Abstract
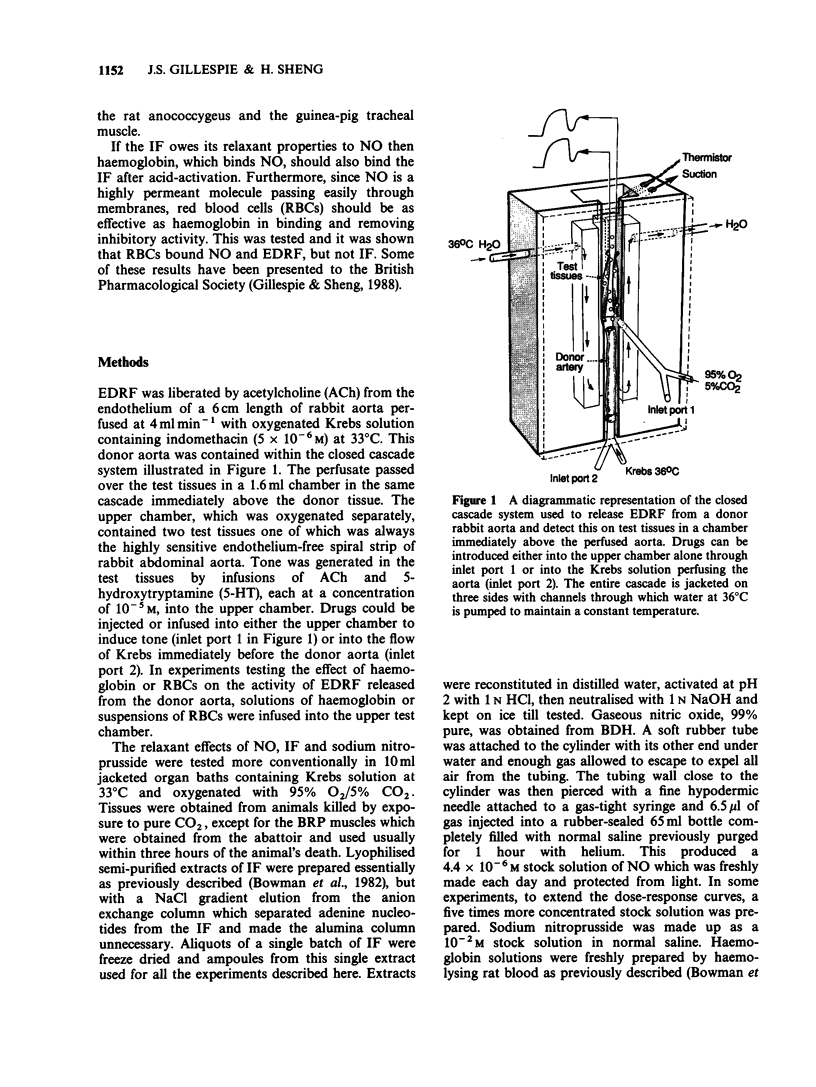
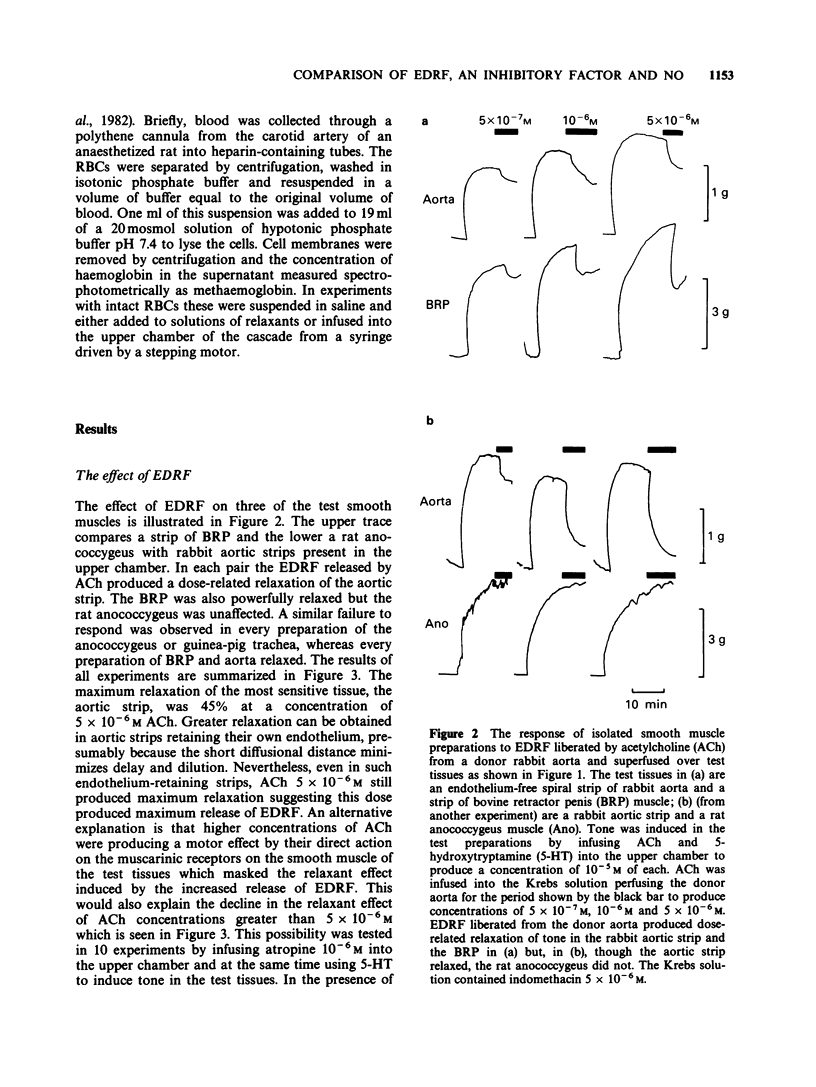
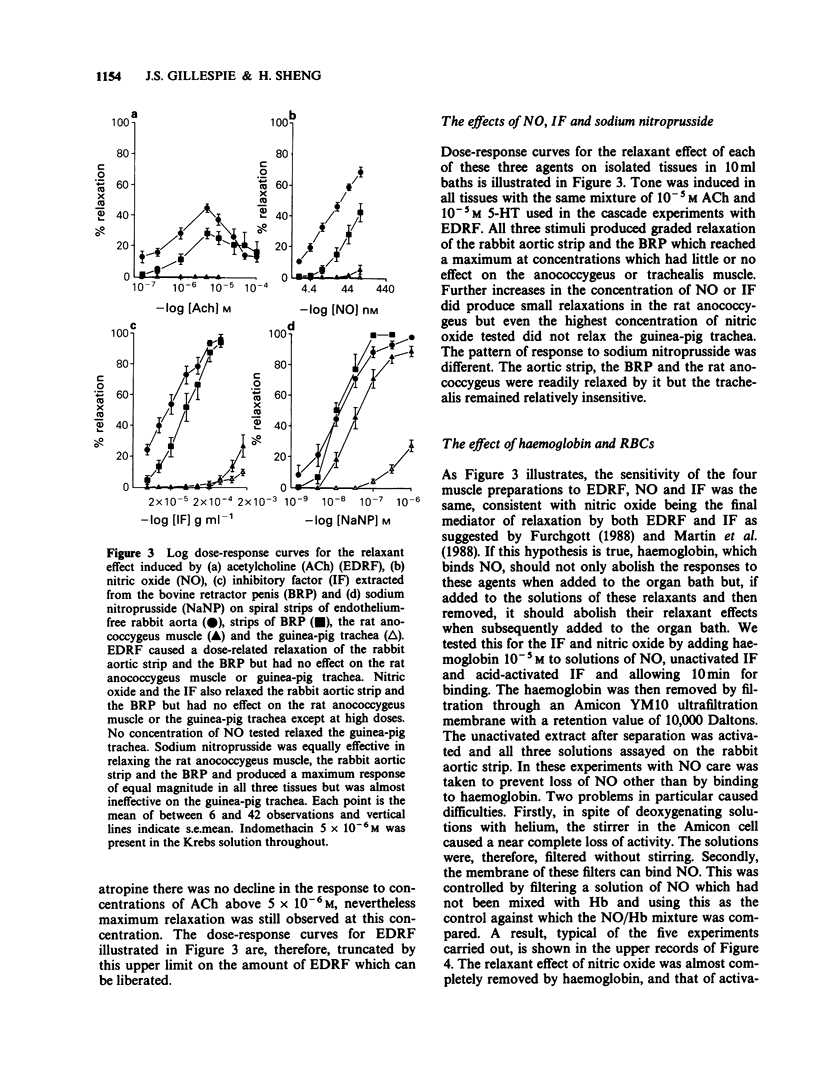
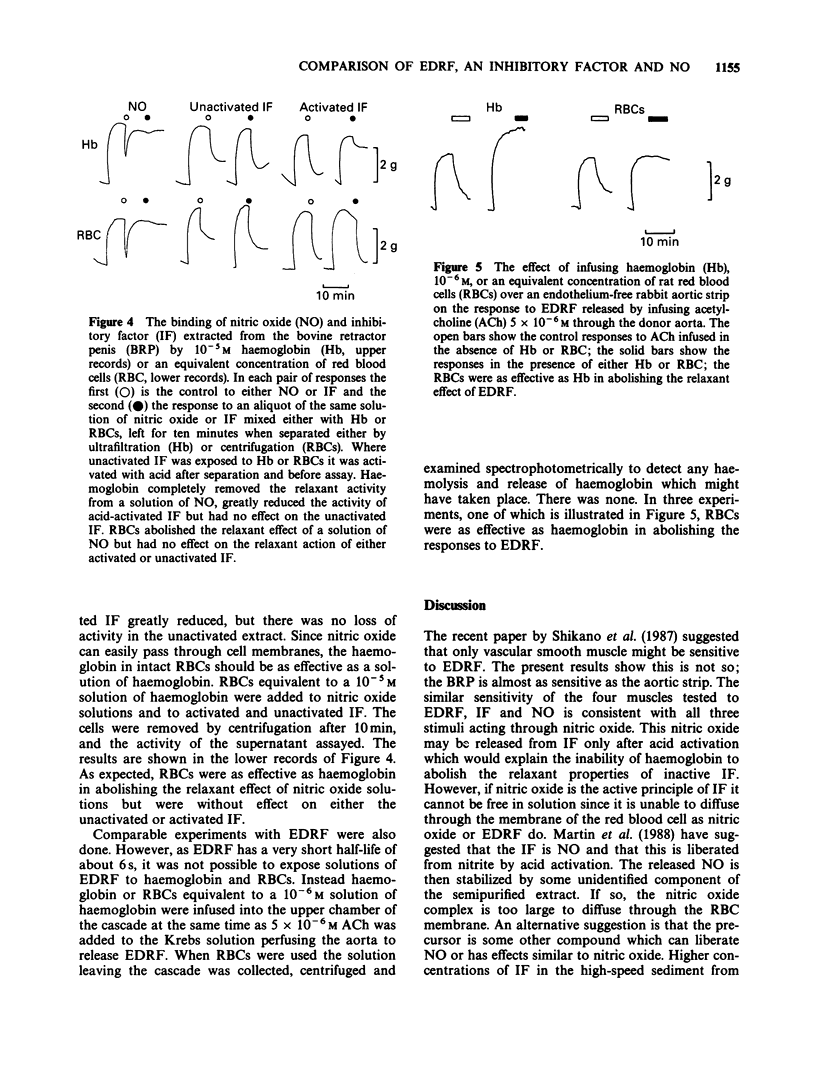
1. The relaxant action of endothelium-derived relaxing factor (EDRF), the smooth muscle inhibitory factor (IF) isolated from the bovine retractor penis (BRP), nitric oxide (NO) and sodium nitroprusside (NaNP) on four vascular and non-vascular smooth muscle preparations has been examined. Their sensitivity to EDRF, the IF and NO was the same, suggesting all might be NO. Sodium nitroprusside produced complete relaxation of the rat anococcygeus at low doses, suggesting an action additional to the intracellular release of NO. 2. Haemoglobin added to solutions of EDRF, activated IF or NO completely removed their relaxant properties, consistent with all three acting by virtue of NO. 3. Suspensions of red blood cells with a haemoglobin concentration equivalent to to that used in the previous experiments were as effective as haemoglobin in abolishing the relaxant effect of EDRF or NO but were ineffective against the activated IF. 4. The similarity in sensitivity of a series of smooth muscles and the binding by haemoglobin are consistent with NO being the active principle of both EDRF and the