Abstract
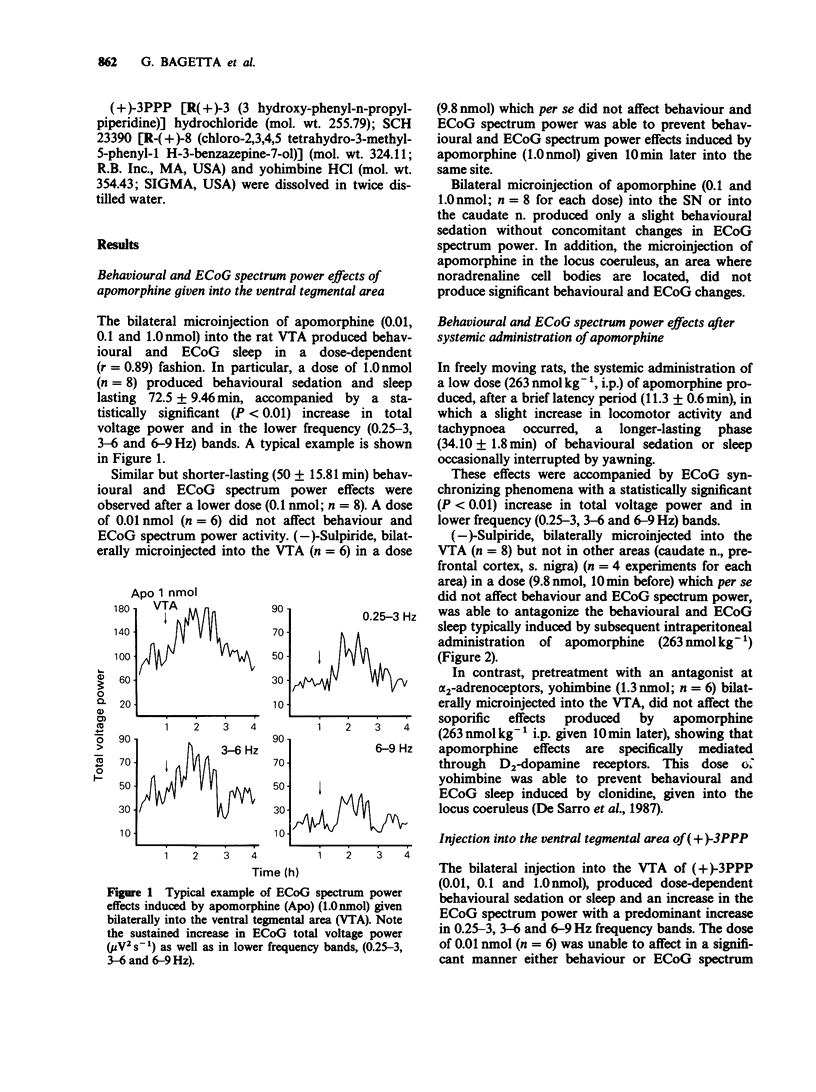
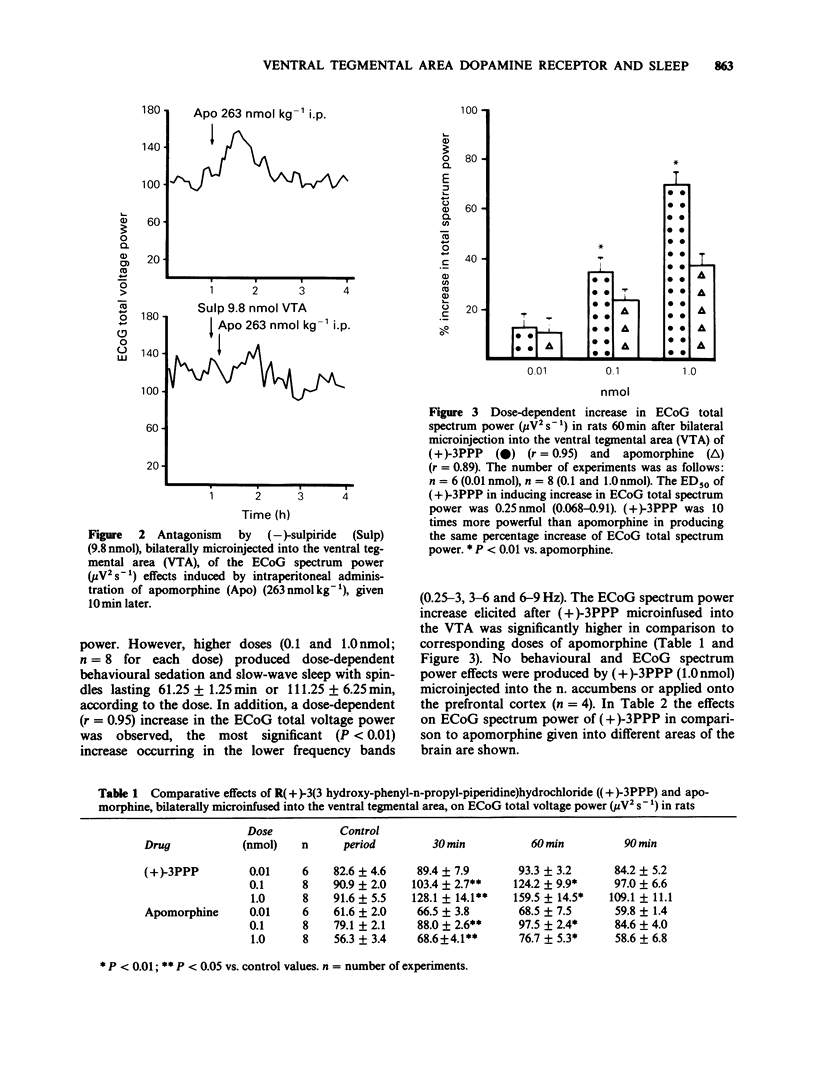
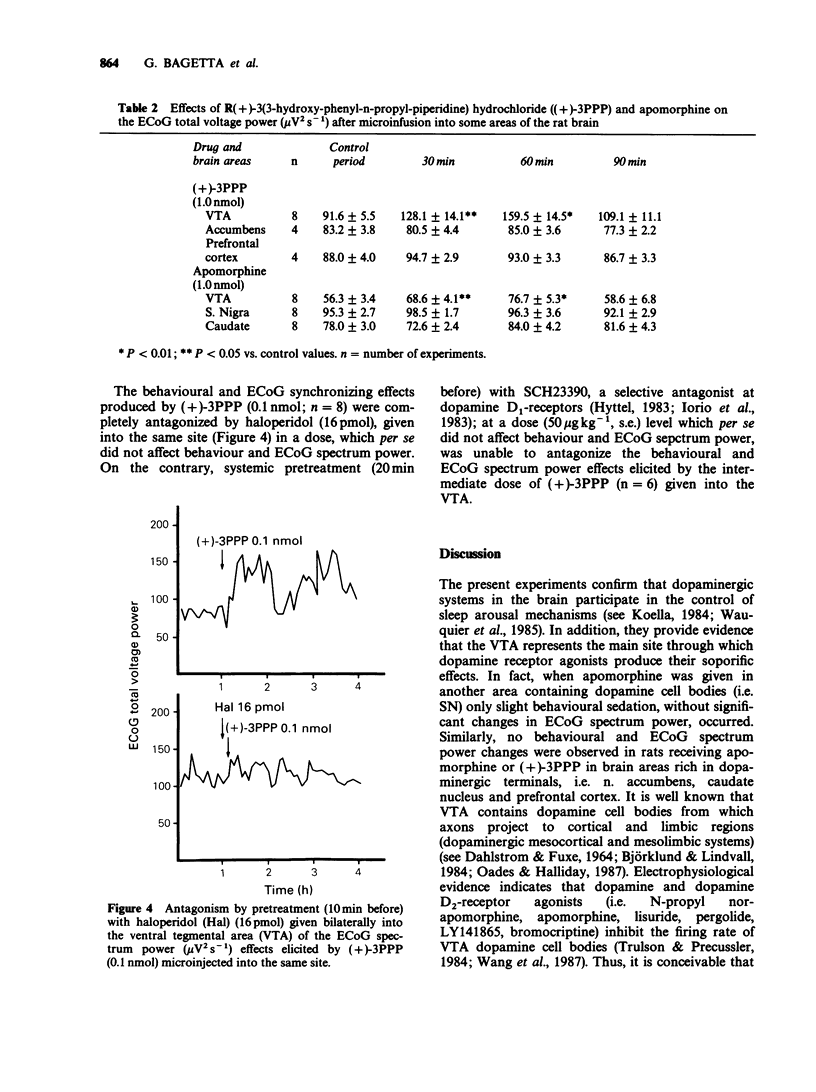
1. In freely moving rats the effects on behaviour and electrocortical (ECoG) spectrum power of some dopamine agonists, i.e. apomorphine and (+)-3PPP, given directly into different areas of the rat brain were studied. In particular, dopamine agonists were microinfused in the ventral tegmental area (VTA) and substantia nigra (SN) or into the caudate nucleus, n. accumbens and prefrontal cortex. The ECoG spectrum power effects were continuously analysed by means of a computerized Berg-Fourier analyser as total spectrum power and power in preselected frequency bands. 2. Apomorphine and (+)-3PPP (0.01, 0.1 and 1.0 nmol) given bilaterally into the VTA produced behavioural and ECoG sleep in a dose-dependent fashion. A statistically significant (P less than 0.01) increase in ECoG total spectrum power with a predominant increase in the lower frequency bands (0.25-3, 3-6 and 6-9 Hz) occurred. No behavioural and ECoG changes were evoked by the same doses of apomorphine bilaterally microinfused into the SN or into the caudate nucleus or by (+)-3PPP (1.0 nml) microinjected into the n. accumbens or applied onto the prefrontal cortex. 3. Behavioural and ECoG sleep was also induced in rats after systemic administration of apomorphine (263 nmol kg-1, i.p.). 4. The behavioural and ECoG spectrum power effects of apomorphine (1.0 nmol) bilaterally micro-infused into the VTA were prevented by a previous microinjection into the same site of (-)-sulpiride (9.8 nmol). Similarly, behavioural and ECoG effects evoked by (+)-3PPP (0.1 nmol) given bilaterally into the VTA, were completely antagonized by a previous injection into the same site of haloperidol (16 pmol given 10 min before).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andén N. E., Butcher S. G., Corrodi H., Fuxe K., Ungerstedt U. Receptor activity and turnover of dopamine and noradrenaline after neuroleptics. Eur J Pharmacol. 1970;11(3):303–314. doi: 10.1016/0014-2999(70)90006-3. [DOI] [PubMed] [Google Scholar]
