Abstract
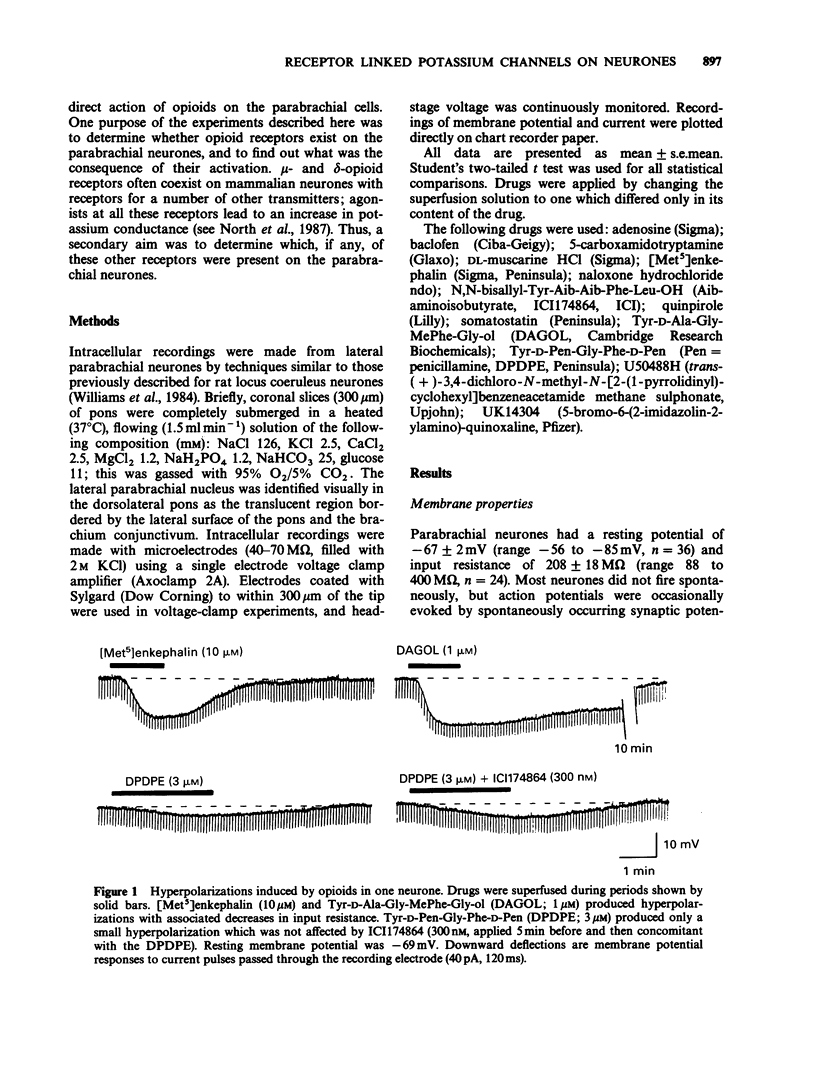
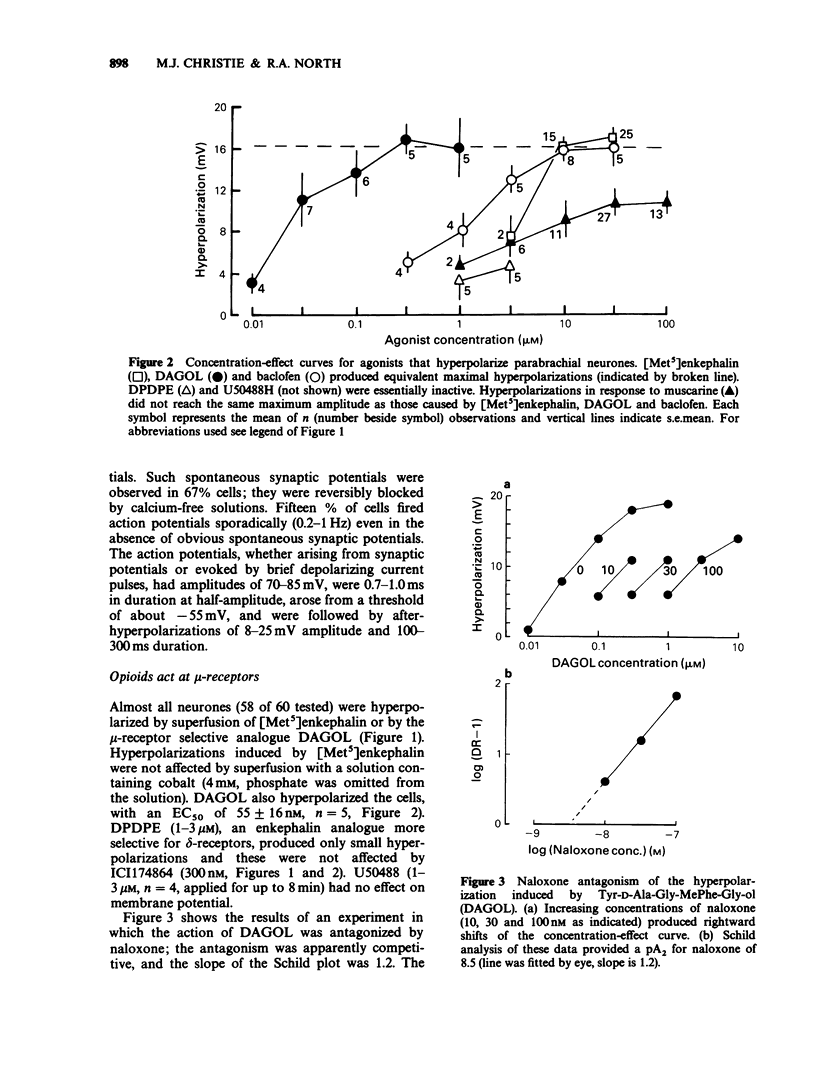
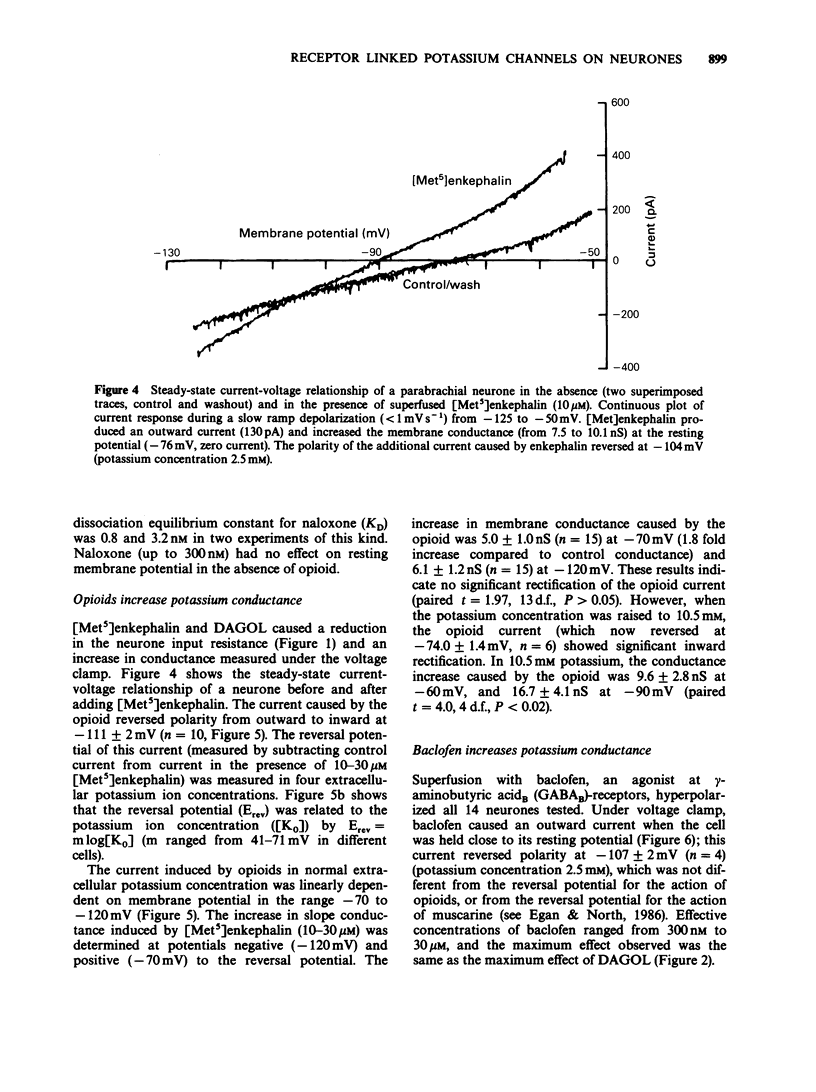
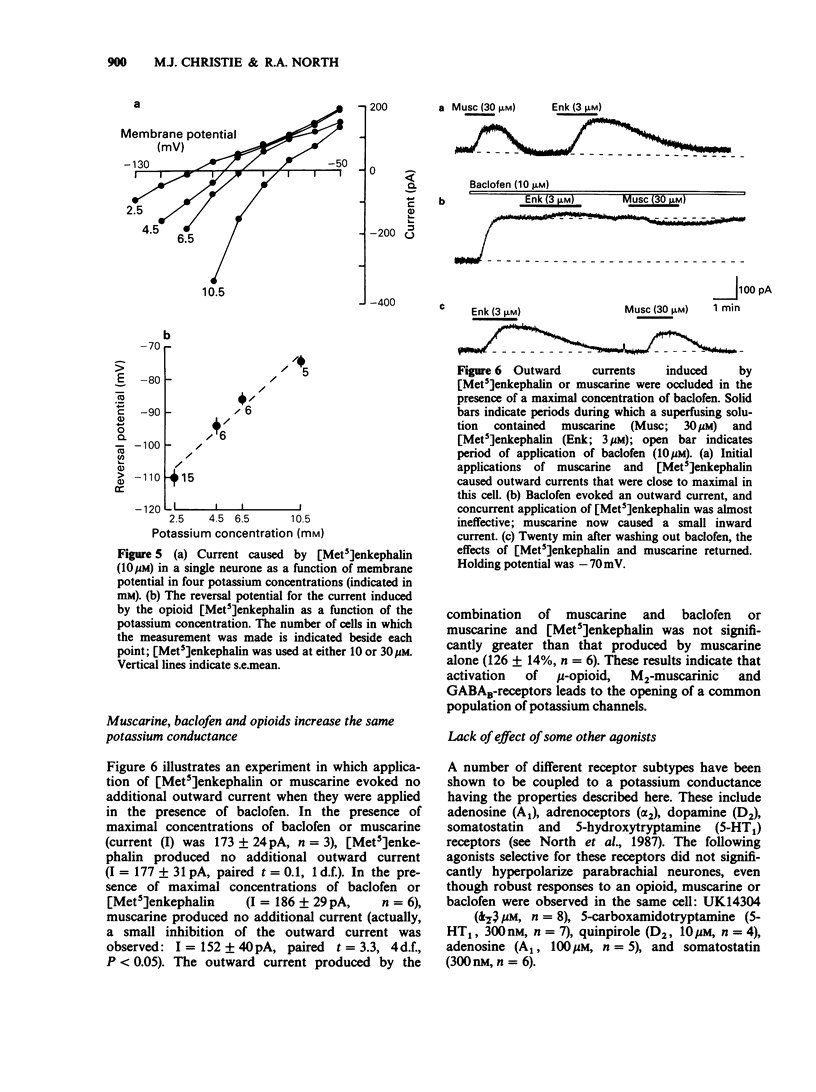
1. Intracellular recordings of membrane potential and current were made from neurones in the lateral parabrachial nucleus in slices of rat brain in vitro. 2. The membrane was hyperpolarized by the opioid peptides Tyr-D-Ala-Gly-MePhe-Gly-ol (DAGOL, 0.01-1 microM) and [Met5]enkephalin (3-30 microM), though not by Tyr-D-Pen-Gly-Phe-D-Pen and U50488. In two experiments, naloxone competitively antagonized the effects of DAGOL and [Met]enkephalin with equilibrium dissociation constants of 0.8 and 3.2 nM, respectively. 3. Baclofen (0.3-30 microM) also hyperpolarized the neurones; this action was unaffected by naloxone. 4. DAGOL, [Met5]enkephalin and baclofen caused outward currents at the resting potential. These currents reversed polarity at a membrane potential which changed with the logarithm of the extracellular potassium concentration. 5. Muscarine has been shown previously to increase the potassium conductance by an action at M2-receptors: the potassium currents induced by maximal concentrations of muscarine, baclofen and [Met5]enkephalin were non-additive, indicating that these agonists opened the same population of potassium channels. 6. Noradrenaline, UK14304, carboxamidotryptamine, dopamine, adenosine and somatostatin had little or no effect on membrane potential. 7. It is concluded that rat lateral parabrachial neurones express mu-opioid, gamma-aminobutyric acidB (GABAB), and M2-muscarinic receptors: activation of any of these receptors increases the potassium conductance of the membrane and inhibits the neurones through hyperpolarization.