Abstract
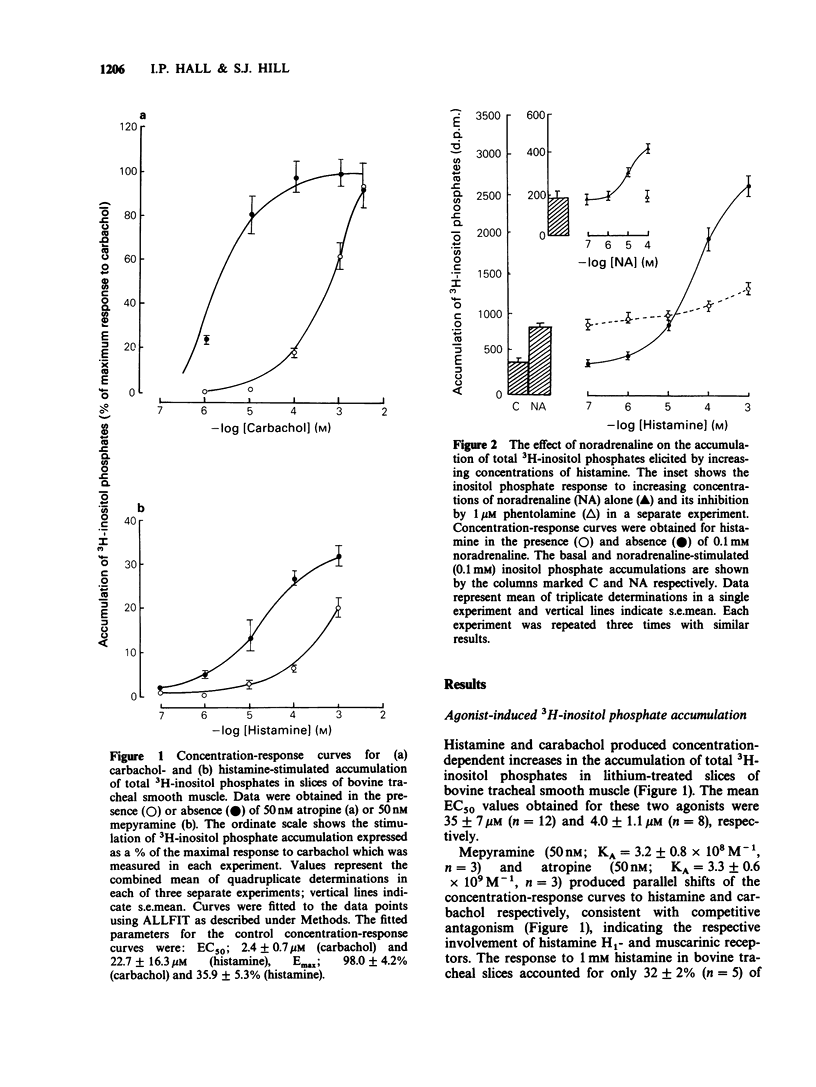
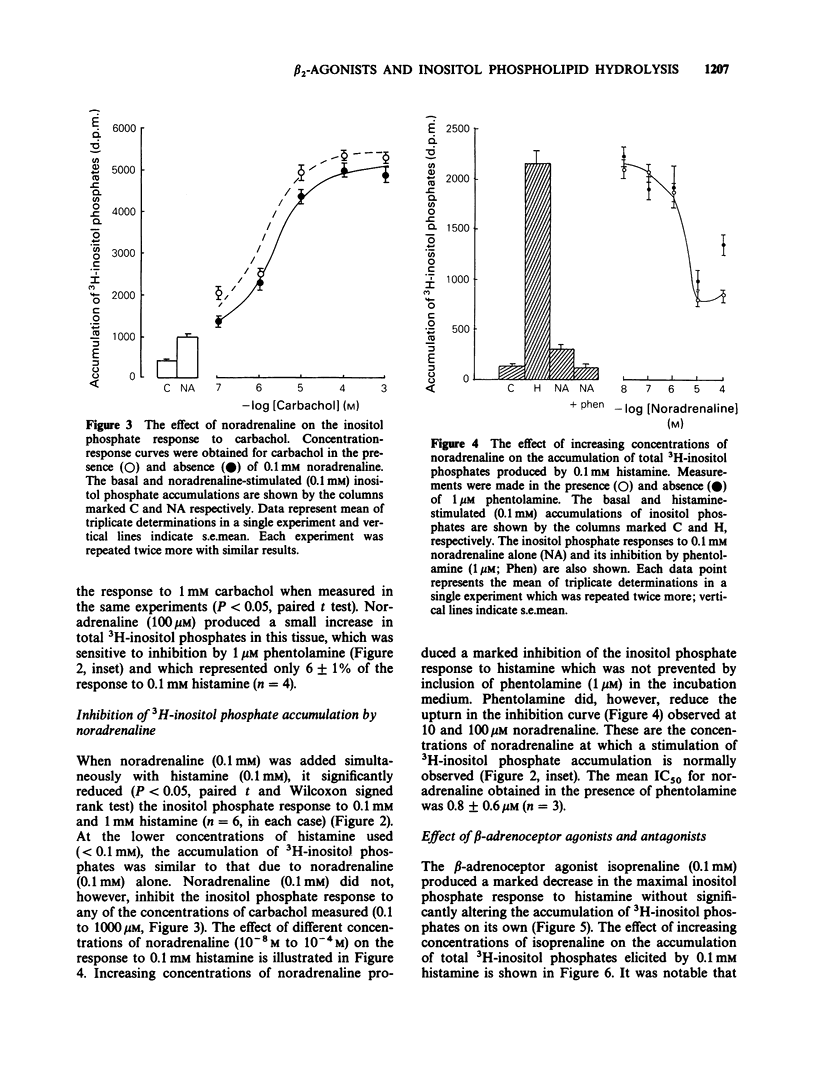
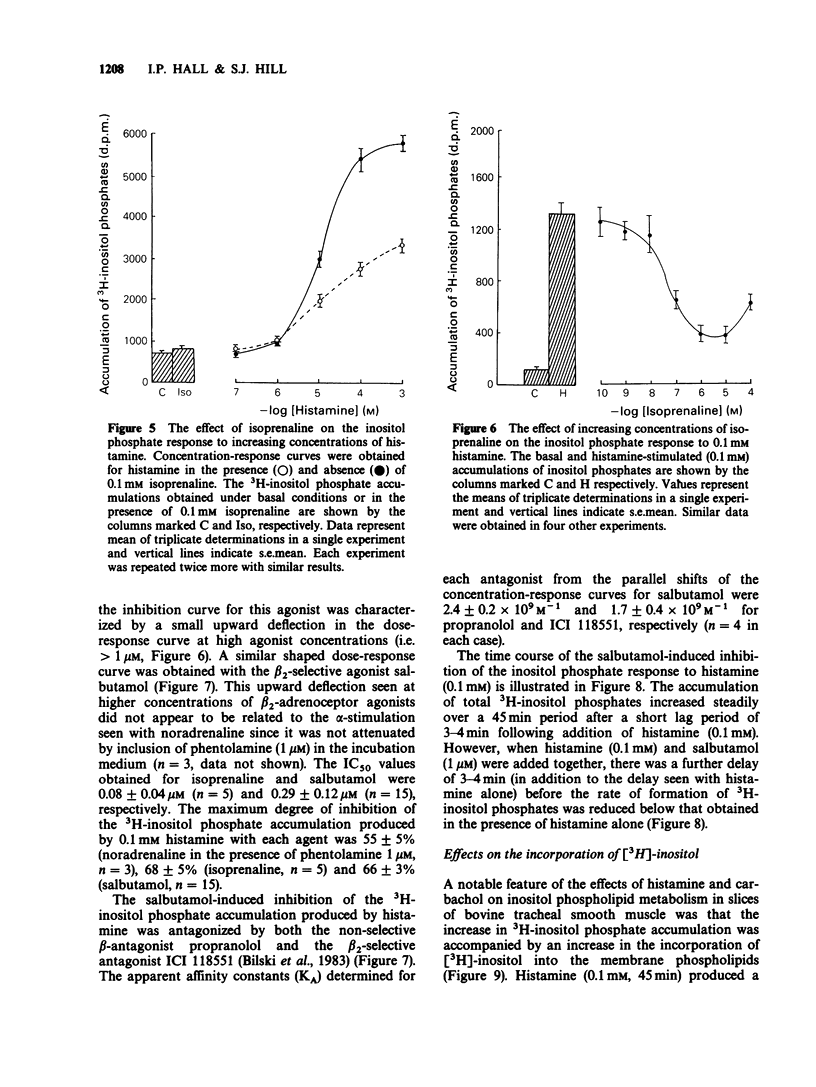
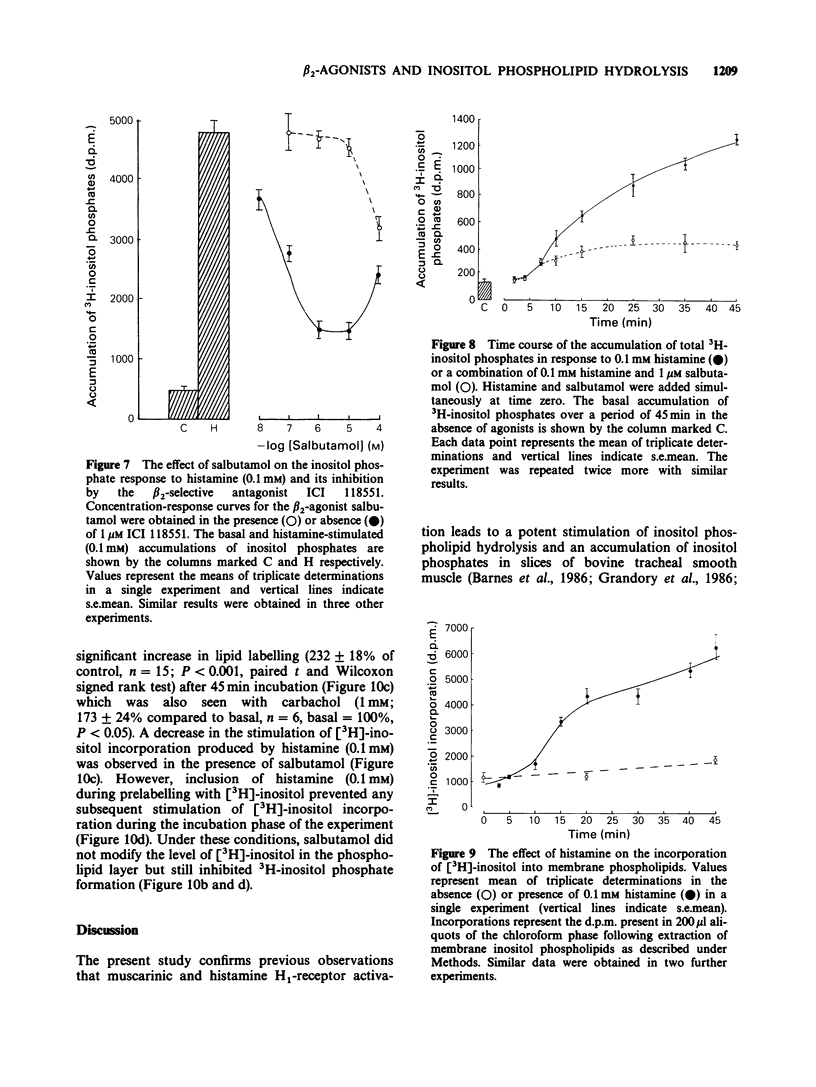
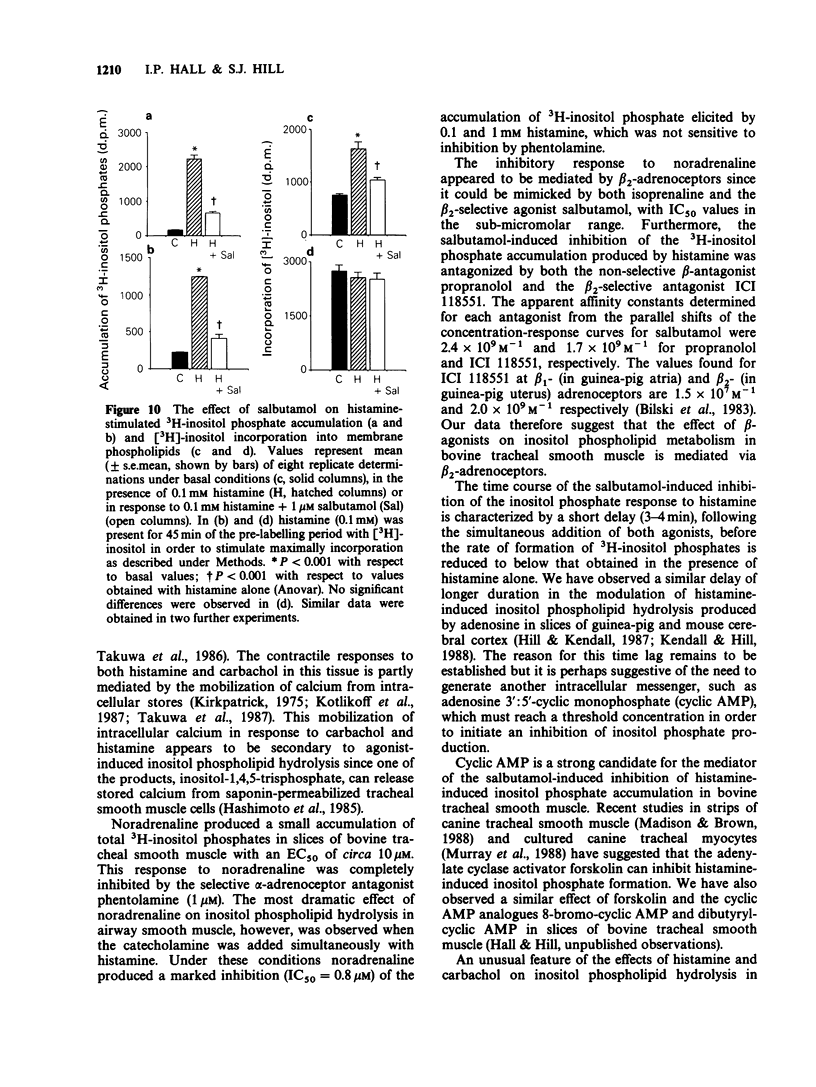
1. Histamine and carbachol produced concentration-related increases in the accumulation of 3H-inositol phosphates in slices of bovine tracheal smooth muscle. 2. Noradrenaline alone produced a small stimulation of 3H-inositol phosphate accumulation which was inhibited by the alpha-adrenoceptor antagonist phentolamine. In contrast, when noradrenaline (0.1 mM) was added simultaneously with histamine it significantly reduced the inositol phosphate response to high (greater than or equal to 0.1 mM) concentrations of histamine. However, noradrenaline had no inhibitory effect on the carbachol-induced inositol phosphate response. 3. The non-selective beta-agonist isoprenaline (IC50 = 0.08 microM) and the beta 2-selective agonist salbutamol (IC50 = 0.29 microM) both produced a dose-related inhibition of the inositol phosphate response to 0.1 mM histamine. The inhibitory effect of salbutamol was antagonized by propranolol (KA = 2.4 x 10(9) M-1) and the beta 2-selective adrenoceptor antagonist ICI 118551 (KA = 1.7 x 10(9) M-1). 