- Argiolas A., Melis M. R., Fadda F., Gessa G. L. Evidence for dopamine autoreceptors controlling dopamine synthesis in the substantia nigra. Brain Res. 1982 Feb 18;234(1):177–181. doi: 10.1016/0006-8993(82)90484-x. [DOI] [PubMed] [Google Scholar]
- Bagetta G., Corasaniti M. T., Strongoli M. C., Sakurada S., Nisticò G. Behavioural and ECoG spectrum power effects after intraventricular injection of drugs altering dopaminergic transmission in rats. Neuropharmacology. 1987 Aug;26(8):1047–1052. doi: 10.1016/0028-3908(87)90247-4. [DOI] [PubMed] [Google Scholar]
- Bagetta G., Corasaniti M. T., Strongoli M. C., Sakurada S., Nisticò G. Electrocortical spectrum power effects of different classes of neuroleptics in rats. J Psychiatr Res. 1987;21(1):93–99. doi: 10.1016/0022-3956(87)90009-4. [DOI] [PubMed] [Google Scholar]
- Bricolo A., Turazzi S., Faccioli F., Odorizzi F., Sciaretta G., Erculiani P. Clinical application of compressed spectral array in long-term EEG monitoring of comatose patients. Electroencephalogr Clin Neurophysiol. 1978 Aug;45(2):211–225. doi: 10.1016/0013-4694(78)90005-6. [DOI] [PubMed] [Google Scholar]
- De Sarro G. B., Ascioti C., Froio F., Libri V., Nisticò G. Evidence that locus coeruleus is the site where clonidine and drugs acting at alpha 1- and alpha 2-adrenoceptors affect sleep and arousal mechanisms. Br J Pharmacol. 1987 Apr;90(4):675–685. doi: 10.1111/j.1476-5381.1987.tb11220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G., Porceddu M. L., Vargiu L., Argiolas A., Gessa G. L. Evidence for dopamine receptors mediating sedation in the mouse brain. Nature. 1976 Dec 9;264(5586):564–567. doi: 10.1038/264564a0. [DOI] [PubMed] [Google Scholar]