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade R., Malenka R. C., Nicoll R. A. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986 Dec 5;234(4781):1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Atweh S. F., Kuhar M. J. Autoradiographic localization of opiate receptors in rat brain. II. The brain stem. Brain Res. 1977 Jun 24;129(1):1–12. doi: 10.1016/0006-8993(77)90965-9. [DOI] [PubMed] [Google Scholar]
- Cechetto D. F. Central representation of visceral function. Fed Proc. 1987 Jan;46(1):17–23. [PubMed] [Google Scholar]
- Chang K. J., Cooper B. R., Hazum E., Cuatrecasas P. Multiple opiate receptors: different regional distribution in the brain and differential binding of opiates and opioid peptides. Mol Pharmacol. 1979 Jul;16(1):91–104. [PubMed] [Google Scholar]
- Denavit-Saubié M., Champagnat J., Zieglgänsberger W. Effects of opiates and methionine-enkephalin on pontine and bulbar respiratory neurones of the cat. Brain Res. 1978 Oct 20;155(1):55–67. doi: 10.1016/0006-8993(78)90305-0. [DOI] [PubMed] [Google Scholar]
- Egan T. M., North R. A. Acetylcholine hyperpolarizes central neurones by acting on an M2 muscarinic receptor. 1986 Jan 30-Feb 5Nature. 319(6052):405–407. doi: 10.1038/319405a0. [DOI] [PubMed] [Google Scholar]
- Fulwiler C. E., Saper C. B. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. 1984 Aug;319(3):229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. R., Sutor B., Zieglgänsberger W. Baclofen reduces post-synaptic potentials of rat cortical neurones by an action other than its hyperpolarizing action. J Physiol. 