4. The accumulation of 3H-inositol phosphates induced by histamine increased steadily over a 40 min period after an initial lag period of 3-4 min. Following the simultaneous addition of histamine and salbutamol there was a further delay of 3-4 min before the appearance of the inhibitory effect of salbutamol. 5. The effect of histamine on inositol phosphate accumulation was accompanied by a stimulation of [3H]-inositol incorporation into membrane phospholipids which was reduced by the presence of salbutamol.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF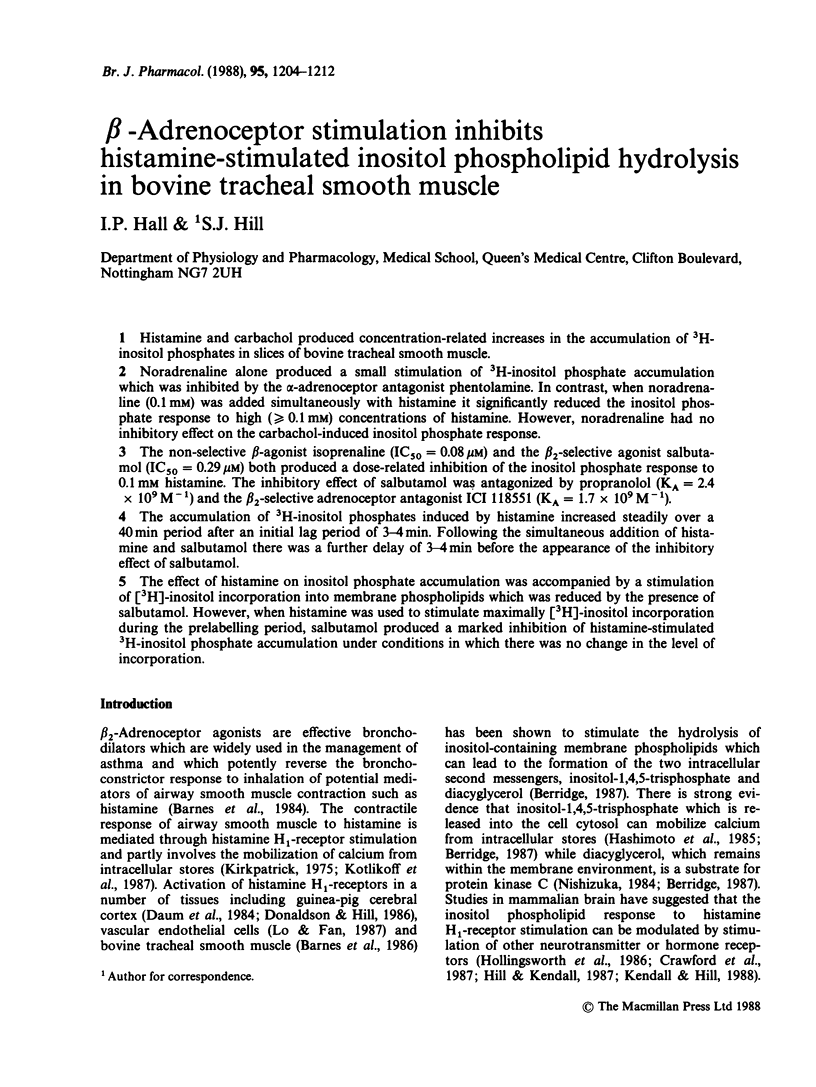



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bilski A. J., Halliday S. E., Fitzgerald J. D., Wale J. L. The pharmacology of a beta 2-selective adrenoceptor antagonist (ICI 118,551). J Cardiovasc Pharmacol. 1983 May-Jun;5(3):430–437. doi: 10.1097/00005344-198305000-00013. [DOI] [PubMed] [Google Scholar]
- Carswell H., Galione A. G., Young J. M. Differential effect of temperature on histamine- and carbachol-stimulated inositol phospholipid breakdown in slices of guinea-pig cerebral cortex. Br J Pharmacol. 