acid activated IF. The abolition of the effect of EDRF by red blood cells (RBCs) is further confirmation for this hypothesis, but the ineffectiveness of RBCs against acid-activated IF suggests that either the latter is not NO or that it is bound in a way which makes it unable to diffuse through cell membranes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N., Killick S. W., Aboo Aar M. Extraction from ox retractor penis of an inhibitory substance which mimics its atropine-resistant neurogenic relaxation. Br J Pharmacol. 1975 Jul;54(3):409–410. doi: 10.1111/j.1476-5381.1975.tb07585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Pollock D. Oxyhaemoglobin blocks non-adrenergic non-cholinergic inhibition in the bovine retractor penis muscle. Eur J Pharmacol. 1982 Nov 19;85(2):221–224. doi: 10.1016/0014-2999(82)90470-8. [DOI] [PubMed] [Google Scholar]
- Cocks T. M., Angus J. A., Campbell J. H., Campbell G. R. Release and properties of endothelium-derived relaxing factor (EDRF) from endothelial cells in culture. J Cell Physiol. 1985 Jun;123(3):310–320. doi: 10.1002/jcp.1041230304. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Hunter J. C., Martin W. Some physical and chemical properties of the smooth muscle inhibitory factor in extracts of the bovine retractor penis muscle. J Physiol. 1981 Jun;315:111–125. doi: 10.1113/jphysiol.1981.sp013736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Hunter J. C., McKnight A. T. The effect of ethanol on inhibitory and motor responses in the rat and rabbit anococcygeus and the bovine retractor penis muscles. Br J Pharmacol. 1982 Jan;75(1):189–198. doi: 10.1111/j.1476-5381.1982.tb08772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Martin W. A smooth muscle inhibitory material from the bovine retractor penis and rat anococcygeus muscles. J Physiol. 1980 Dec;309:55–64. doi: 10.1113/jphysiol.1980.sp013493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Martin W., Smith J. A., Lewis M. J., Henderson A. H. Evidence that inhibitory factor extracted from bovine retractor penis is nitrite, whose acid-activated derivative is stabilized nitric oxide. Br J Pharmacol. 1988 Mar;93(3):579–586. doi: 10.1111/j.1476-5381.1988.tb10313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Shikano K., Ohlstein E. H., Berkowitz B. A. Differential selectivity of endothelium-derived relaxing factor and nitric oxide in smooth muscle. Br J Pharmacol. 1987 Nov;92(3):483–485. doi: 10.1111/j.1476-5381.1987.tb11347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