- Gessa G. L., Porceddu M. L., Collu M., Mereu G., Serra M., Ongini E., Biggio G. Sedation and sleep induced by high doses of apomorphine after blockade of D-1 receptors by SCH 23390. Eur J Pharmacol. 1985 Feb 26;109(2):269–274. doi: 10.1016/0014-2999(85)90429-7. [DOI] [PubMed] [Google Scholar]
- Hjorth S., Carlsson A., Wikström H., Lindberg P., Sanchez D., Hacksell U., Arvidsson L. E., Svensson U., Nilsson J. L. 3-PPP, a new centrally acting DA-receptor agonist with selectivity for autoreceptors. Life Sci. 1981 Mar 16;28(11):1225–1238. doi: 10.1016/0024-3205(81)90448-3. [DOI] [PubMed] [Google Scholar]
- Hjorth S. On the mode of action of 3-(3-hydroxyphenyl)-N-n-propylpiperidine, 3-PPP, and its enantiomers. With particular reference to dopaminergic mechanisms in the central nervous system. Acta Physiol Scand Suppl. 1983;517:1–52. [PubMed] [Google Scholar]
- Hyttel J. SCH 23390 - the first selective dopamine D-1 antagonist. Eur J Pharmacol. 1983 Jul 15;91(1):153–154. doi: 10.1016/0014-2999(83)90381-3. [DOI] [PubMed] [Google Scholar]
- Iorio L. C., Barnett A., Leitz F. H., Houser V. P., Korduba C. A. SCH 23390, a potential benzazepine antipsychotic with unique interactions on dopaminergic systems. J Pharmacol Exp Ther. 1983 Aug;226(2):462–468. [PubMed] [Google Scholar]
- Kafi S., Gaillard J. M. Brain dopamine receptors and sleep in the rat: effects of stimulation and blockade. Eur J Pharmacol. 1976 Aug;38(2):357–363. doi: 10.1016/0014-2999(76)90340-x. [DOI] [PubMed] [Google Scholar]
- Koella W. P. The organization and regulation of sleep. A review of the experimental evidence and a novel integrated model of the organizing and regulating apparatus. Experientia. 1984 Apr 15;40(4):309–338. doi: 10.1007/BF01952538. [DOI] [PubMed] [Google Scholar]