1987 Jan;90(1):175–182. doi: 10.1111/j.1476-5381.1987.tb16838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell H., Nahorski S. R. Beta-adrenoceptor heterogeneity in guinea-pig airways: comparison of functional and receptor labelling studies. Br J Pharmacol. 1983 Aug;79(4):965–971. doi: 10.1111/j.1476-5381.1983.tb10542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum P. R., Downes C. P., Young J. M. Histamine stimulation of inositol 1-phosphate accumulation in lithium-treated slices from regions of guinea pig brain. J Neurochem. 1984 Jul;43(1):25–32. doi: 10.1111/j.1471-4159.1984.tb06674.x. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Donaldson J., Hill S. J. Histamine-induced hydrolysis of polyphosphoinositides in guinea-pig ileum and brain. Eur J Pharmacol. 1986 May 27;124(3):255–265. doi: 10.1016/0014-2999(86)90226-8. [DOI] [PubMed] [Google Scholar]
- Donaldson J., Hill S. J. Histamine-induced inositol phospholipid breakdown in the longitudinal smooth muscle of guinea-pig ileum. Br J Pharmacol. 1985 Jun;85(2):499–512. doi: 10.1111/j.1476-5381.1985.tb08887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Sumimoto K., Itoh T., Suzuki H., Kuriyama H. Relaxing actions of procaterol, a beta 2-adrenoceptor stimulant, on smooth muscle cells of the dog trachea. Br J Pharmacol. 