1987 Mar;384:539–569. doi: 10.1113/jphysiol.1987.sp016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterlitz H. W., Watt A. J. Kinetic parameters of narcotic agonists and antagonists, with particular reference to N-allylnoroxymorphone (naloxone). Br J Pharmacol Chemother. 1968 Jun;33(2):266–276. doi: 10.1111/j.1476-5381.1968.tb00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. On the potassium conductance increase activated by GABAB and dopamine D2 receptors in rat substantia nigra neurones. J Physiol. 1988 Jul;401:437–453. doi: 10.1113/jphysiol.1988.sp017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie F. M., Chavkin C., Cox B. M. Opioid binding properties of brain and peripheral tissues: evidence for heterogeneity in opioid ligand binding sites. J Pharmacol Exp Ther. 1980 Aug;214(2):395–402. [PubMed] [Google Scholar]
- Murakami S., Okamura H., Yanaihara C., Yanaihara N., Ibata Y. Immunocytochemical distribution of met-enkephalin-Arg6-Gly7-Leu8 in the rat lower brainstem. J Comp Neurol. 1987 Jul 8;261(2):193–208. doi: 10.1002/cne.902610203. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Williams J. T. On the potassium conductance increased by opioids in rat locus coeruleus neurones. J Physiol. 1985 Jul;364:265–280. doi: 10.1113/jphysiol.1985.sp015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Williams J. T., Surprenant A., Christie M. J. Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5487–5491. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmanović S. S., Shefner S. A. Baclofen increases the potassium conductance of rat locus coeruleus neurons recorded in brain slices. Brain Res. 1988 Jan 12;438(1-2):124–136. doi: 10.1016/0006-8993(88)91331-5. [DOI] [PubMed] [Google Scholar]