- Laduron P. M., Leysen J. E. Domperidone, a specific in vitro dopamine antagonist, devoid of in vivo central dopaminergic activity. Biochem Pharmacol. 1979 Jul 15;28(14):2161–2165. doi: 10.1016/0006-2952(79)90198-9. [DOI] [PubMed] [Google Scholar]
- Marley E., Nistico G. Effects of catecholamines and adenosine derivatives given into the brain of fowls. Br J Pharmacol. 1972 Dec;46(4):619–636. doi: 10.1111/j.1476-5381.1972.tb06888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu G. P., Scarnati E., Paglietti E., Quarantotti B. P., Chessa P., Di Chiara G., Gessa G. L. Sleep induced by low doses of apomorphine in rats. Electroencephalogr Clin Neurophysiol. 1979 Feb;46(2):214–219. doi: 10.1016/0013-4694(79)90071-3. [DOI] [PubMed] [Google Scholar]
- Monti J. M. Catecholamines and the sleep-wake cycle. I. EEG and behavioral arousal. Life Sci. 1982 Apr 5;30(14):1145–1157. doi: 10.1016/0024-3205(82)90656-7. [DOI] [PubMed] [Google Scholar]
- Nisticò G., Stephenson J. D., Preziosi P. Behavioural, electrocortical and body temperature effects of cholera toxin. Eur J Pharmacol. 1976 Apr;36(2):459–462. doi: 10.1016/0014-2999(76)90103-5. [DOI] [PubMed] [Google Scholar]
- Oades R. D., Halliday G. M. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987 May;434(2):117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Trulson M. E., Preussler D. W. Dopamine-containing ventral tegmental area neurons in freely moving cats: activity during the sleep-waking cycle and effects of stress. Exp Neurol. 1984 Feb;83(2):367–377. doi: 10.1016/S0014-4886(84)90105-5. [DOI] [PubMed] [Google Scholar]
- Wiegand H., Erdmann G., Wellhöner H. H. 125I-labelled botulinum A neurotoxin: pharmacokinetics in cats after intramuscular injection. Naunyn Schmiedebergs Arch Pharmacol. 1976;292(2):161–165. doi: 10.1007/BF00498587. [DOI] [PubMed] [Google Scholar]