1988 Jan;93(1):199–209. doi: 10.1111/j.1476-5381.1988.tb11422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandordy B. M., Cuss F. M., Barnes P. J. Breakdown of phosphoinositides in airway smooth muscle: lack of influence of anti-asthmatic drugs. Life Sci. 1987 Sep 28;41(13):1621–1627. doi: 10.1016/0024-3205(87)90730-2. [DOI] [PubMed] [Google Scholar]
- Grandordy B. M., Cuss F. M., Sampson A. S., Palmer J. B., Barnes P. J. Phosphatidylinositol response to cholinergic agonists in airway smooth muscle: relationship to contraction and muscarinic receptor occupancy. J Pharmacol Exp Ther. 1986 Jul;238(1):273–279. [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Ito Y. A role for inositol 1,4,5-trisphosphate in the initiation of agonist-induced contractions of dog tracheal smooth muscle. Br J Pharmacol. 1985 Sep;86(1):191–199. doi: 10.1111/j.1476-5381.1985.tb09449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S. J., Kendall D. A. Studies on the adenosine-receptor mediating the augmentation of histamine-induced inositol phospholipid hydrolysis in guinea-pig cerebral cortex. Br J Pharmacol. 1987 Jul;91(3):661–669. doi: 10.1111/j.1476-5381.1987.tb11260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth E. B., De la Cruz R. A., Daly J. W. Accumulations of inositol phosphates and cyclic AMP in brain slices: synergistic interactions of histamine and 2-chloroadenosine. Eur J Pharmacol. 