phosphono dipeptides and piperazine derivatives as antagonists of amino acid-induced and synaptic excitation in mammalian and amphibian spinal cord. Neurosci Lett. 1984 Nov 23;52(1-2):79–84. doi: 10.1016/0304-3940(84)90354-9. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Role of excitatory amino acid receptors in mono- and polysynaptic excitation in the cat spinal cord. Exp Brain Res. 1983;49(2):280–290. doi: 10.1007/BF00238587. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Hynes M. A., King G. L. Involvement of N-methyl-D-aspartate receptors in epileptiform bursting in the rat hippocampal slice. J Physiol. 1986 Nov;380:175–189. doi: 10.1113/jphysiol.1986.sp016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. H., Evans S. J., Pook P. C., Sunter D. C. A comparison of excitatory amino acid antagonists acting at primary afferent C fibres and motoneurones of the isolated spinal cord of the rat. Br J Pharmacol. 1987 Jul;91(3):531–537. doi: 10.1111/j.1476-5381.1987.tb11246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Hunt K., Oakes D. J., Watkins J. C. Antagonism of excitatory amino acid-induced responses and of synaptic excitation in the isolated spinal cord of the frog. Br J Pharmacol. 1979 Dec;67(4):591–603. doi: 10.1111/j.1476-5381.1979.tb08706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984 Jan;42(1):1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res. 1984 May;319(2):103–164. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Ganong A. H., Cotman C. W. Kynurenic acid and quinolinic acid act at N-methyl-D-aspartate receptors in the rat hippocampus. J Pharmacol Exp Ther. 1986 Jan;236(1):293–299. [PubMed] [Google Scholar]
- Harris E. W., Ganong A. H., Cotman C. W. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984 Dec 3;323(1):132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Simmonds M. A. Quantitative studies on some antagonists of N-methyl D-aspartate in slices of rat cerebral cortex. Br J Pharmacol. 1985 Feb;84(2):381–391. doi: 10.1111/j.1476-5381.1985.tb12922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley P. M., Parsons C. G., West D. C. The role of N-methylaspartate receptors in mediating responses of rat and cat spinal neurones to defined sensory stimuli. J Physiol. 1987 Apr;385:169–188. doi: 10.1113/jphysiol.1987.sp016490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron C. E., Lester R. A., Coan E. J., Collingridge G. L. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature. 1986 Jul 17;322(6076):265–268. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- Herron C. E., Williamson R., Collingridge G. L. A selective N-methyl-D-aspartate antagonist depresses epileptiform activity in rat hippocampal slices. Neurosci Lett. 1985 Nov 11;61(3):255–260. doi: 10.1016/0304-3940(85)90473-2. [DOI] [PubMed] [Google Scholar]
- Jones R. S. Complex synaptic responses of entorhinal cortical cells in the rat to subicular stimulation in vitro: demonstration of an NMDA receptor-mediated component. Neurosci Lett. 1987 Oct 16;81(1-2):209–214. doi: 10.1016/0304-3940(87)90000-0. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P., Honoré T., Hansen J. J., Curtis D. R., Lodge D. New class of glutamate agonist structurally related to ibotenic acid. Nature. 1980 Mar 6;284(5751):64–66. doi: 10.1038/284064a0. [DOI] [PubMed] [Google Scholar]
- Martin D., Lodge D. Ketamine acts as a non-competitive N-methyl-D-aspartate antagonist on frog spinal cord in vitro. Neuropharmacology. 1985 Oct;24(10):999–1003. doi: 10.1016/0028-3908(85)90128-5. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- McLennan H., Lodge D. The antagonism of amino acid-induced excitation of spinal neurones in the cat. Brain Res. 1979 Jun 15;169(1):83–90. doi: 10.1016/0006-8993(79)90375-5. [DOI] [PubMed] [Google Scholar]
- McLennan H. Receptors for the excitatory amino acids in the mammalian central nervous system. Prog Neurobiol. 1983;20(3-4):251–271. doi: 10.1016/0301-0082(83)90004-7. [DOI] [PubMed] [Google Scholar]
- Mody I., Heinemann U. NMDA receptors of dentate gyrus granule cells participate in synaptic transmission following kindling. Nature. 1987 Apr 16;326(6114):701–704. doi: 10.1038/326701a0. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Yao D., Cotman C. W. L-[3H]Glutamate binds to kainate-, NMDA- and AMPA-sensitive binding sites: an autoradiographic analysis. Brain Res. 1985 Aug 12;340(2):378–383. doi: 10.1016/0006-8993(85)90936-9. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Perkins M. N., Stone T. W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982 Sep 9;247(1):184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- Salt T. E. Mediation of thalamic sensory input by both NMDA receptors and non-NMDA receptors. Nature. 1986 Jul 17;322(6076):263–265. doi: 10.1038/322263a0. [DOI] [PubMed] [Google Scholar]
- Stone T. W., Burton N. R. NMDA receptors and ligands in the vertebrate CNS. Prog Neurobiol. 1988;30(4):333–368. doi: 10.1016/0301-0082(88)90027-5. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., West D. C., Lodge D. An N-methylaspartate receptor-mediated synapse in rat cerebral cortex: a site of action of ketamine? Nature. 1985 Feb 7;313(6002):479–481. doi: 10.1038/313479a0. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]