- Pappano A. J., Matsumoto K., Tajima T., Agnarsson U., Webb W. Pertussis toxin-insensitive mechanism for carbachol-induced depolarization and positive inotropic effect in heart muscle. Trends Pharmacol Sci. 1988 Feb;Suppl:35–39. [PubMed] [Google Scholar]
- Standaert D. G., Watson S. J., Houghten R. A., Saper C. B. Opioid peptide immunoreactivity in spinal and trigeminal dorsal horn neurons projecting to the parabrachial nucleus in the rat. J Neurosci. 1986 May;6(5):1220–1226. doi: 10.1523/JNEUROSCI.06-05-01220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. R., Gallagher J. P., Shinnick-Gallagher P. Further studies on the action of baclofen on neurons of the dorsolateral septal nucleus of the rat, in vitro. Brain Res. 1985 Dec 9;358(1-2):360–363. doi: 10.1016/0006-8993(85)90984-9. [DOI] [PubMed] [Google Scholar]
- Travers J. B., Travers S. P., Norgren R. Gustatory neural processing in the hindbrain. Annu Rev Neurosci. 1987;10:595–632. doi: 10.1146/annurev.ne.10.030187.003115. [DOI] [PubMed] [Google Scholar]
- Watson S. J., Khachaturian H., Taylor L., Fischli W., Goldstein A., Akil H. Pro-dynorphin peptides are found in the same neurons throughout rat brain: immunocytochemical study. Proc Natl Acad Sci U S A. 1983 Feb;80(3):891–894. doi: 10.1073/pnas.80.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. T., North R. A. Opiate-receptor interactions on single locus coeruleus neurones. Mol Pharmacol. 1984 Nov;26(3):489–497. [PubMed] [Google Scholar]
- Williams J. T., North R. A., Shefner S. A., Nishi S., Egan T. M. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984 Sep;13(1):137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]