1986 Mar 11;122(1):45–50. doi: 10.1016/0014-2999(86)90156-1. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K., Schultz G. GTP-dependent inhibition of cardiac adenylate cyclase by muscarinic cholinergic agonists. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):113–119. doi: 10.1007/BF00500275. [DOI] [PubMed] [Google Scholar]
- Kendall D. A., Hill S. J. Adenosine inhibition of histamine-stimulated inositol phospholipid hydrolysis in mouse cerebral cortex. J Neurochem. 1988 Feb;50(2):497–502. doi: 10.1111/j.1471-4159.1988.tb02939.x. [DOI] [PubMed] [Google Scholar]
- Kendall D. A., Nahorski S. R. Inositol phospholipid hydrolysis in rat cerebral cortical slices: II. Calcium requirement. J Neurochem. 1984 May;42(5):1388–1394. doi: 10.1111/j.1471-4159.1984.tb02799.x. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. T. Excitation and contraction in bovine tracheal smooth muscle. J Physiol. 1975 Jan;244(2):263–281. doi: 10.1113/jphysiol.1975.sp010796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlikoff M. I., Murray R. K., Reynolds E. E. Histamine-induced calcium release and phorbol antagonism in cultured airway smooth muscle cells. Am J Physiol. 1987 Oct;253(4 Pt 1):C561–C566. doi: 10.1152/ajpcell.1987.253.4.C561. [DOI] [PubMed] [Google Scholar]
- Lo W. W., Fan T. P. Histamine stimulates inositol phosphate accumulation via the H1-receptor in cultured human endothelial cells. Biochem Biophys Res Commun. 1987 Oct 14;148(1):47–53. doi: 10.1016/0006-291x(87)91074-6. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Olianas M. C., Onali P., Neff N. H., Costa E. Adenylate cyclase activity of synaptic membranes from rat striatum. Inhibition by muscarinic receptor agonists. Mol Pharmacol. 1983 Mar;23(2):393–398. [PubMed] [Google Scholar]
- Takuwa Y., Takuwa N., Rasmussen H. Carbachol induces a rapid and sustained hydrolysis of polyphosphoinositide in bovine tracheal smooth muscle measurements of the mass of polyphosphoinositides, 1,2-diacylglycerol, and phosphatidic acid. J Biol Chem. 1986 Nov 5;261(31):14670–14675. [PubMed] [Google Scholar]
- Takuwa Y., Takuwa N., Rasmussen H. Measurement of cytoplasmic free Ca2+ concentration in bovine tracheal smooth muscle using aequorin. Am J Physiol. 1987 Dec;253(6 Pt 1):C817–C827. doi: 10.1152/ajpcell.1987.253.6.C817. [DOI] [PubMed] [Google Scholar]


